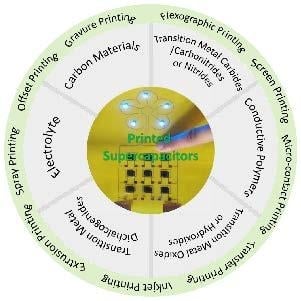
Printable Nanomaterials for the Fabrication of High-Performance Supercapacitors
Abstract
:1. Introduction
2. Advances of Printing Technology
2.1. Plate-Based Printing Techniques
2.2. Digital Printing Techniques
3. Printable Nanomaterials in Fabricating Supercapacitors
3.1. Carbon Materials
3.1.1. Activated Carbon
3.1.2. Graphene
3.1.3. Carbon Nanotubes
3.1.4. Carbon Fiber
3.2. Transition Metal Carbides/Carbonitrides or Nitrides (MXenes)
3.3. Conductive Polymers
3.4. Transition Metal Oxides or Hydroxides
3.4.1. RuO2
3.4.2. MnO2
3.4.3. NiO
3.4.4. Co(OH)2
3.5. Transition Metal Dichalcogenides
3.6. Printable Electrolyte
4. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Martin, W.; Brodd, R.J. What are batteries, fuel cells, and supercapacitors? Chem. Rev. 2004, 104, 4245–4269. [Google Scholar]
- Nishide, H.; Oyaizu, K. Toward flexible batteries. Science 2008, 319, 737–738. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Zhi, L.J. Graphene hybridization for energy storage applications. Chem. Soc. Rev. 2018, 47, 3189–3216. [Google Scholar] [CrossRef] [PubMed]
- Oganov, A.R.; Chen, J.; Gatti, C.; Ma, Y.; Ma, Y.; Glass, C.W.; Liu, Z.; Yu, T.; Kurakevych, O.O.; Solozhenko, V.L. Ionic high-pressure form of elemental boron. Nature 2009, 457, 863–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.; Zhou, Z. Micro/nanostructured materials for sodium ion batteries and capacitors. Small 2014, 14, 1702961. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hu, J.; Cheng, Y.; Yang, H.Y.; Yao, Y.; Yang, S.A. Borophene as an extremely high capacity electrode material for Li-ion and Na-ion batteries. Nanoscale 2016, 8, 15340–15347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, K.; Zou, R.; Li, W.; Liu, Q.; Liu, X.; An, L.; Hu, J. Design and synthesis of 3D interconnected mesoporous NiCo2O4@CoxNi1−x(OH)2 core-shell nanosheet arrays with large areal capacitance and high rate performance for supercapacitors. J. Mater. Chem. A 2014, 2, 10090–10097. [Google Scholar] [CrossRef]
- Nagaraju, G.; Ko, Y.H.; Cha, S.M.; Im, S.H.; Yu, J.S. A facile one-step approach to hierarchically assembled core-shell-like MnO2@MnO2 nanoarchitectures on carbon fibers: An efficient and flexible electrode material to enhance energy storage. Nano Res. 2016, 9, 1507–1522. [Google Scholar] [CrossRef]
- Wang, S.L.; Liu, N.S.; Su, J.; Li, L.Y.; Long, F.; Zou, Z.G.; Jiang, X.L.; Gao, Y.H. Highly tretchable and self-healable supercapacitor with reduced graphene oxide based fiber springs. ACS Nano 2017, 11, 2066–2074. [Google Scholar] [CrossRef] [PubMed]
- Sumboja, A.; Foo, C.Y.; Wang, X.; Lee, P.S. Large areal mass, flexible and free-standing reduced graphene oxide/manganese dioxide paper for asymmetric supercapacitor device. Adv. Mater. 2013, 25, 2809–2815. [Google Scholar] [CrossRef] [PubMed]
- Du, C.F.; Liang, Q.H.; Luo, Y.B.; Zheng, Y.; Yan, Q.Y. Recent advances in printable secondary batteries. J. Mater. Chem. A 2017, 5, 22442–22458. [Google Scholar] [CrossRef]
- Tian, X.C.; Jin, J.; Yuan, S.Q.; Chua, C.K.; Tor, S.B.; Zhou, K. Emerging 3D-printed electrochemical energy storage devices: A critical review. Adv. Energy Mater. 2017, 7, 1700127. [Google Scholar] [CrossRef]
- Zhang, F.; Wei, M.; Viswanathan, V.V.; Swart, B.; Shao, Y.Y.; Wu, G.; Zhou, C. 3D printing technologies for electrochemical energy storage. Nano Energy 2017, 40, 418–431. [Google Scholar] [CrossRef]
- Menard, E.; Meitl, M.A.; Sun, Y.G.; Park, J.U.; Shir, D.J.L.; Nam, Y.S.; Jeon, S.; Rogers, J.A. Micro- and nanopatterning techniques for organic electronic and optoelectronic systems. Chem. Rev. 2007, 107, 1117–1160. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Haverinen, H.M.; Dhagat, P.; Jabbour, G.E. Inkjet printing-process and its applications. Adv. Mater. 2010, 22, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.Z.; Bao, B.; He, M.; Zhou, H.H.; Song, Y.L. Recent advances in controlling the depositing morphologies of inkjet droplets. ACS Appl. Mater. Interfaces 2015, 7, 28086–28099. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Lee, W.H.; Cho, K. Recent advances in organic transistor printing processes. ACS Appl. Mater. Interfaces 2013, 5, 2302–2315. [Google Scholar] [CrossRef] [PubMed]
- Nie, Z.H.; Kumacheva, E. Patterning surfaces with functional polymers. Nat. Mater. 2008, 7, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Sun, J.F.; Huang, Y.; Zhu, M.S.; Pei, Z.X.; Li, H.F.; Wang, Y.K.; Li, N.; Zhang, H.Y.; Zhi, C.Y. Recent progress on flexible and wearable supercapacitors. Small 2017, 13, 1701827. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Yoon, H. Nanostructured electrode materials for electrochemical capacitor applications. Nanomaterials 2015, 5, 906–936. [Google Scholar] [CrossRef] [PubMed]
- Kamyshny, A.; Magdassi, S. Conductive nanomaterials for printed electronics. Small 2014, 10, 3515–3535. [Google Scholar] [CrossRef] [PubMed]
- Hammock, M.L.; Chortos, A.; Tee, B.C.K.; Tok, J.B.H.; Bao, Z.N. The evolution of electronic skin (e-skin): A brief history, design considerations, and recent progress. Adv. Mater. 2013, 25, 5997–6038. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Zhang, C.; Han, C.B.; Fan, F.R.; Tang, W.; Wang, Z.L. Woven structured triboelectric nanogenerator for wearable devices. ACS Appl. Mater. Interfaces 2014, 6, 14695–14701. [Google Scholar] [CrossRef] [PubMed]
- Aricò, A.S.; Bruce, P.; Scrosati, B.; Tarascon, J.M.; Van Schalkwijk, W. Nanostructured materials for advanced energy conversion and storage devices. Nat. Mater. 2005, 4, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wu, Z.-S.; Yang, S.; Dong, R.; Feng, X.; Müllen, K. Ultraflexible in-plane micro-supercapacitors by direct printing of solution-processable electrochemically exfoliated graphene. Adv. Mater. 2016, 28, 2217–2222. [Google Scholar] [CrossRef] [PubMed]
- Rocha, V.G.; Tuñón, E.G.; Botas, C.; Markoulidis, F.; Feilden, E.; D’Elia, E.; Ni, N.; Shaffer, M.; Saiz, E. Multimaterial 3D printing of graphene-based electrodes for electrochemical energy storage using thermoresponsive inks. ACS Appl. Mater. Interfaces 2017, 9, 37136–37145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.C.; Han, S.; Wang, C.; Li, J.P.; Xu, G.B. Single-walled carbon nanohorns for energy applications. Nanomaterials 2015, 5, 22442–22458. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, N.; Xia, Q.; Savilov, S.V.; Aldoshin, S.M.; Yu, Y.; Xia, H. Enhanced pseudocapacitive performance of α-MnO2 by cation preinsertion. ACS Appl. Mater. Interfaces 2016, 8, 33732–33740. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, S.T.; Fu, N.; Liu, Y.; Wang, Y.; Zhou, L.; Huang, H. Flexible fiber hybrid supercapacitor with NiCo2O4 nanograss@carbon fiber and bio-waste derived high surface area porous carbon. Electrochim. Acta 2016, 211, 411–419. [Google Scholar] [CrossRef]
- Liu, L.; Yu, Y.; Yan, C.; Li, K.; Zheng, Z. Wearable energy-dense and power-dense supercapacitor yarns enabled by scalable graphene-metallic textile composite electrodes. Nat. Commun. 2015, 6, 7260. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, S.; Kubob, A.; Noharac, K.; Zhang, X.T.; Taneichi, N.; Okuib, T.; Liu, Z.Y.; Nakata, K.; Sakai, H.; Murakamia, T.; et al. TiO2-based superhydrophobic–superhydrophilic patterns: Fabrication via an ink-jet technique and application in offset printing. Appl. Surf. Sci. 2009, 255, 6221–6225. [Google Scholar] [CrossRef]
- Tian, D.L.; Song, Y.L.; Jiang, L. Patterning of controllable surface wettability for printing techniques. Chem. Soc. Rev. 2013, 42, 5184–5209. [Google Scholar] [CrossRef] [PubMed]
- Zielke, D.; Hüblerb, A.C.; Hahn, U.; Brandt, N.; Bartzsch, M.; Fügmann, U.; Fischer, T. Polymer-based organic field-effect transistor using offset printed source/drain structures. Appl. Phys. Lett. 2005, 87, 123508. [Google Scholar] [CrossRef]
- Cen, J.L.; Kitsomboonloha, R.; Subramanian, V. Cell filling in gravure printing for printed electronics. Langmuir 2014, 30, 13716–13726. [Google Scholar] [CrossRef] [PubMed]
- Kitsomboonloha, R.; Morris, S.J.S.; Rong, X.Y.; Subramanian, V. Femtoliter-scale patterning by high-speed, highly scaled inverse gravure printing. Langmuir 2012, 28, 16711–16723. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ramm, A.; Lim, S.; Xie, W.; Ahn, B.Y.; Xu, W.C.; Mahajan, A.; Suszynski, W.J.; Kim, C.; Lewis, J.A.; et al. Wettability contrast gravure printing. Adv. Mater. 2015, 45, 7420–7425. [Google Scholar] [CrossRef] [PubMed]
- Tobjörk, D.; Österbacka, R. Paper electronics. Adv. Mater. 2011, 23, 1935–1961. [Google Scholar] [CrossRef] [PubMed]
- Polte, J.; Tuaev, X.; Wuithschick, M.; Fischer, A.; Thuenemann, A.F.; Rademann, K.; Kraehnert, R.; Emmerling, F. Formation mechanism of colloidal silver nanoparticles: Analogies and differences to the growth of gold nanoparticles. ACS Nano 2012, 6, 5791–5802. [Google Scholar] [CrossRef] [PubMed]
- Frederik, C.K. Fabrication and processing of polymer solar cells: A review of printing and coating techniques. Sol. Energy Mater. Sol. Cells 2009, 93, 394–412. [Google Scholar]
- Chen, L.; Ji, F.; Xu, Y.; He, L.; Mi, Y.F.; Bao, F.; Sun, B.Q.; Zhang, X.H.; Zhang, Q. High-yield seedless synthesis of triangular gold nanoplates through oxidative etching. Nano Lett. 2014, 14, 7201–7206. [Google Scholar] [CrossRef] [PubMed]
- Li, S.W.; Yu, X.L.; Zhang, G.J.; Ma, Y.; Yao, J.N.; Keita, B.; Louis, N.; Zhao, H. Green chemical decoration of multiwalled carbon nanotubes with polyoxometalate-encapsulated gold nanoparticles: Visible light photocatalytic activities. J. Mater. Chem. 2011, 21, 2282–2287. [Google Scholar] [CrossRef]
- Xu, H.; Ling, X.Y.; van Bennekom, J.; Duan, X.; Ludden, M.J.W.; Reinhoudt, D.N.; Wessling, M.; Lammertink, R.G.H.; Huskens, J. Microcontact printing of dendrimers, proteins, and nanoparticles by porous stamps. J. Am. Chem. Soc. 2009, 131, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Cho, K.S.; Lee, E.K.; Lee, S.J.; Chae, J.S.; Kim, J.W.; Kim, D.; Kwon, H.J.Y.; Amaratunga, G.; Lee, S.Y.; et al. Full-colour quantum dot displays fabricated by transfer printing. Nat. Mater. 2011, 5, 176–182. [Google Scholar] [CrossRef]
- Hwang, J.K.; Cho, S.; Dang, J.M.; Kwak, E.B.; Song, K.; Moon, J.; Sung, M.M. Direct nanoprinting by liquid-bridge-mediated nanotransfer moulding. Nat. Nanotechnol. 2010, 5, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Derby, B. Inkjet printing of functional and structural materials: Fluid property requirements, feature stability, and resolution. Annu. Rev. Mater. Res. 2010, 40, 395–414. [Google Scholar] [CrossRef]
- Sun, J.Z.; Guo, Y.Z.; Cui, B.; Chu, F.Q.; Li, H.Z.; Li, Y.; He, M.; Ding, D.; Liu, R.P.; Li, L.H.; et al. Inkjet printing bendable circuits based on an oil-water interface reaction. Appl. Surf. Sci. 2018, 445, 391–397. [Google Scholar] [CrossRef]
- Kucherov, F.A.; Gordeev, E.G.; Kashin, A.S.; Ananikov, V.P. Three-dimensional printing with biomass-derived PEF for carbon neutral manufacturing. Angew. Chem. Int. Ed. 2017, 56, 1–6. [Google Scholar]
- Onses, M.S.; Sutanto, E.; Ferreira, P.M.; Alleyne, A.G.; Rogers, J.A. Mechanisms, capabilities, and applications of high-resolution electrohydrodynamic jet printing. Small 2015, 11, 4237–4266. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.Y.; Zhang, Y.Z.; Wang, J.X.; Ren, Y.B.; Song, Y.L.; Jiang, L. Ultra-fast fabrication of colloidal photonic crystals by spray coating. Macromol. Rapid. Commun. 2009, 30, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.H.; Jiang, K.; Wei, R.; Tahir, M.; Wu, X.G.; Shen, M.; Wang, X.Z.; Cao, C.B. Popcorn-derived porous carbon flakes with an ultrahigh specific surface area for superior performance supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 30626–30634. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.Y.; Jin, M.; Zhang, Y.; Niu, Y.B.; Gao, J.C.; Li, C.M. Chemically exfoliating biomass into a graphene-like porous active carbon with rational pore structure, good conductivity, and large surface area for high-performance supercapacitors. Adv. Energy Mater. 2018, 8, 1702545. [Google Scholar] [CrossRef]
- Yin, C.M.; Tao, C.A.; Cai, F.L.; Song, C.C.; Gong, H.; Wang, J.F. Effects of activation temperature on the deoxygenation, specific surface area and supercapacitor performance of graphene. Carbon 2016, 109, 558–565. [Google Scholar] [CrossRef]
- Lee, S.S.; Choi, K.H.; Kim, S.H.; Lee, S.Y. Wearable supercapacitors printed on garments. Adv. Funct. Mater. 2018, 28, 1705571. [Google Scholar] [CrossRef]
- Choi, K.H.; Yoo, J.T.; Chang, K.L.; Lee, S.Y. All-inkjet-printed, solid-state flexible supercapacitors on paper. Energy Environ. Sci. 2016, 9, 2812–2821. [Google Scholar] [CrossRef]
- Jin, H.; Wang, X.M.; Gu, Z.R.; Fan, Q.H.; Luo, B. A facile method for preparing nitrogen-doped graphene and its application in supercapacitors. J. Power Sources 2015, 273, 1156–1162. [Google Scholar] [CrossRef]
- Abdelkader, A.M.; Karim, N.; Vallés, C.; Afroj, S.; Novoselov, K.S.; Yeates, S.G. Ultraflexible and robust graphene supercapacitors printed on textiles for wearable electronics applications. 2D Mater. 2017, 4, 035016. [Google Scholar] [CrossRef] [Green Version]
- Sollami, D.S.; Smith, A.D.; Li, J.T.; Östling, M. Inkjet printed highly transparent and flexible graphene micro-supercapacitors. Nanoscale 2017, 9, 6998–7005. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Zhang, B.B.; Xu, Q.; Hou, Y.Y.; Seyedin, S.Y.; Qin, S.; Wallace, G.G.; Beirne, S.; Razal, J.M.; Chen, J. Development of graphene oxide/polyaniline inks for high performance flexible microsupercapacitors via extrusion printing. Adv. Funct. Mater. 2018, 28, 1706592. [Google Scholar] [CrossRef]
- Li, W.B.; Li, Y.H.; Su, M.; An, B.X.; Liu, J.; Su, D.; Li, L.H.; Li, F.Y.; Song, Y.L. Printing assembly and structural regulation of graphene towards three-dimensional flexible micro-supercapacitors. J. Mater. Chem. A 2017, 5, 16281–16288. [Google Scholar] [CrossRef]
- Kim, J.H.; Nam, K.W.; Ma, S.B.; Kim, K.B. Fabrication and electrochemical properties of carbon nanotube film electrodes. Carbon 2006, 44, 1963–1968. [Google Scholar] [CrossRef]
- Wang, X.Y.; Lu, Q.Q.; Chen, C.; Han, M.; Wang, Q.R.; Li, H.X.; Niu, Z.Q.; Chen, J. A Consecutive Spray Printing strategy to construct and integrate diverse supercapacitors on various substrates. ACS Appl. Mater. Interfaces 2017, 9, 28612–28619. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Yoon, J.Y.; Lee, G.B.; Paik, S.H.; Choi, G.W.; Kim, D.; Kim, B.M.; Zi, G.S.; Ha, J.S. Encapsulated, High-Performance, Stretchable array of stacked planar micro-supercapacitors as waterproof wearable energy storage devices. ACS Appl. Mater. Interfaces 2016, 8, 16016–16025. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.G.; Guo, H.Y.; Wang, M.J.; Liu, J.L.; Wang, G.; Hu, C.G.; Xi, Y. A flexible micro-supercapacitor based on a pen ink-carbon fiber thread. J. Mater. Chem. A 2014, 2, 19665–19669. [Google Scholar] [CrossRef]
- Noh, J.; Yoon, C.M.; Yun, K.K.; Jang, J.S. High performance asymmetric supercapacitor twisted from carbon fiber/MnO2, and carbon fiber/MoO3. Carbon 2017, 116, 470–478. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 42, 4248–4253. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Kent, P.R.C. Hybrid density functional study of structural and electronic properties of functionalized Tin+1Xn, (X = C, N) monolayers. Phys. Rev. B 2013, 87, 939–949. [Google Scholar] [CrossRef]
- Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional transition metal carbides. ACS Nano 2012, 6, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.B.; Li, X.F.; Bai, Z.M.; Lu, S.G. Recent advances in layered Ti3C2Tx MXene for electrochemical energy storage. Small 2018, 14, 1703419. [Google Scholar] [CrossRef] [PubMed]
- Ivanovskii, A.L.; Enyashin, A.N. Graphene-like transition-metal nanocarbides and nanonitrides. Russ. Chem. Rev. 2013, 82, 735–746. [Google Scholar] [CrossRef]
- Anasori, B.; Xie, Y.; Beidaghi, M.; Lu, J.; Hosler, B.C.; Hultman, L.; Kent, P.R.C.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional, ordered, double transition metals carbides (MXenes). ACS Nano 2015, 9, 9507–9516. [Google Scholar] [CrossRef] [PubMed]
- Hantanasirisakul, K.; Zhao, M.Q.; Urbankowski, P.; Halim, J.; Anasori, B.; Kota, S.; Ren, C.E.; Barsoum, M.W.; Gogotsi, Y. Fabrication of Ti3C2Tx MXene transparent thin films with tunable optoelectronic properties. Adv. Electron. Mater. 2016, 2, 1600050. [Google Scholar] [CrossRef]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.Q.; Gogotsi, Y.; Barsoum, M.W. Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature 2014, 516, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.M.; Li, Z.J.; Li, G.X.; Hu, T.; Zhang, C.; Wang, X.H. All-solid-state flexible fiber-based MXene supercapacitors. Adv. Mater. Technol. 2017, 2, 1700143. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Mashtalir, O.; Ren, C.E.; Agnese, Y.D.; Rozier, P.; Taberna, P.L.; Naguib, M.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. Cation intercalation and high volumetric capacitance of two-dimensional titanium carbide. Science 2013, 341, 1502–1505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boota, M.; Anasori, B.; Voigt, C.; Zhao, M.Q.; Barsoum, M.W.; Gogotsi, Y. Pseudocapacitive electrodes produced by oxidant-free polymerization of pyrrole between the layers of 2D titanium carbide (MXene). Adv. Mater. 2016, 28, 1517–1522. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.F.; Kremer, M.P.; Seral-Ascaso, A.; Park, S.H.; McEvoy, N.; Anasori, B.; Gogotsi, Y.; Nicolosi, V. Stamping of flexible, coplanar micro-supercapacitors using MXene inks. Adv. Funct. Mater. 2018, 28, 1705506. [Google Scholar] [CrossRef]
- Yan, J.; Ren, C.E.; Maleski, K.; Hatter, C.B.; Anasori, B.; Urbankowski, P.; Sarycheva, A.; Gogotsi, Y. Flexible MXene/graphene films for ultrafast supercapacitors with outstanding volumetric capacitance. Adv. Funct. Mater. 2017, 27, 1701264. [Google Scholar] [CrossRef]
- Luo, J.M.; Zhang, W.K.; Yuan, H.D.; Jin, C.B.; Zhang, L.Y.; Huang, H.; Liang, C.; Xia, Y.; Zhang, J.; Gan, Y.P.; et al. Pillared structure design of MXene with ultra-large interlayer spacing for high performance lithium-ion capacitors. ACS Nano 2017, 11, 2459–2469. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, H.; Louis, E.J.; MacDiarmid, A.G.; Chiang, C.K.; Heeger, A.J. Synthesis of electrically conducting organic polymers: Halogen derivatives of polyacetylene, (CH)X. J. Chem. Soc. 1977, 16, 578–580. [Google Scholar] [CrossRef]
- Chiang, C.K.; Fincher, C.R.; Park, J.Y.W.; Heeger, A.J.; Shirakawa, H.; Louis, E.J.; Gau, S.C.; MacDiarmid, A.G. Electrical conductivity in doped polyacetylene. Phys. Rev. Lett. 1977, 39, 1098. [Google Scholar] [CrossRef]
- Cui, X.Y.; Hetke, J.F.; Wiler, J.A.; Anderson, D.J.; Martin, D.C. Electrochemical deposition and characterization of conducting polymer polypyrrole/PSS on multichannel neural probes. Sensor Actuat. A Phys. 2001, 93, 8–18. [Google Scholar] [CrossRef]
- Macdiarmid, A.G.; Chiang, J.C.; Richter, A.F.; Epstein, A.J. Polyaniline: A new concept in conducting polymers. Synth. Met. 1987, 18, 285–290. [Google Scholar] [CrossRef]
- Kaneto, K.; Yoshino, K.; Inuishi, Y. Electrical properties of conducting polymer, poly-thiophene, prepared by electrochemical polymerization. Jpn. J. Appl. Phys. 1982, 21, 567–568. [Google Scholar] [CrossRef]
- Janata, J.; Josowicz, M. Conducting polymers in electronic chemical sensors. Nat. Mater. 2003, 2, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Jurewicz, K.; Lota, K.; Béguin, F. Supercapacitors based on conducting polymers/nanotubes composites. J. Power Sources 2006, 153, 413–418. [Google Scholar]
- Mastragostino, M.; Arbizzani, C.; Soavi, F. Conducting polymers as electrode materials in supercapacitors. Solid State Ion. 2002, 148, 493–498. [Google Scholar] [CrossRef]
- Snook, G.A.; Kao, P.; Best, A.S. Conducting-polymer-based supercapacitor devices and electrodes. J. Power Sources 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Talbi, H.; Just, P.E.; Dao, L.H. Electropolymerization of aniline on carbonized polyacrylonitrile aerogel electrodes: Applications for supercapacitors. J. Appl. Electrochem. 2003, 33, 465–473. [Google Scholar] [CrossRef]
- Potphode, D.D.; Mishra, S.P.; Sivaraman, P.; Patri, M. Asymmetric supercapacitor devices based on dendritic conducting polymer and activated carbon. Electrochim. Acta 2017, 230, 29–38. [Google Scholar] [CrossRef]
- Meng, Q.F.; Cai, K.F.; Chen, Y.X.; Chen, L.D. Research progress on conducting polymer based supercapacitor electrode materials. Nano Energy 2017, 36, 268–285. [Google Scholar] [CrossRef]
- Chi, K.; Zhang, Z.Y.; Xi, J.B.; Huang, Y.A.; Xiao, F.; Wang, S.; Liu, Y.Q. Freestanding graphene paper supported three-dimensional porous graphene-polyaniline nanocomposite synthesized by inkjet printing and in flexible all-solid-state supercapacitor. ACS Appl. Mater. Interfaces 2014, 6, 16312–16319. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Huang, Y.S.; Liu, J.J.; Sarfraz, M.; Agboola, P.O.; Shakir, I.; Xu, Y.X. A three-dimensional graphene framework-enabled high-performance stretchable asymmetric supercapacitor. J. Mater. Chem. A 2018, 6, 1802–1808. [Google Scholar] [CrossRef]
- Maeng, J.; Kim, Y.J.; Meng, C.Z.; Irazoqui, P.P. Three-dimensional microcavity array electrodes for high-capacitance all-solid-state flexible microsupercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 13458–13465. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.Y.; Kong, D.B.; Xu, Y.; Xie, X.Y.; Tao, Y.; Xiao, Z.C.; Lv, W.; Jang, H.D.; Huang, J.X.; Yang, Q.H. Disassembly-reassembly spproach to RuO2/Graphene composites for ultrahigh volumetric capacitance supercapacitor. Small 2017, 13, 1701026. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Kim, M.; Jang, J. Screen-printable and flexible RuO2 nanoparticle-decorated PEDOT:PSS/Graphene nanocomposite with enhanced electrical and electrochemical performances for high-capacity supercapacitor. ACS Appl. Mater. Interfaces 2015, 7, 10213–10227. [Google Scholar] [CrossRef] [PubMed]
- He, Y.M.; Chen, W.J.; Li, X.D.; Zhang, Z.X.; Fu, J.C.; Zhao, C.H.; Xie, E.Q. Freestanding three-dimensional graphene/MnO2 composite networks as ultralight and flexible supercapacitor electrodes. ACS Nano 2013, 7, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Liu, L.; Yang, S.L.; Liu, J.; Tian, Q.Y.; Yao, W.J.; Xue, Q.W.; Li, M.X.; Wu, W. Facile synthesis of amorphous FeOOH/MnO2, composites as screen-printed electrode materials for all-printed solid-state flexible supercapacitors. J. Power Sources 2017, 361, 31–38. [Google Scholar] [CrossRef]
- Sundriyal, P.; Bhattacharya, S. Inkjet printed electrodes on A4 paper substrates for low cost, disposable and flexible asymmetric supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 38507–38521. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.F.; Wang, H.E.; Wang, C.; Deng, Z.; Chen, L.H.; Li, Y.; Hasan, T.; Su, B.L. 3D interconnected macro-mesoporous electrode with self-assembled NiO nanodots for high-performance supercapacitor-like Li-ion battery. Nano Energy 2016, 22, 269–277. [Google Scholar] [CrossRef]
- Meng, G.; Yang, Q.; Wu, X.C.; Wan, P.B.; Li, Y.P.; Lei, X.D.; Sun, X.M.; Liu, J.F. Hierarchical mesoporous NiO nanoarrays with ultrahigh capacitance for aqueous hybrid supercapacitor. Nano Energy 2016, 30, 831–839. [Google Scholar] [CrossRef]
- Pang, H.; Li, X.R.; Zhao, Q.X.; Xue, H.G.; Lai, W.Y.; Hu, Z.; Huang, W. One-pot synthesis of heterogeneous Co3O4-nanocube/Co(OH)2-nanosheet hybrids for high-performance flexible asymmetric all-solid-state supercapacitors. Nano Energy 2017, 35, 138–145. [Google Scholar] [CrossRef]
- Ji, X.B.; Hallam, P.M.; Houssein, S.M.; Kadara, R.; Lang, L.M.; Banks, C.E. Printable thin film supercapacitors utilizing single crystal cobalt hydroxide, nanosheets. RSC Adv. 2012, 2, 1508–1515. [Google Scholar] [CrossRef]
- Lu, X.; Utama, M.I.B.; Lin, J.H.; Luo, X.; Zhao, Y.Y.; Zhang, J.; Pantelides, S.T.; Zhou, W.; Quek, S.Y.; Xiong, Q.H. Rapid and nondestructive identification of polytypism and stacking sequences in few-layer molybdenum diselenide by raman spectroscopy. Adv. Mater. 2015, 27, 4502–4508. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, Z.J.; Liu, J.X.; Wei, G.J.; Du, J.; Li, Y.P.; An, C.H.; Zhang, J. Synthesis of few-layer 1T’-MoTe2 ultrathin nanosheets for high-performance pseudocapacitors. J. Mater. Chem. A 2017, 5, 1035–1042. [Google Scholar] [CrossRef]
- Zhang, X.M.; Kar, M.; Mendes, T.C.; Wu, Y.T.; MacFarlane, D.R. Supported Ionic Liquid Gel Membrane Electrolytes for Flexible Supercapacitors. Adv. Energy Mater. 2018, 8, 1702702. [Google Scholar] [CrossRef]
- Shen, K.; Ding, J.W.; Yang, S.B. 3D printing quasi-solid-state asymmetric micro supercapacitors with ultrahigh areal energy density. Adv. Energy Mater. 2018. [Google Scholar] [CrossRef]



















| Feature | Advantage | Disadvantage | |
|---|---|---|---|
| Material | |||
| Carbon | Large specific surface area High conductivity Electrochemical stability Cheap price | Low energy density Poor dispersity | |
| MXenes | Large specific surface area High conductivity Good dispersity Higher area capacitance | Complex production High prices Lower mass capacitance | |
| Conductive polymers | High specific capacitance Unique solution processability Filming has good flexibility | Low conductivity Poor electrochemical stability | |
| Transition metal oxides or hydroxides | High specific capacitance High energy density Wide potential window Low cost Easy to prepare | Low conductivity Poor electrochemical stability | |
| Transition metal dichalcogenides | Large specific surface area High specific capacitance Good electrochemical stability | Preparing method is not mature. Physical and chemical properties are easily affected by the environment. | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Cui, B.; Chu, F.; Yun, C.; He, M.; Li, L.; Song, Y. Printable Nanomaterials for the Fabrication of High-Performance Supercapacitors. Nanomaterials 2018, 8, 528. https://doi.org/10.3390/nano8070528
Sun J, Cui B, Chu F, Yun C, He M, Li L, Song Y. Printable Nanomaterials for the Fabrication of High-Performance Supercapacitors. Nanomaterials. 2018; 8(7):528. https://doi.org/10.3390/nano8070528
Chicago/Turabian StyleSun, Jiazhen, Bo Cui, Fuqiang Chu, Chenghu Yun, Min He, Lihong Li, and Yanlin Song. 2018. "Printable Nanomaterials for the Fabrication of High-Performance Supercapacitors" Nanomaterials 8, no. 7: 528. https://doi.org/10.3390/nano8070528
APA StyleSun, J., Cui, B., Chu, F., Yun, C., He, M., Li, L., & Song, Y. (2018). Printable Nanomaterials for the Fabrication of High-Performance Supercapacitors. Nanomaterials, 8(7), 528. https://doi.org/10.3390/nano8070528