3.1. Phase Structure, Thermodynamic Properties, and Microstructure
Figure 1a shows the XRD patterns of both pristine AgSbTe
2 thick films developed at linear speed of 5 and 10 ms
−1, and Se-doped AgSbTe
1.98Se
0.02 film prepared at 10 ms
−1. Selenium was chosen as a dopant because previous work displayed that 1% of Se is capable of inhibiting the formation of AgTe
2 impurities and improving the thermoelectric properties in bulk samples prepared by spark plasma sintering [
9,
14]. To be clear, the three films were labeled as SAT-5, SAT-10, and SATS-10 in the following discussion, respectively. All the XRD peaks are well assigned to a cubic phase (fcc lattice, Fm
m group, formula AgSbTe
2) and no perceptible peaks with respect to known impurities were detected, such as AgTe
2 and Ag
0.35Sb
0.09Te
0.56. However, due to the subtlety of the Ag
2Te-Sb
2Te
3 pseudo-binary phase diagram and limited test accuracy of XRD itself, the possibility of traces amount of impurity precipitation in the as-prepared films could not be ruled out completely.
Figure 2a,b displays the microstructure of both sides of the SAT-5 films, one is the wheel side where film had contact with copper wheel during solidifying from liquid, the other is the free side where grains were growing more freely during melt spinning. Dendritic patterns were observed on the wheel side of AST-5 film, suggesting that the cooling rate was not high enough to completely ‘freeze’ the grains nucleated on the surface of chilled copper wheel, resulting in the merge between the adjoining grains, and development of dendritic AgSbTe
2. This result was unwelcome when we tried to achieve impurity-free cubic AgSbTe
2 phase by rapid cooling. To make matters worse, small round particles marked by ovals in
Figure 2a were observed between large grains on the free side. The appearance of these particles casts more doubt on the XRD results and led us to re-consider the phase structure of all the films.
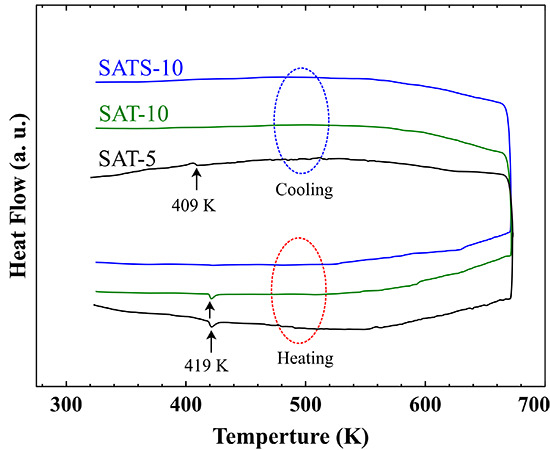
In order to determine the phases of the obtained films accurately, the DSC analysis was performed and the heat flow curves are shown in
Figure 1b. For SAT-5 film, endothermic and exothermic peaks corresponding to the α–β phase transition of Ag
2Te were detected at 419 and 409 K during both heating and cooling processes, respectively [
24,
25]. This suggests that the small particles around large grains on free side were α-Ag
2Te precipitations, and the impurity cannot be integrated into the AgSbTe
2 matrix during heat treatment up to 673 K. For SAT-10 film achieved at higher cooling rate, the DSC curve only revealed an endothermic peak at 419 K in the heating process, while the exothermic peak was disappeared completely in the cooling process. Surely, the SAT-10 film consists of traces of α-Ag
2Te impurity. However, it dissolved into the matrix gradually in the heating process irreversibly, which means that the SAT-10 film has rather homogeneous element distribution, though weak phase separation occurred during the formation process. This analysis was consistent with the EDS results obtained on both the wheel side and free side. The actual composition of SAT-10 film varied within 2% of the nominal one, and fell well within the expected range. Thus, a linear speed of 10 ms
−1 was used to prepare Se-doped film. The thickness of the melt-spun film decreased with the increase of the linear speed of the copper wheel, and further increase of the linear speed damaged the completeness of the film, and generated large portions of dust-like materials.
No peaks were observed during DSC measurement, and the result suggests that the SATS-10 film is a phase-pure cubic AgSbTe
2 compound free from impurity Ag
2Te. That implies that 1% Se is capable of preventing the precipitation of the α-Ag
2Te, just as it does in the melt-quench-spark-plasma-sintering bulk samples [
9]. Early research reported that partial substitution of Te by Se leads to the stabilization of the cubic structure of the AgSbTe
2-AgSbSe
2 pseudo-binary system [
14]. However, the DSC result revealed multiple peaks corresponding to the existence of both Ag
2Te, Ag
0.35Sb
0.09Te
0.56, and possible other precipitations in the samples prepared by melt-slow-cooling route. So both a high cooling rate and suitable dopant were crucial to develop the high purity cubic AgSbTe
2 phase.
As shown in
Figure 2c,d, 400–600 nm grains with clear boundaries were observed on the free side of the SAT-10 film. On the wheel side, 20–100 nm nano-grains are packed tightly with no signs of any dendritic pattern. Interestingly, 20–40 nm fine surface texture and pores/voids in between grains were observed on the wheel side grain surface. These features are similar with SAT-5 film. The solo difference is that the SAT-10 film is free of perceptible AgTe
2 particles at the grain boundaries due to the higher cooling rate.
Figure 2e,f shows FESEM photographs of the free side (e) and wheel side (f) of the SATS-10 film. Unlike the pristine films SAT-5 and SAT-10, the wheel side of Se-doped film looks rather flat, free of pores and voids in between grains. All grains with fine surface texture were packed tightly as the morphology of the wheel side of two pristine films, if the grain boundaries were neglected. This suggests that Se doping has significant influences on the grain growth and restrains the free growth of grains on the free side toward the direction perpendicular to the surface of the film. So the grains have to expand and grow parallel to the surface, which leads to the flat features of the free side. The influence is also reflected in the wheel side image. Unlike the homogeneous distribution of grains in SAT-10 film, gully-like structures were formed on the wheel side due to the strain accompanying the refrain of free growth of the grains on the opposite side.
To have an overall understanding of the melt-spun films. The cross section of SAT-10 film is displayed in
Figure 3a. The film had clear boundary on both wheel side and free side, and a thickness of about 13.2 μm was measured for SAT-10 film. TEM images of SAT-10 and SATS-10 films are shown in
Figure 3b,c, which display the structural details of the interior of the films by exfoliate the surface layer using ion bombardment. Both SAT-10 and SATS-10 films displayed fine nanostructure. In addition to the 20–100 nano-grains on the wheel side and 20–40 nm grain surface texture on the free side disclosed by SEM, grains as small as 5–10 nm were observed in the TEM image of film SAT-10. This suggests that the cooling rate was so huge that grains ranging from 5–100 nm were frozen during melt spinning. The SATS-10 film showed an even finer and more homogeneous nanostructure. The observed fine grains as small as 5 nm among a likely amorphous background indicated that the films might have inherited part of the structural character of the molten phase due to the ultrahigh cooling rate. This result is corresponding to the analysis on SEM image and confirms that Se have substantial influence on the growth of AgSbTe
2 thick films. We conjectured that bulk samples synthesized by combination use of melt spinning and spark plasma sintering, benefits a lot from the fine and hieratical microstructure of AgSbTe
2 thick films. So the low lattice thermal conductivity is not only related to the nanopores on the grain surface in the bulk sample, but also owing to the fine and hieratical microstructure in the melt-spun thick films/ribbons [
4].
3.2. Transport Properties
The temperature dependence of the conductivity
σ and the Seebeck coefficient
α for melt-spun films and reference samples are shown in
Figure 4a,b. All films had similar electrical conductivities at room temperature. However, the evolution trend of electrical conductivity upon temperature varied from film to film. With the increasing temperature, electrical conductivity of SAT-5 film decreased steadily, while these of SAT-10 and SATS-10 had the opposite trend. Moreover, for the SAT-10 film, the electrical conductivity showed an apparently weaker dependence of temperature below 400 K and a stronger one above 400 K. Combined with the phase structure elaborated in
Section 3.1 and the abnormal behaviors of samples reported in the literature [
4,
9,
10,
14,
16,
19,
25,
26], we deduced that the contrasting conductivity features are closely associated with Ag
2Te precipitates in the films. A small amount of Ag
2Te had strong influence on the transport properties of film SAT-5 and covered the intrinsic character of AgSbTe
2 completely. Meanwhile, traces of Ag
2Te impurities changed the temperature dependence of electrical conductivity of film SAT-10 below 400 K. However, the influences diminished gradually above 400 K because of the merging of Ag
2Te impurities with the matrix in SAT-10. This result is in accord with the DSC analysis on the thermodynamic properties film SAT-10. So, only the film SATS-10 film exhibits the intrinsic properties of AgSbTe
2 compound in the whole characterization temperature range. No reference samples are immune to the influences from Ag
2Te precipitate. The above discussion confirmed that the Ag
2Te precipitate in AgSbTe
2 has strong influences on the transport properties, and traces of it is enough to cover the intrinsic properties of AgSbTe
2 compound.
All films exhibited large positive Seebeck coefficients ranging from 230 to 300 μVK−1, comparable to the values achieved in SPS, MS-SPS and MA-SPS bulk samples. As expected, samples with low electrical conductivity had higher Seebeck coefficients and vice versa. Moreover, a similar bifurcation behavior was observed in the Seebeck coefficient, similar with what we have seen in the case of electrical conductivity.
To gain more insight into the electrical transport behavior, Hall effect measurements were carried out at room temperature. The results are presented in
Table 1. All films show very small Hall coefficient, and the value changes from positive one for Se-doped SATS-10 film to negative one for pristine SATS-5, and SAT-5 films. According to the phase structure and electron band structure, there are two factors contributing to the abnormality of Hall coefficient. One is the
n-type impurity Ag
2Te, the other is the distinct transport nature of the AgSbTe
2 compound itself, in which both electrons and holes take part in the charge transport [
11].
When regarding the SAT-5 and SAT-10 films as composites between cubic AgSbTe
2 and α-Ag
2Te, the small amount of
n-type impurity is far from enough to shift the Hall coefficient from positive to negative. So, the Seebeck coefficient and Hall coefficient can be described by formulae (1) and (2) as in a typical two-carrier system, where the Seebeck coefficient is the sum of first-order terms in the carrier mobility, whereas the Hall coefficient is the sum of quadratic terms in the carrier mobility [
11].
Here,
p and
n are the electron and hole concentrations;
μe and
μh are the electron and hole mobilities;
αe and
αh are the partial electron and hole Seebeck coefficients, and
b is the ratio between hole and electron mobilities (
b =
μe/
μh). So, the Hall coefficient can have an opposite sign with respect to the Seebeck coefficient when the mobility of electrons (minority-carrier) is much higher than the mobility of holes (majority carrier). Negative Hall coefficients have been observed in Se-doped AgSbTe
2 samples prepared by spark plasma sintering and other methods [
9,
11]. Meanwhile, Jovovic and Heremans found that the mobility of electrons and hole are 2200 and 11 cmV
−1s
−1, respectively, with the
b value as high as 200 [
11]. So, both pristine films have small negative Hall coefficients. However, the 1% Se-doping shifted the Hall coefficient of SATS-10 film to a small positive one. Based on the formula (2), an increase of hole concentration
p or a decrease of mobility ratio
b are capable of shifting the Hall coefficient from negative to positive. In AgSbTe
2, Selenium is an acceptor-type dopant and can increase the hole concentration
p [
9]. Also, point defects accompanying the incorporation of Selenium in the Tellurium sublattice enhance the scattering of the charge carrier, especially the high-mobility minority-carrier electron, and decrease the difference between the two mobilities (decrease
b). Though it is impossible to quantify the contributions of each factor. We are confident that the Se has both substantial impact on the nature of transport in AgSbTe
2 films.
Figure 4c presents the temperature dependence of power factor
α2σ for films and reference bulk samples. Both SAT-10 and SATS-10 film have very high power factors compared with bulk samples, owing to their excellent electrical conductivities. A power factor of ~1.3 was achieved at 500 K for film SATS-10. Due to the fragility and size of the films, no thermal conductivity data were collected. In semiconductors, the total thermal conductivity
κ can be written as
κ =
κlatt +
κcarr, where
κlatt and
κcarr are the lattice and carrier contributions, respectively. The carrier component can be calculated using the Wiedemann-Franz law as
κcarr =
LTσ, where the Lorenz number
L = 0.7
L0,
L0 equals 2.45 × 10
−8 V
2/K
2 for the fully degenerate semiconductor. Assuming the
κcarr is constant for all films and bulk samples prepared by MS-SPS, the
κ value for film samples can be given by a formula,
κ =
κlatt (bulk sample) +
κcarr. Here, we roughly estimated the dimensionless figure-of-merit
zTs based on the measured values of Seebeck coefficients, electrical conductivity, and calculated data of thermal conductivity. A
zT of 1.40 was obtained by film SAT-10 at 500 K, which is higher than any other reference samples at the same temperature. Though the maximum
zT of 1.35 of film SATS-10, estimated using same lattice thermal conductivity, is lower than that of SAT-10 at 500 K, it may have a higher
zT value when taking into account of the effect of point defect scattering on phonon transport accompanying with the substitution of Te by Se, which has been well demonstrated in bulk AgSbSe
xTe
2−x samples prepared by SPS [
9]. This suggest that reviving the intrinsic properties of the cubic AgSbTe
2 compound and refraining the precipitation of Ag
2Te impurity by Se doping and repaid cooling are potential ways to optimize the thermoelectric properties.