Time-Dependent Growth of Silica Shells on CdTe Quantum Dots
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Synthesis of CdTe Nanocrystals
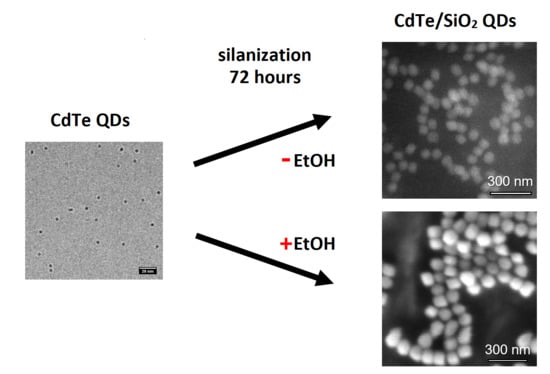
2.3. Synthesis of CdTe/SiO2 Nanocrystals
2.4. Ethanol Precipitations of CdTe/SiO2 Nanocrystals
2.5. Photophysical and Size Characterization
3. Results and Discussion
3.1. Photophysical Properties
3.2. Size Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Drbohlavová, J.; Adam, V.; Kizek, R.; Hubálek, J. Quantum dots—Characterization, preparation and usage in biological systems. Int. J. Mol. Sci. 2009, 10, 656–673. [Google Scholar] [CrossRef] [PubMed]
- Hobson, D.W. Commercialization of nanotechnology. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Farka, Z.; Juřík, T.; Kovář, D.; Trnková, L.; Skládal, P. Nanoparticle-Based Immunochemical Biosensors and Assays: Recent Advances and Challenges. Chem. Rev. 2017, 117, 9973–10042. [Google Scholar] [CrossRef] [PubMed]
- Klepárník, K.; Voráčová, I.; Lišková, M.; Přikryl, J.; Hezinová, V.; Foret, F. Capillary electrophoresis immunoassays with conjugated quantum dots. Electrophoresis 2011, 32, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Lišková, M.; Voráčová, I.; Klepárník, K.; Hezinová, V.; Přikryl, J.; Foret, F. Conjugation reactions in the preparations of quantum dot-based immunoluminescent probes for analysis of proteins by capillary electrophoresis. Anal. Bioanal. Chem. 2011, 400, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Voráčová, I.; Klepárník, K.; Lišková, M.; Foret, F. Determination of ζ-potential, charge, and number of organic ligands on the surface of water soluble quantum dots by capillary electrophoresis. Electrophoresis 2015, 36, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Nazzal, A.Y.; Wang, X.; Qu, L.; Yu, W.; Wang, Y.; Peng, X.; Xiao, M. Environmental Effects on Photoluminescence of Highly Luminescent CdSe and CdSe/ZnS Core/Shell Nanocrystals in Polymer Thin Films. J. Phys. Chem. B 2004, 108, 5507–5515. [Google Scholar] [CrossRef]
- Derfus, A.M.; Chan, W.C.W.; Bhatia, S.N. Probing the Cytotoxicity of Semiconductor Quantum Dots. Nano Lett. 2004, 4, 11–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha, T.L.; Mestre, N.C.; Sabóia-Morais, S.M.T.; Bebianno, M.J. Environmental behaviour and ecotoxicity of quantum dots at various trophic levels: A review. Environ. Int. 2017, 98, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Modlitbová, P.; Novotný, K.; Pořízka, P.; Klus, J.; Zlámalová-Gargošová, H.; Kaiser, J. Comparative investigation of toxicity and bioaccumulation of Cd-based quantum dots and Cd salt in freshwater plant Lemna minor L. Ecotoxicol. Environ. Saf. 2018, 147, 334–341. [Google Scholar] [CrossRef]
- Mattoussi, H.; Matthew Mauro, J.; Goldman, E.R.; Anderson, G.P.; Sundar, V.C.; Mikulec, F.V.; Bawendi, M.G. Self-assembly of CdSe-ZnS quantum dot bioconjugates using an engineered recombinant protein. J. Am. Chem. Soc. 2000, 122, 12142–12150. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y. Encapsulation of quantum nanodots in polystyrene and silica micro-/nanoparticles. Langmuir 2004, 20, 6071–6073. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Yang, C.; Qiao, R.; Niu, M.; Du, M.; Wang, D.; Gao, M. Highly fluorescent CdTe@SiO2 particles prepared via reverse microemulsion method. Chem. Mater. 2010, 22, 420–427. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, M. Preparation of fluorescent SiO2 particles with single CdTe nanocrystal cores by the reverse microemulsion method. Adv. Mater. 2005, 17, 2354–2357. [Google Scholar] [CrossRef]
- Wolcott, A.; Gerion, D.; Visconte, M.; Sun, J.; Schwartzberg, A.; Chen, S.; Zhang, J.Z. Silica-coated CdTe quantum dots functionalized with thiols for bioconjugation to IgG proteins. J. Phys. Chem. B 2006, 110, 5779–5789. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, S.J.; Chang, J.C.; Kovtun, O.; McBride, J.R.; Tomlinson, I.D. Biocompatible quantum dots for biological applications. Chem. Biol. 2011, 18, 10–24. [Google Scholar] [CrossRef] [PubMed]
- De Koninck, P.; Labrecque, S.; Heyes, C.D.; Wiseman, P.W. Probing synaptic signaling with quantum dots. HFSP J. 2007, 1, 5–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nann, T.; Mulvaney, P. Single quantum dots in spherical silica particles. Angew. Chem. Int. Ed. 2004, 43, 5393–5396. [Google Scholar] [CrossRef] [PubMed]
- Gerion, D.; Pinaud, F.; Williams, S.C.; Parak, W.J.; Zanchet, D.; Weiss, S.; Alivisatos, A.P. Synthesis and properties of biocompatible water-soluble silica-coated CdSe/ZnS semiconductor quantum dots. J. Phys. Chem. B 2001, 105, 8861–8871. [Google Scholar] [CrossRef]
- Rogach, A.L.; Nagesha, D.; Ostrander, J.W.; Giersig, M.; Kotov, N.A. “Raisin bun”-type composite spheres of silica and semiconductor nanocrystals. Chem. Mater. 2000, 12, 2676–2685. [Google Scholar] [CrossRef]
- Correa-Duarte, M.A.; Giersig, M.; Liz-Marzán, L.M. Stabilization of CdS semiconductor nanoparticles against photodegradation by a silica coating procedure. Chem. Phys. Lett. 1998, 286, 497–501. [Google Scholar] [CrossRef]
- Modlitbová, P.; Pořízka, P.; Novotný, K.; Drbohlavová, J.; Chamradová, I.; Zlámalová-Gargošová, H.; Romih, T.; Kaiser, J. Short-term assessment of cadmium toxicity and uptake from di ff erent types of Cd-based Quantum Dots in the model plant Allium cepa L. Ecotoxicol. Environ. Saf. 2018, 153, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Selvan, S.T.; Tan, T.T.; Ying, J.Y. Robust, non-cytotoxic, silica-coated CdSe quantum dots with efficient photoluminescence. Adv. Mater. 2005, 17, 1620–1625. [Google Scholar] [CrossRef]
- Mulvaney, P.; Liz-Marzán, L.M.; Giersig, M.; Ung, T. Silica encapsulation of quantum dots and metal clusters. J. Mater. Chem. 2000, 10, 1259–1270. [Google Scholar] [CrossRef]
- Giersig, M.; Ung, T.; Liz-Marzhn, L.M.; Mulvaney, P. Direct Observation of Chemical Reactions in Silica-Coated Gold and Silver Nanoparticles. Adv. Mater. Commun. 1997, 9, 570–575. [Google Scholar] [CrossRef]
- Bootz, A.; Vogel, V.; Schubert, D.; Kreuter, J. Comparison of scanning electron microscopy, dynamic light scattering and analytical ultracentrifugation for the sizing of poly(butyl cyanoacrylate) nanoparticles. Eur. J. Pharm. Biopharm. 2004, 57, 369–375. [Google Scholar] [CrossRef]
- Mahl, D.; Diendorf, J.; Meyer-Zaika, W.; Epple, M. Possibilities and limitations of different analytical methods for the size determination of a bimodal dispersion of metallic nanoparticles. Colloids Surfaces A Physicochem. Eng. Asp. 2011, 377, 386–392. [Google Scholar] [CrossRef]
- Lange, H. Comparative test of methods to determine particle size and particle size distribution in the submicron range. Part. Part. Syst. Charact. 1995, 12, 148–157. [Google Scholar] [CrossRef]
- Bowen, P. Particle size distribution measurement from millimeters to nanometers and from rods to platelets. J. Dispers. Sci. Technol. 2002, 23, 631–662. [Google Scholar] [CrossRef]




| QDs | Time of Silica Shell Growth (h) | DLS (nm) | SEM/TEM * (nm) |
|---|---|---|---|
| CdTe | / | 3.5 ± 0.2 | 3.8 ± 0.3 * |
| CdTe/SiO2 | 24 | 28.8 ± 2.2 | 53.7 ± 4.2 |
| CdTe/SiO2 | 72 | 29.2 ± 2.2 | 59.7 ± 4.0 |
| CdTe/SiO2 + EtOH | 24 | 50.1 ± 9.3 | 62.3 ± 7.5 |
| CdTe/SiO2 + EtOH | 72 | 115.1 ± 13.7 | 83.1 ± 6.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Modlitbová, P.; Klepárník, K.; Farka, Z.; Pořízka, P.; Skládal, P.; Novotný, K.; Kaiser, J. Time-Dependent Growth of Silica Shells on CdTe Quantum Dots. Nanomaterials 2018, 8, 439. https://doi.org/10.3390/nano8060439
Modlitbová P, Klepárník K, Farka Z, Pořízka P, Skládal P, Novotný K, Kaiser J. Time-Dependent Growth of Silica Shells on CdTe Quantum Dots. Nanomaterials. 2018; 8(6):439. https://doi.org/10.3390/nano8060439
Chicago/Turabian StyleModlitbová, Pavlína, Karel Klepárník, Zdeněk Farka, Pavel Pořízka, Petr Skládal, Karel Novotný, and Jozef Kaiser. 2018. "Time-Dependent Growth of Silica Shells on CdTe Quantum Dots" Nanomaterials 8, no. 6: 439. https://doi.org/10.3390/nano8060439
APA StyleModlitbová, P., Klepárník, K., Farka, Z., Pořízka, P., Skládal, P., Novotný, K., & Kaiser, J. (2018). Time-Dependent Growth of Silica Shells on CdTe Quantum Dots. Nanomaterials, 8(6), 439. https://doi.org/10.3390/nano8060439