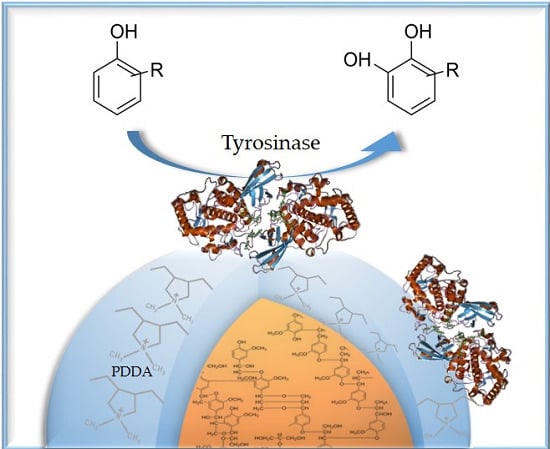
Functionalized Tyrosinase-Lignin Nanoparticles as Sustainable Catalysts for the Oxidation of Phenols
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Reactivity of Tyrosinase with Organosolv Lignin
2.3. Preparation of Catalyst I
2.4. Preparation of Catalyst II
2.5. Preparation of Catalyst III
2.6. Preparation of Catalyst IV
2.7. SEM Characterization
2.8. DLS and Zeta Potential
2.9. Determination of Activity Parameters
2.10. Determination of the Kinetic Constants
2.11. Electrochemical Characterization
2.12. General Procedure for the Oxidation of Phenols
3. Results and Discussion
3.1. Reactivity of Tyrosinase towards Organosolv Lignin (OL)
3.2. Immobilization Procedures
3.2.1. Encapsulation and Direct Adsorption Procedures
3.2.2. Layer-By-Layer Immobilization Procedure
3.3. Activity Parameters of Catalysts I–IV
3.4. Cyclic Voltammetry Analysis
3.5. Kinetic Properties of Catalysts I–IV and Synthesis of Catechol Derivatives
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ghisellini, P.; Cialani, C.; Ulgiati, S. A review on circular economy: The expected transition to a balanced interplay of environmental and economic systems. J. Clean. Prod. 2016, 114, 11–32. [Google Scholar] [CrossRef]
- Hu, T.Q. (Ed.) Chemical Modification, Properties, and Usage of Lignin; Kluwer Academic-Plenum Publishers: New York, NY, USA, 2002. [Google Scholar]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H. Lignin Valorization: Improving Lignin Processing in the Biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Uppal, S.K. Structural characterization and antioxidant activity of lignin from sugarcane bagasse. Colloid Polym. Sci. 2015, 293, 2585–2592. [Google Scholar] [CrossRef]
- Li, M.F.; Sun, S.N.; Xu, F.; Sun, R.C. Microwave-assisted organic acid extraction of lignin from bamboo: Structure and antioxidant activity investigation. Food Chem. 2012, 134, 1392–1398. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, H.; Hatakeyama, T. Biopolymers: Lignin, Proteins, Bioactive Nanocomposites; Abe, A., Dusek, K., Kobayashi, S., Eds.; Springer: Berlin, Germany, 2010; Volume 232, pp. 1–63. [Google Scholar]
- Zimniewska, M.; Batog, J.; Bogacz, E.; Romanowska, B. Functionalization of natural fibres textiles by improvement of nanoparticles fixation on their surface. J. Fiber Bioeng. Inf. 2012, 5, 321–339. [Google Scholar] [CrossRef]
- Ding, J.; Gu, L.; Dong, W.; Yu, H. Epoxidation Modification of Renewable Lignin to Improve the Corrosion Performance of Epoxy Coating. Int. J. Electrochem. Sci. 2016, 11, 6256–6265. [Google Scholar] [CrossRef]
- Milczarek, G.; Nowicki, M. Carbon nanotubes/kraft lignin composite: Characterization and charge storage properties. Mater. Res. Bull. 2013, 48, 4032–4038. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K.; Raghavan, P.; Kessler, P. Progress in Green Polymer Composites from Lignin for Multifunctional Applications: A Review. ACS Sustain. Chem. Eng. 2014, 2, 1072–1092. [Google Scholar] [CrossRef]
- Lievonen, M.; Valle-Delgado, J.J.; Mattinen, M.; Hult, E.; Lintinen, K.; Kostiainen, M.A.; Paananen, A.; Szilvay, G.R.; Setälä, H.; Österberg, M. A simple process for lignin nanoparticle preparation. Green Chem. 2015, 18, 1416–1422. [Google Scholar] [CrossRef]
- Doungporn, Y.; Grit, B.; Eckhard, T.; Katharina, L.; Wurm, F.R. Biodegradable lignin nanocontainers. RSC Adv. 2014, 4, 11661–11663. [Google Scholar] [CrossRef] [Green Version]
- Qian, Y.; Zhong, X.; Li, Y.; Qiu, X. Fabrication of uniform lignin colloidal spheres for developing natural broad-spectrum sunscreens with high sun protection factor. Ind. Crops Prod. 2017, 101, 54–60. [Google Scholar] [CrossRef]
- Xiong, F.; Han, Y.; Wang, S.; Li, G.; Qin, T.; Chen, Y. Preparation and formation mechanism of size-controlled lignin nanospheres by self-assembly. Ind. Crops Prod. 2017, 100, 146–152. [Google Scholar] [CrossRef]
- Myint, A.A.; Lee, H.W.; Seo, B.; Son, W.-S.; Yoon, J.; Yoon, T.J.; Park, H.J.; Yu, J.; Yoon, J.; Lee, Y.-W. One pot synthesis of environmentally friendly lignin nanoparticles with compressed liquid carbon dioxide as an antisolvent. Green Chem. 2016, 18, 2129–2146. [Google Scholar] [CrossRef]
- Zhang, S.; Jiang, Z.; Wang, X.; Yan, C.; Shi, J. A facile method to prepare microcapsules inspired by polyphenol chemistry for efficient enzyme immobilization. ACS Appl. Mater. Interface. 2015, 7, 19570–19578. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, C.S.; Gentil de Farias Lemos, M.G.; de Sousa, M.; Barros Goncalves, L.R. Ezyme immobilization onto renewable polymeric matrixes: Past, present, and future trends. J. Appl. Polym. Sci. 2015, 42125, 1–15. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Isozaki, K.; Nakamura, M.; Takaya, H.; Watanabe, T. Discovery of 12-mer peptides that bind to wood lignin. Sci. Rep. 2016, 6, 21833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leskinen, T.; Witos, J.; Valle Delgato, J.J.; Lintinen, K.S.; Kostiainen, M.A.; Wiedmer, S.K.; Osterberg, M.; Mattinen, M.L. Adsorption of proteins on colloidal lignin particles for advanced biomaterials. Biomacromolecules 2017, 18, 2767–2776. [Google Scholar] [CrossRef] [PubMed]
- Strobel, K.L.; Pfeiffer, K.A.; Blanch, H.W.; Clark, D.S. Structural Insights into the Affinity of Cel7A Carbohydrate-binding Module for Lignin. J. Biol. Chem. 2015, 290, 22818–22826. [Google Scholar] [CrossRef] [PubMed]
- Westereng, B.; Cannella, D.; Agger, J.W.; Jørgensen, H.; Andersen, M.L.; Eijsink, V.G.H.; Felby, C. Enzymatic cellulose oxidation is linked to lignin by long-range electron transfer. Sci. Rep. 2015, 5, 18561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frommhagen, M.; Mutte, S.K.; Westphal, A.H.; Koetsier, M.J.; Hinz, S.W.A.; Visser, J.; Vincken, J.-P.; Weijers, D.; van Berkel, W.J.H.; Gruppen, H.; et al. Boosting LPMO-driven lignocellulose degradation by polyphenol oxidase-activated lignin building blocks. Biotechnol. Biofuels 2017, 10, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frommhagen, M.; Koetsier, M.J.; Westphal, A.H.; Visser, J.; Hinz, S.W.A.; Vincken, J.-P. Lytic Polysaccharide monooxygenase from Myceliophthora thermophila C1 differ in substrate preference and reducing agent specificity. Biotechnol. Biofuels 2016, 9, 186. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, D.; Gallone, A.; Fiocco, D.; Palazzo, G.; Mallardi, A. Mushroom tyrosinase in polyelectrolyte multilayers as an optical biosensor for o-diphenols. Biosens. Bioelectron. 2010, 25, 2033–2037. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Zhou, Y.; Xu, J.; Ai, S.; Cui, L.; Zhu, L. Amperometric biosensor based on tyrosinase immobilized onto multiwalled carbon nanotubes-cobalt phthalocyanine-silk fibroin film and its application to determine bisphenol A. Anal. Chim. Acta 2010, 659, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Dinçer, A.; Becerik, S.; Aydemir, T. Immobilization of tyrosinase on chitosan–clay composite beads. Int. J. Biol. Macromol. 2012, 50, 815–820. [Google Scholar] [CrossRef]
- Guazzaroni, M.; Crestini, C.; Saladino, R. Layer-by-Layer coated tyrosinase: An efficient and selective synthesis of catechols. Bioorg. Med. Chem. 2012, 20, 157–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guazzaroni, M.; Pasqualini, M.; Botta, G.; Saladino, R. A Novel Synthesis of Bioactive Catechols by Layer-by-Layer Immobilized Tyrosinase in an Organic Solvent Medium. Chem. Cat. Chem. 2012, 4, 89–99. [Google Scholar] [CrossRef]
- Subrizi, F.; Crucianelli, M.; Grossi, V.; Passacantando, M.; Pesci, L.; Saladino, R. Carbon Nanotubes as Activating Tyrosinase Supports for the Selective Synthesis of Catechols. ACS Catal. 2014, 4, 810–822. [Google Scholar] [CrossRef]
- Botta, G.; Delfino, M.; Guazzaroni, M.; Crestini, C.; Onofri, S.; Saladino, R. Selective Synthesis of DOPA and DOPA Peptides by Native and Immobilized Tyrosinase in Organic Solvent. ChemPlusChem 2013, 78, 325–330. [Google Scholar] [CrossRef] [Green Version]
- Bizzarri, B.M.; Martini, A.; Serafini, F.; Aversa, D.; Piccinino, D.; Botta, L.; Berretta, N.; Guatteo, E.; Saladino, R. Tyrosinase mediated oxidative functionalization in the synthesis of DOPA-derived peptidomimetics with anti-Parkinson activity. RSC Adv. 2017, 7, 20502–20509. [Google Scholar] [CrossRef] [Green Version]
- Bozzini, T.; Botta, G.; Delfino, M.; Onofri, S.; Saladino, R.; Amatore, D.; Sgarbanti, R.; Nencioni, L.; Palamara, A.T. Tyrosinase and Layer-by-Layer supported tyrosinases in the synthesis of lipophilic catechols with antiinfluenza activity. Bioorg. Med. Chem. 2013, 21, 7699–7708. [Google Scholar] [CrossRef] [PubMed]
- Botta, G.; Bizzarri, B.M.; Garozzo, A.; Timpanaro, R.; Bisignano, B.; Amatore, D.; Palamara, A.T.; Nencioni, L.; Saladino, R. Carbon nanotubes supported tyrosinase in the synthesis of lipophilic hydroxytyrosol and dihydrocaffeoyl catechols with antiviral activity against DNA and RNA viruses. Bioorg. Med. Chem. 2015, 23, 5345–5351. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, B.M.; Rotelli, L.; Botta, G.; Saladino, R. Current advances in DOPA and DOPA-peptidomimetics: Chemistry, applications and biological activity. Curr. Med. Chem. 2015, 22, 4138–4165. [Google Scholar] [CrossRef] [PubMed]
- Botta, L.; Bizzarri, B.M.; Crucianelli, M.; Saladino, R. Advances in biotechnological synthetic applications of carbon nanostructured systems. J. Mater. Chem. B 2017, 5, 6490–6510. [Google Scholar] [CrossRef]
- Ismaya, W.T.; Rozeboom, H.J.; Schurink, M.; Boeriu, C.G.; Wichers, H.; Dijkstra, B.W. Crystallization and preliminary X-ray crystallographic analysis of tyrosinase from the mushroom Agaricus bisporus. Acta Crystallogr. 2011, 67, 575–578. [Google Scholar] [CrossRef]
- El Hage, R.; Brosse, N.; Chrusciel, L.; Sanchez, C.; Sannigrahi, P.; Rag, A. Characterization of milled wood lignin and ethanol organosolv lignin from Miscanthus. Polym. Degrad. Stab. 2009, 94, 1632–1638. [Google Scholar] [CrossRef]
- Hu, J.; Shen, D.; Wu, S.; Zhang, H.; Xiao, R. Composition Analysis of Organosolv Lignin and Its Catalytic Solvolysis in Supercritical Alcohol. Energy Fuels 2014, 28, 4260–4266. [Google Scholar] [CrossRef]
- Granata, A.; Argyropoulos, D.S. 2-Chloro-4,4,5,5-tetramethyl-1,3,2-dioxaphospholane, a Reagent for the Accurate Determination of the Uncondensed and Condensed Phenolic Moieties in Lignins. J. Agric. Food Chem. 1995, 43, 1538–1544. [Google Scholar] [CrossRef]
- Lu, F.; Ralph, J. The DFRC method for lignin analysis. 2. Monomers from isolated lignins. J. Agric. Food Chem. 1998, 46, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; He, H.; Jiang, H.; Ma, L.; Jia, D.M. Nano-lignin filled natural rubber composites: Preparation and characterization. Express Polym. Lett. 2013, 7, 480–493. [Google Scholar] [CrossRef] [Green Version]
- Tortolini, C.; Bollella, P.; Antiochia, R.; Favero, G.; Mazzei, F. Inhibition-based biosensor for atrazine detection. Sens. Actuators B Chem. 2016, 224, 552–558. [Google Scholar] [CrossRef]
- Leboukh, S.; Gouzi, H.; Coradin, T.; Yahia, H. An optical catechol biosensor based on a desert truffle tyrosinase extract immobilized into a sol–gel silica layered matrix. J. Sol-Gel Sci. Technol. 2018, 86, 675–681. [Google Scholar] [CrossRef]
- Narukawa, Y.; Komatsu, C.; Yamauchi, R.; Shibayama, S.; Hachisuka, M.; Kiuchi, F. Two new lignans and melanogenesis inhibitors from Schisandra nigra. J. Nat. Med. 2016, 70, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.J.; Kwon, H.S. Tyrosinase Inhibitory Activities of Safrole from Myristica fragrans Houtt. J. Appl. Biol. Chem. 2015, 58, 295–301. [Google Scholar] [CrossRef]
- Malik, A.; Khan, M.T.H.; Khan, S.B.; Ahmad, A.; Choudhary, M.I. Tyrosinase inhibitory lignans from the methanol extract of the roots of Vitex negundo Linn and their structure-activity relationship. Phytomedicine 2006, 13, 255–260. [Google Scholar] [CrossRef]
- Grönqvist, S.; Viikari, L.; Niku-Paavola, M.-L.; Orlandi, M.; Canevali, C.; Buchert, J. Oxidation of milled wood lignin with laccase, tyrosinase and horseradish peroxidase. Appl. Microbiol. Biotechnol. 2005, 67, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Min, K.; Yum, T.; Kim, J.; Woo, H.M.; Kim, Y.; Sang, B.-I.; Yoo, Y.J.; Kim, Y.H.; Um, Y. Perspectives for biocatalytic lignin utilization: Cleaving 4-O-5 and Cα-Cβ bonds in dimeric lignin model compounds catalyzed by a promiscuous activity of tyrosinase. Biotech. Biofuels 2017, 10, 212. [Google Scholar] [CrossRef] [PubMed]
- Cieńska, M.; Labus, K.; Lewańczuk, M.; Koźlecki, T.; Liesiene, J.; Bryjak, J.; Legault, C. Effective L-Tyrosine hydroxylation of native and immobilized tyrosinase. PLoS ONE 2016, 11, e0164213. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Liu, R.; Hu, L.-Q.; Zou, Z.-F.; Si, C.-L. Lignin Nanoparticle as a Novel Green Carrier for the Efficient Delivery of Resveratrol. ACS Sustain. Chem. Eng. 2017, 5, 8241–8249. [Google Scholar] [CrossRef]
- Figueiredo, P.; Lintinen, K.; Kiriazis, A.; Hynninen, V.; Liu, Z.; Bauleth-Ramos, T.; Yli-Kauhaluoma, J. In vitro evaluation of biodegradable lignin-based nanoparticles for drug delivery and enhanced antiproliferation effect in cancer cells. Biomaterials 2017, 121, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qiu, X.; Qian, Y.; Xiong, W.; Yang, D. pH-responsive lignin-based complex micelles: Preparation, characterization and application in oral drug delivery. Chem. Eng. J. 2017, 327, 1176–1183. [Google Scholar] [CrossRef]
- Caluey, A.N.; Wilson, J.N. Functionalized lignin biomaterials for enhancing optical properties and cellular interactions of dyes. Biomater. Sci. 2017, 5, 2114–2121. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Sakaguchi, M.; Maitani, Y. Folate-linked lipid-based nanoparticles deliver a NFκB decoy into activated murine macrophage-like RAW264 7 cells. Biol. Pharm. Bull. 2006, 29, 1516–1520. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, L.A.; Radotic, K. Fluorescence lifetime imaging of lignin autofluorescence in normal and compression wood. J. Microsc. 2013, 251, 178–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piccinino, D.; Delfino, M.; Botta, G.; Crucianelli, M.; Grossi, V.; Passacantando, M.; Saladino, R. Highly efficient synthesis of aldehydes by layer-by-layer multi-walled carbon nanotubes (MWCNTs) laccase mediator systems. Appl. Catal. A Gen. 2015, 499, 77–88. [Google Scholar] [CrossRef]
- Pillai, K.V.; Renneckar, S. Cation p-interactions as a mechanism in technical lignin adsorption to cationic surfaces. Biomacromolecules 2009, 10, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-M.; Yen, M.-J.; Chen, L.-C. A bioanode based on MWCNT/protein-assisted co-immobilization of glucose oxidase and 2, 5-dihydroxybenzaldehyde for glucose fuel cells. Biosens. Bioelectron. 2010, 25, 2515–2521. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Solanki, K.; Gupta, M.N. Enhancement of lipase activity in non-aqueous media upon immobilization on multi-walled carbon nanotubes. Chem. Cent. J. 2007, 1, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Masamoto, Y.; Kubo, M. Inhibitory effect of Chinese crude drugs on tyrosinase. Planta Med. 1980, 40, 361–365. [Google Scholar] [CrossRef] [PubMed]
- Arslan, A.; Kiralp, S.; Toppare, L.; Yagci, Y. Immobilization of tyrosinase in polysiloxane/polypyrrole copolymer matrices. Int. J. Biol. Macromol. 2005, 35, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Tischer, W. Immobilized enzymes: Crystals or carriers? Trends Biotechnol. 1999, 117, 326–335. [Google Scholar] [CrossRef]
- Kim, M.A.; Lee, W.Y. Amperometric phenol biosensor based on sol–gel silicate/Nafion composite film. Anal. Chim. Acta 2003, 479, 143–150. [Google Scholar] [CrossRef]
- Yu, J.H.; Liu, S.Q.; Ju, H.X. Mediator-free phenol sensor based on titania sol–gel encapsulation matrix for immobilization of tyrosinase by a vapor deposition method. Biosens. Bioelectron. 2003, 19, 509–514. [Google Scholar] [CrossRef]
- Fernández-Mar, M.I.; Mateos, R.; García-Parrilla, M.C.; Puertas, B.; Cantos-Villar, E. Bioactive compounds in wine: Resveratrol, hydroxytyrosol and melatonin: A review. Food Chem. 2012, 130, 797–813. [Google Scholar] [CrossRef] [Green Version]
- Lekse, J.; Xia, L.; Stark, J.; Morrow, J.D.; May, J.M. Plant catechols prevent lipid peroxidation in human plasma and erythrocytes. Mol. Cell. Biochem. 2001, 226, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Farhoosh, R.; Johnny, S.; Asnaashari, M.; Molaahmadibahraseman, N.; Sharif, A. Structure–antioxidant activity relationships of o-hydroxyl, o-methoxy, and alkyl ester derivatives of p-hydroxybenzoic acid. Food Chem. 2016, 194, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Nolan, L.C.; O’Connor, K.E. Dioxygenase-and monooxygenase-catalysed synthesis of cis-dihydrodiols, catechols, epoxides and other oxygenated products. Biotechnol. Lett. 2008, 30, 1879–1891. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, M.P.; Barril, C.; Clark, A.C.; Prenzler, P.D.; Scollary, G.R. Ascorbic acid: A review of its chemistry and reactivity in relation to a wine environment. Crit. Rev. Food Sci. Nutr. 2011, 51, 479–498. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.G.; Li, S.B.; Xue, Y.; Zhang, H.T.; Quan, J.; Nie, H.L.; Branford-White, C. 4-Hydroxyphenylacetic Acid as a Monophenolase Inhibitor and a Diphenolase Activator on Mushroom Tyrosinase. In Proceedings of the 3rd International Conference on Bioinformatics and Biomedical Engineering, Beijing, China, 11–13 June 2009. [Google Scholar] [CrossRef]
- Habeeb, A.F.S.A.; Hiramoto, R. Reaction of proteins with glutaraldehyde. Arch. Biochem. Biophys. 1968, 126, 16–26. [Google Scholar] [CrossRef]
- Borges, J.; Mano, J.F. Molecular Interactions Driving the Layer-by-Layer Assembly of Multilayers. Chem. Rev. 2014, 114, 8883–8942. [Google Scholar] [CrossRef] [PubMed]
- Kotov, N.A. Layer-by-layer self-assembly: The contribution of hydrophobic interactions. Nanostructured Mater. 1999, 12, 789–796. [Google Scholar] [CrossRef]
- Zhong, X.; Qian, Y.; Huang, J.; Yang, D.; Deng, Y.; Qiu, X. Fabrication of Lignosulfonate vesicular reverse micelles to immobilize Horseradish Peroxidase. Ind. Eng. Chem. Res. 2016, 55, 2731–2737. [Google Scholar] [CrossRef]








| Sample | Carboxylic Acids | Aliphatic -OH | Condensed -OH | Phenolic-OH b | |||
|---|---|---|---|---|---|---|---|
| Para-Hydroxy Phenyl -OH | Guaiacyl -OH | Total Phenolic -OH | Total Group -OH | ||||
| OL | 0.22 | 0.99 | 1.95 | 0.24 | 0.68 | 2.87 | 4.08 |
| OL-tyr | 0.19 | 0.97 | 1.79 | 0.17 | 0.59 | 2.55 | 3.71 |
| Sample | Mw | Mn | Mw/Mn |
|---|---|---|---|
| OL | 154.670 | 15.104 | 10.2 |
| OL-tyr | 202.828 | 18.803 | 18.8 |
| Entry | Catalyst | Immobilization Yield % | Activity Yield % a | Activity (Units/mg) b |
|---|---|---|---|---|
| 1 | I | 69 | 34 | 6.8 |
| 2 | II | 71 | 31 | 6.2 |
| 3 | III | 90 | 42 | 8.4 |
| 4 | IV | 87 | 58 | 11.6 (11.3) c |
| Entry | Catalyst | Km (mM) | Vmax × 10−3 (∆Abs min µgenzyme)−1 | Vmax/Km (×10−3) |
|---|---|---|---|---|
| 1 | Tyr | 0.18 | 6.02 | 33.44 |
| 2 | I | 0.51 | 3.40 | 6.77 |
| 3 | II | 0.67 | 1.14 | 1.70 |
| 4 | III | 0.37 | 3.89 | 10.51 |
| 5 | IV | 0.25 | 4.10 | 16.40 |
| Entry | Catalyst | Substrate | Product(s) | % Conversion | % Yield |
|---|---|---|---|---|---|
| 1 | Tyr | 1 | 5a | 99 | 98 |
| 2 | I | 1 | 5a[5b] | 91 | 20 [18] |
| 3 | II | 1 | 5a | 40 | 39 |
| 4 | III | 1 | 5a | 59 | 59 |
| 5 | IV | 1 | 5a | 75 | 75 |
| 6 | Tyr | 2 | 6 | 96 | 96 |
| 7 | I | 2 | 6 | 35 | 25 |
| 8 | II | 2 | 6 | 20 | 18 |
| 9 | III | 2 | 6 | 38 | 37 |
| 10 | IV | 2 | 6 | 46 | 46 |
| 11 | Tyr | 3 | 7 | 77 | 77 |
| 12 | I | 3 | 7 | 18 | 7 |
| 13 | II | 3 | 7 | 15 | 15 |
| 14 | III | 3 | 7 | 21 | 20 |
| 15 | IV | 3 | 7 | 28 | 28 |
| 16 | Tyr | 4 | 8 | 78 | 78 |
| 17 | I | 4 | 8 | 34 | 12 |
| 18 | II | 4 | 8 | 30 | 30 |
| 19 | III | 4 | 8 | 57 | 56 |
| 20 | IV | 4 | 8 | 67 | 65 |
| Entry | Run | Catalyst I (Yield %) | Catalyst I (Yield %) | Catalyst III (Yield %) | Catalyst IV (Yield %) |
|---|---|---|---|---|---|
| 1 | 1 | 20 | 39 | 59 | 75 |
| 2 | 2 | 20 | 39 | 59 | 75 |
| 3 | 3 | 15 | 32 | 51 | 70 |
| 4 | 4 | 11 | 27 | 46 | 67 |
| 5 | 5 | 9 | 21 | 42 | 61 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capecchi, E.; Piccinino, D.; Delfino, I.; Bollella, P.; Antiochia, R.; Saladino, R. Functionalized Tyrosinase-Lignin Nanoparticles as Sustainable Catalysts for the Oxidation of Phenols. Nanomaterials 2018, 8, 438. https://doi.org/10.3390/nano8060438
Capecchi E, Piccinino D, Delfino I, Bollella P, Antiochia R, Saladino R. Functionalized Tyrosinase-Lignin Nanoparticles as Sustainable Catalysts for the Oxidation of Phenols. Nanomaterials. 2018; 8(6):438. https://doi.org/10.3390/nano8060438
Chicago/Turabian StyleCapecchi, Eliana, Davide Piccinino, Ines Delfino, Paolo Bollella, Riccarda Antiochia, and Raffaele Saladino. 2018. "Functionalized Tyrosinase-Lignin Nanoparticles as Sustainable Catalysts for the Oxidation of Phenols" Nanomaterials 8, no. 6: 438. https://doi.org/10.3390/nano8060438
APA StyleCapecchi, E., Piccinino, D., Delfino, I., Bollella, P., Antiochia, R., & Saladino, R. (2018). Functionalized Tyrosinase-Lignin Nanoparticles as Sustainable Catalysts for the Oxidation of Phenols. Nanomaterials, 8(6), 438. https://doi.org/10.3390/nano8060438