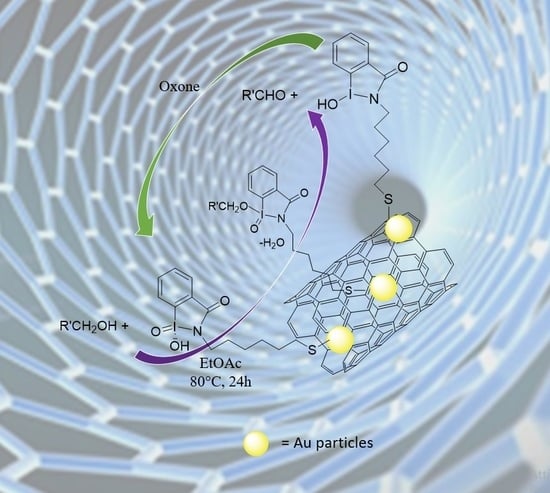
Iodoxybenzoic Acid Supported on Multi Walled Carbon Nanotubes as Biomimetic Environmental Friendly Oxidative Systems for the Oxidation of Alcohols to Aldehydes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of oxMWCNTs I
2.3. Preparation of Oxidizing Solid Reagents IV A–B
2.4. Preparation of Oxidizing Solid Reagents VIII A–B
2.5. Preparation of Oxidizing Solid Reagent VIII–C
2.6. Transmission Electron Microscopy (TEM), Scanning Electron Microscopy (SEM), and X-Ray Photoelectron Spectroscopy (XPS) Analyses
2.7. Inductively Coupled Plasma Mass-Spectrometry (ICP–MS) Analysis
2.8. Oxidation of Aromatic Alcohols
3. Results
3.1. Preparation of IBX Supported MWCNTs and MWCNTs–Au Oxidizing Solid Reagents
3.2. Determination of the Electron Binding Energies of the Elements by XPS Analysis
3.3. Determination of the Iodine Loading Factor by ICP–MS Analysis
3.4. Oxidation of Aromatic Alcohols with IV A–B and VIII A–C
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Choudhary, V.R.; Dumbre, D.K. Solvent-free selective oxidation of primary alcohols-to-aldehydes and aldehydes-to-carboxylic acids by molecular oxygen over MgO-supported nano-gold catalyst. Catal. Commun. 2011, 13, 82–86. [Google Scholar] [CrossRef]
- Christopher, R.G.; Bi-Shun, Z.; SonBinh, T.N. Organic Syntheses by Oxidation with Metal Compounds. J. Am. Chem. Soc. 2006, 128, 12596–12597. [Google Scholar]
- Meyer, S.D.; Schreiber, S.L. Acceleration of the Dess-Martin Oxidation by Water Schreiber. J. Org. Chem. 1994, 59, 7549–7552. [Google Scholar] [CrossRef]
- Crich, D.; Neelamkavil, S. The fluorous Swern and Corey-Kim reaction: Scope and mechanism. Tetrahedron 2002, 58, 3865–3870. [Google Scholar] [CrossRef]
- Sultane, P.R.; Bielawski, C.W. Bielawski. Burgess Reagent Facilitated Alcohol Oxidations in DMSO. J. Org. Chem. 2017, 82, 1046–1052. [Google Scholar]
- Noyori, R.; Ohkuma, T. Angew. Asymmetric Catalysis by Architectural and Functional Molecular Engineering: Practical Chemo- and Stereoselective Hydrogenation of Ketones. Chem. Int. Ed. 2001, 40, 40–73. [Google Scholar] [CrossRef]
- De Graauw, C.F.; Peters, J.A.; Van Bekkum, H.; Huskens, J. Meerwein-Ponndorf-Verley Reductions and Oppenauer Oxidations: An Integrated Approach. Synthesis 1994, 10, 1007–1017. [Google Scholar] [CrossRef]
- Pereira, J.P.C.; Van der Wielen, L.A.M.; Straathof, A.J.J. Perspectives for the microbial production of methyl propionate integratedwith product recovery. Bioresour. Technol. 2018, 256, 187–194. [Google Scholar] [CrossRef] [PubMed]
- D’ Acunzo, F.; Baiocco, P.; Fabbrini, M.; Galli, C.; Gentili, P. A Mechanistic Survey of the Oxidation of Alcohols and Ethers with the Enzyme Laccase and Its Mediation by TEMPO. Eur. J. Org. Chem. 2002, 2002, 4195–4201. [Google Scholar] [CrossRef]
- Gorrebeeck, C.; Spanoghe, M.; Lanens, D.; Lemière, G.L.; Dommisse, R.A.; Lepoivre, J.A.; Alderweireldt, F.C. Permeability studies on horse liver alcohol dehydrogenase (HLAD) in polyacrylamide gel beads. Recl. Trav. Chim. Pays-Bas 1991, 110, 231–235. [Google Scholar] [CrossRef]
- Snijder-Lambers, A.M.; Vulfson, E.N.; Dodema, H.J. Optimization of alcohol dehydrogenase activity and NAD(H) regeneration in organic solvents. Recl. Trav. Chim. Pays-Bas 1991, 110, 226–230. [Google Scholar] [CrossRef]
- Liu, R.; Liang, X.; Dong, C.; Hu, X. Transition-Metal-Free: A Highly Efficient Catalytic Aerobic Alcohol Oxidation Process. J. Am. Chem. Soc. 2004, 126, 4112–4113. [Google Scholar] [CrossRef] [PubMed]
- Mulbaier, M.; Giannis, A. The synthesis and Oxidative Properties of Polymer-Supportes IBX. Angew. Chem. Int. Ed. 2001, 40, 23. [Google Scholar] [CrossRef]
- Jang, H.-A.; Kim, Y.-A.; Kim, Y.-O.; Lee, S.-M.; Kim, J.W.; Chung, W.; Lee, Y. Polymer-supported IBX amide for mild and efficient oxidation reactions. J. Ind. Eng. Chem. 2014, 20, 29–36. [Google Scholar] [CrossRef]
- Verma, N.; Kumar, S.; Ahmed, N. LiBr/β-CD/IBX/H2O-DMSO: A new approach for one-pot biomimetic regioselective ring opening of chalcone epoxides to bromohydrins and conversion to 1,2,3-triketones. Synth. Commun. 2017, 47, 1110–1120. [Google Scholar] [CrossRef]
- Kumar, S.; Ahmed, N. β-Cyclodextrin/IBX in water: Highly facile biomimetic one pot deprotection of THP/MOM/Ac/Ts ethers and concomitant oxidative cleavage of chalcone epoxides and oxidative dehydrogenation of alcohols. Green Chem. 2016, 18, 648–656. [Google Scholar] [CrossRef]
- Koguchi, S.; Mihoya, A.; Mimura, M. Alcohol oxidation via recyclable hydrophobic ionic liquid-supported IBX. Tetrahedron 2016, 72, 7633–7637. [Google Scholar] [CrossRef]
- Zhdankin, V.V. Organoiodine (V) Reagents in Organic Synthesis. J. Org. Chem. 2011, 76, 1185–1197. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, M. Polymer-Supported Hypervalent Iodine as Green Oxidant in Organic Synthesis. Synlett 2011, 16, 2433–2434. [Google Scholar] [CrossRef]
- More, J.D.; Finney, N.S. A Simple and Advantageous Protocol for the Oxidation of Alcohols with o-Iodoxybenzoic Acid (IBX). Org. Lett. 2002, 4, 3001–3003. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Jang, H.S.; Kim, Y.; Ahn, H.; Yeo, Y.; Lee, S.; Lee, Y. Heterogeneous Transition-Metal-Free Alcohol Oxidation by Graphene Oxide Supported Iodoxybenzoic Acid in Water. Synlett 2013, 24, 2282–2286. [Google Scholar]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Z.; Dubonos, S.V. Electric Field Effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Botta, L.; Bizzarri, B.M.; Crucianelli, M.; Saladino, R. Advances in biotechnological synthetic applications of carbon nanostructured systems. J. Mater. Chem. B 2017, 5, 6490. [Google Scholar] [CrossRef]
- Bao, Y.; Pang, H.; Xu, L.; Cui, C.-H.; Jiang, X.; Yan, D.-X.; Li, Z.-M. Influence of surface polarity of carbon nanotubes on electric field induced aligned conductive network formation in a polymer melt. RSC Adv. 2013, 3, 24185. [Google Scholar] [CrossRef]
- Parra, E.A.; Blondeau, P.; Crespo, G.A.; Rius, F.X. An effective nanostructured assembly for ion-selective electrodes. An ionophore covalently linked to carbon nanotubes for Pb2+ determination. Chem. Commun. 2011, 47, 2438–2440. [Google Scholar] [CrossRef] [PubMed]
- D’Archivio, A.A.; Maggi, M.A.; Odoardi, A.; Santucci, S.; Passacantando, M. Adsorption of triazine herbicides from aqueous solution by functionalized multiwall carbon nanotubes grown on silicon substrate. Nanotechnology 2018, 29, 065701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cegłowski, M.; Narkiewicz, U.; Pełech, I.; Schroeder, G. Functionalization of gold-coated carbon nanotubes with self-assembled monolayers of thiolates. J. Mater. Sci. 2012, 47, 3463–3467. [Google Scholar] [CrossRef]
- Zhdankin, V.V.; Koposov, A.Y.; Netzel, B.C.; Yashin, N.V.; Rempel, B.P.; Ferguson, M.J.; Tykwinski, R.R. IBX amides: A new family of ipervalent iodine reagents. Angew. Chem. Int. Ed. 2003, 42, 2194–2196. [Google Scholar] [CrossRef] [PubMed]
- De Munari, S.; Frigerio, M.; Santagostino, M. Hypervalent iodine oxidants: structure and kinetics of the reactive intermediates in the oxidation of alcohols and 1,2-Diolsby o-iodoxybenzoic acid (IBX) and Dess-Martin periodinane. A comparative 1H-NMR Study. J. Org. Chem. 1996, 61, 9272–9279. [Google Scholar] [CrossRef]
- Bayrut, T.; Carlson, J.; Godfrey, B.; Retzlaff, M.S.; Sligar, S.G. Structure, behavior, and manipulation of nanoscale biological assemblies. In Nanostructured Materials and Nanotechnology, 1st ed.; Nalwa, H.S., Ed.; Academic Press: San Diego, CA, USA, 2002; p. 808. ISBN 9780125139205. [Google Scholar]
- Khoo, K.H.; Chelikowsky, J.R. Electron transport across carbon nanotube junctions decorated with Au nanoparticles: Density functional calculations. Phys. Rew. B 2009, 79, 205422. [Google Scholar] [CrossRef]
- Dos Santos, J.C.S.; Barbosa, O.; Ortiz, C.; Berenguer-Murcia, A.; Rodrigues, C.R.; Fernandez-Lafuente, R. Importance of the Support Properties for Immobilization or Purification of Enzymes. ChemCatChem 2015, 7, 2413–2432. [Google Scholar] [CrossRef] [Green Version]
- Román-Martínez, M.C.; De Le Cea, S.M. Heterogenization of homogeneous catalysts on carbon material. In New and Future Developments in Catalysis, 1st ed.; Suib, S.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 55–75. ISBN 9780444538765. [Google Scholar]







| Element | Reagent | Assignments | |||
|---|---|---|---|---|---|
| III B | IV B | VII A | VIII A | ||
| N 1s | 400.5 | 400.5 | 400.5 | 400.5 | N–H (amide) |
| S 2p3/2 | - | - | 164.4 | - | C–S–C (sulfide) |
| 168.6 | 168.6 | C–SOx–C | |||
| Au 4f7/2 | - | - | 84.0 | 84.0 | Au-Au |
| I 3d5/2 | 619.2 | - | 619.2 | - | I2 |
| 621.5 | 621.5 | 621.5 | 621.5 | I-O | |
| Entry | Compound | ICP-MSc | LF a | |
|---|---|---|---|---|
| Iodine (%) | Gold (%) | |||
| 1 | IV A | 0.03 | - | 0.3 |
| 2 | IV B | 0.21 | - | 2.1 |
| 3 | VIII A | 0.04 | 0.38 | 0.4 |
| 4 | VIII B | 0.07 | 4.7 | 0.7 |
| 5 | VIII C | 0.03 | 2.10 | 0.3 |
| Products a | m/z (%) |
|---|---|
| Benzaldehyde (9) | 107 (10) [M+1], 106 (80) [M], 105 (72) (M-1) |
| 4 Methoxy Benzaldehyde (10) | 136 (20) [M], 135 (69) [M-1], 134 (100) [M-2], 133 (95) [M-3], 132 (77) [M-4], 131 (95) [M-5] |
| 3-4 Dimethoxy Benzaldehyde (11) | 167 (10) [M+1], 166 (52) [M], 165 (80) [M-1], 164 (92) [M-2], 163 (99) [M-3], 162 (87) [M-4], 161 (50) [M-5], 160 (17) [M-6] |
| 3-4-5 Trimethoxy Benzaldehyde (12) | 197 (2) [M+1], 196 (85) |
| 4 HydroxyBenzaldehyde (13) | 124 (2) [M+2], 123 (1) [M+1], 122 (100) [M], 121 (63) [M-1], 120 (10) [M-2] |
| 4 Chlorobenzaldehyde (14) | 142 (10) [M+2], 141 (12) [M+1], 140 (123) [M], 139 (45) [M-1], 138 (90) [M-2], 137 (50) [M-3], 136 (100) [M-4], 135 (1) [M-5] |
| 4-Hydroxyphenylacetaldehyde (15) | 136 (100) |
| Phenylacetaldehyde (16) | 121 (2) [M+1], 120 (35) [M], |
| Entry | Substrate | Oxidant | Product | Yield (%) b |
|---|---|---|---|---|
| 1 | Benzyl alcohol (1) | IBX | 9 | 95 |
| 2 | Benzyl alcohol (1) | sIBX c | 9 | 25 |
| 3 | Benzyl alcohol (1) | IV A | 9 | 29 |
| 4 | 4-Methoxy benzyl alcohol (2) | IV A | 10 | 35 |
| 5 | 3,4-Dimethoxy benzyl alcohol (3) | IV A | 11 | 39 |
| 6 | 3,4,5-Trimethoxy benzy alcohol (4) | IV A | 12 | 41 |
| 7 | 4-Hydroxy benzyl alcohol (5) | IV A | 13 | 50 |
| 8 | 4-Chloro benzyl alcohol (6) | IV A | 14 | 18 |
| 9 | Tyrosol (7) | IV A | 15 | 5 |
| 10 | Phenethyl alcohol (8) | IV A | 16 | 7 |
| 11 | Benzyl alcohol (1) | IV B | 9 | 52 |
| 12 | 4-Methoxy benzyl alcohol (2) | IV B | 10 | 60 |
| 13 | 3,4-Dimethoxy benzyl alcohol (3) | IV B | 11 | 62 |
| 14 | 3,4,5-Trimethoxy benzyl alcohol (4) | IV B | 12 | 63 |
| 15 | 4-Hydroxy benzyl alcohol (5) | IV B | 13 | 80 |
| 16 | 4-Chloro benzyl alcohol (6) | IV B | 14 | 45 |
| 17 | Tyrosol (7) | IV B | 15 | 10 |
| 18 | Phenethyl alcohol (8) | IV B | 16 | 15 |
| Entry | Substrate | Oxidant | Product | Yield (%) b |
|---|---|---|---|---|
| 1 | Benzyl alcohol (1) | IBX | 9 | 95 |
| 2 | Benzyl alcohol (1) | sIBX c | 9 | 25 |
| 3 | Benzyl alcohol (1) | VIII A | 9 | 38 |
| 4 | 4-Methoxy benzyl alcohol (2) | VIII A | 10 | 44 |
| 5 | 3,4-Dimethoxy benzyl alcohol (3) | VIII A | 11 | 48 |
| 6 | 3,4,5-Trimethoxy benzy alcohol (4) | VIII A | 12 | 50 |
| 7 | 4-Hydroxy benzyl alcohol (5) | VIII A | 13 | 68 |
| 8 | 4-Chloro benzyl alcohol (6) | VIII A | 14 | 27 |
| 9 | Tyrosol (7) | VIII A | 15 | 9 |
| 10 | Phenethyl alcohol (8) | VIII A | 16 | 10 |
| 11 | Benzyl alcohol (1) | VIII B | 9 | 98 |
| 12 | 4-Methoxy benzyl alcohol (2) | VIII B | 10 | >99 |
| 13 | 3,4-Dimethoxy benzyl alcohol (3) | VIII B | 11 | >99 |
| 14 | 3,4,5-Trimethoxy benzyl alcohol (4) | VIII B | 12 | >99 |
| 15 | 4-Hydroxy benzyl alcohol (5) | VIII B | 13 | >99 |
| 16 | 4-Chloro benzyl alcohol (6) | VIII B | 14 | 95 |
| 17 | Tyrosol (7) | VIII B | 15 | 20 |
| 18 | Phenethyl alcohol (8) | VIII B | 16 | 24 |
| 19 | Benzyl alcohol (1) | VIII C | 9 | <3 |
| 20 | Benzyl alcohol (1) | VIII B | 9 | 96 d |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bizzarri, B.M.; Abdalghani, I.; Botta, L.; Taddei, A.R.; Nisi, S.; Ferrante, M.; Passacantando, M.; Crucianelli, M.; Saladino, R. Iodoxybenzoic Acid Supported on Multi Walled Carbon Nanotubes as Biomimetic Environmental Friendly Oxidative Systems for the Oxidation of Alcohols to Aldehydes. Nanomaterials 2018, 8, 516. https://doi.org/10.3390/nano8070516
Bizzarri BM, Abdalghani I, Botta L, Taddei AR, Nisi S, Ferrante M, Passacantando M, Crucianelli M, Saladino R. Iodoxybenzoic Acid Supported on Multi Walled Carbon Nanotubes as Biomimetic Environmental Friendly Oxidative Systems for the Oxidation of Alcohols to Aldehydes. Nanomaterials. 2018; 8(7):516. https://doi.org/10.3390/nano8070516
Chicago/Turabian StyleBizzarri, Bruno Mattia, Issam Abdalghani, Lorenzo Botta, Anna Rita Taddei, Stefano Nisi, Marco Ferrante, Maurizio Passacantando, Marcello Crucianelli, and Raffaele Saladino. 2018. "Iodoxybenzoic Acid Supported on Multi Walled Carbon Nanotubes as Biomimetic Environmental Friendly Oxidative Systems for the Oxidation of Alcohols to Aldehydes" Nanomaterials 8, no. 7: 516. https://doi.org/10.3390/nano8070516
APA StyleBizzarri, B. M., Abdalghani, I., Botta, L., Taddei, A. R., Nisi, S., Ferrante, M., Passacantando, M., Crucianelli, M., & Saladino, R. (2018). Iodoxybenzoic Acid Supported on Multi Walled Carbon Nanotubes as Biomimetic Environmental Friendly Oxidative Systems for the Oxidation of Alcohols to Aldehydes. Nanomaterials, 8(7), 516. https://doi.org/10.3390/nano8070516