Nanoporous Ni with High Surface Area for Potential Hydrogen Storage Application
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of SiO2 Aerogel
2.3. Preparation of Nanoporous Ni
2.4. Characterization
3. Results
3.1. Structural Characterization of Nanoporous Ni
3.2. Magnetic Properties of Nanoporous Ni
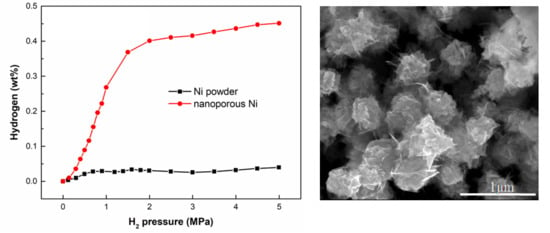
3.3. Hydrogen Adsorption
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Tappan, B.C.; Steiner, S.A.; Luther, E.P. Nanoporous Metal Foams. Angew. Chem. Int. Ed. 2010, 49, 4544–4565. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, C.M. Nanoporous metals: Fabrication strategies and advanced electrochemical applications in catalysis, sensing and energy systems. Chem. Soc. Rev. 2012, 41, 7016–7031. [Google Scholar] [CrossRef] [PubMed]
- Rolison, D.R.; Long, J.W.; Lytle, J.C.; Fischer, A.E.; Rhodes, C.P.; McEvoy, T.M.; Bourg, M.E.; Lubers, A.M. Multifunctional 3D nanoarchitectures for energy storage and conversion. Chem. Soc. Rev. 2009, 38, 226–252. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Ceder, G. Battery materials for ultrafast charging and discharging. Nature 2009, 458, 190–193. [Google Scholar] [CrossRef] [PubMed]
- Sakintuna, B.; Lamaridarkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrogen Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Bérubé, V.; Radtke, G.; Dresselhaus, M.; Chen, G. Size effects on the hydrogen storage properties of nanostructured metal hydrides: A review. Int. J. Energy Res. 2007, 31, 637–663. [Google Scholar] [CrossRef]
- Wollack, E.J.; Fixsen, D.J.; Henry, R.; Kogut, A.; Limon, M.; Mirel, P. Electromagnetic and Thermal Properties of a Conductively Loaded Epoxy. Int. J. Infrared Millim. Waves 2007, 29, 51–61. [Google Scholar] [CrossRef]
- Zhu, C.; Shi, Q.; Fu, S.; Song, J.; Xia, H.; Du, D.; Lin, Y. Efficient Synthesis of MCu (M = Pd, Pt, and Au) Aerogels with Accelerated Gelation Kinetics and their High Electrocatalytic Activity. Adv. Mater. 2016, 28, 8779–8783. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cao, Y.; Yang, L.; Huang, Z.; Long, N.V. Synthesis of Pt-Pd Bimetallic Porous Nanostructures as Electrocatalysts for the Methanol Oxidation Reaction. Nanomaterials 2018, 8, 208. [Google Scholar] [CrossRef] [PubMed]
- Wittstock, A.; Biener, J.; Baumer, M. Nanoporous gold: A new material for catalytic and sensor applications. Phys. Chem. Chem. Phys. 2010, 12, 12919–12930. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.-B.; Cheng, J.-B.; Wang, Y.-Z. Biomass-derived Co@crystalline carbon@carbon aerogel composite with enhanced thermal stability and strong microwave absorption performance. J. Alloys Compd. 2018, 736, 71–79. [Google Scholar] [CrossRef]
- Kashid, S.B.; Raut, R.W.; Malghe, Y.S. Microwave assisted synthesis of nickel nanostructures by hydrazine reduction route: Effect of solvent and capping agent on morphology and magnetic properties. Mater. Chem. Phys. 2016, 170, 24–31. [Google Scholar] [CrossRef]
- Tang, Y.; Yeo, K.L.; Chen, Y.; Yap, L.W.; Xiong, W.; Cheng, W. Ultralow-density copper nanowire aerogel monoliths with tunable mechanical and electrical properties. J. Mater. Chem. A 2013, 1, 6723–6726. [Google Scholar] [CrossRef]
- Zhao, H.-B.; Chen, M.; Chen, H.-B. Thermally Insulating and Flame-Retardant Polyaniline/Pectin Aerogels. ACS Sustain. Chem. Eng. 2017, 5, 7012–7019. [Google Scholar] [CrossRef]
- Zhao, H.-B.; Zhou, X.-C.; Fu, Z.-B.; Mi, R.; Wang, C.-Y. Freestanding monolithic Ni aerogel with large surface areas from cellulose aerogel templates. Mater. Lett. 2017, 196, 296–299. [Google Scholar] [CrossRef]
- Liu, W.; Herrmann, A.K.; Bigall, N.C.; Rodriguez, P.; Wen, D.; Oezaslan, M.; Schmidt, T.J.; Gaponik, N.; Eychmuller, A. Noble metal aerogels-synthesis, characterization, and application as electrocatalysts. Acc. Chem. Res. 2015, 48, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.R.; Hodge, A.M.; Biener, J.; Hamza, A.V.; Sieradzki, K. Monolithic nanoporous copper by dealloying Mn-Cu. J. Mater. Res. 2011, 21, 2611–2616. [Google Scholar] [CrossRef]
- Zhu, J.; Yang, X.; Fu, Z.; He, J.; Wang, C.; Wu, W.; Zhang, L. Three-Dimensional Macroassembly of Sandwich-Like, Hierarchical, Porous Carbon/Graphene Nanosheets towards Ultralight, Superhigh Surface Area, Multifunctional Aerogels. Chemistry 2016, 22, 2515–2524. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jeong, H.Y.; Imura, M.; Wang, L.; Radhakrishnan, L.; Fujita, N.; Castle, T.; Terasaki, O.; Yamauchi, Y. Shape- and size-controlled synthesis in hard templates: Sophisticated chemical reduction for mesoporous monocrystalline platinum nanoparticles. J. Am. Chem. Soc. 2011, 133, 14526–14529. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Luo, H.; Kou, R.; Gil, M.P.; Xiao, S.; Golub, V.O.; Yang, Z.; Brinker, C.J.; Lu, Y. A general route to macroscopic hierarchical 3D nanowire networks. Angew. Chem. Int. Ed. Engl. 2004, 43, 6169–6173. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, H.Y.; Huang, Y.C.; Ho, R.M.; Lai, C.H.; Makida, T.; Hasegawa, H. Nanoporous gyroid nickel from block copolymer templates via electroless plating. Adv. Mater. 2011, 23, 3041–3046. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Cizeron, J.; Bertone, J.F.; Colvin, V.L. Preparation of Macroporous Metal Films from Colloidal Crystals. J. Am. Chem. Soc. 1999, 121, 7957–7958. [Google Scholar] [CrossRef]
- Huang, X.; Fu, Z.; Yang, X.; Sun, Z.; Zhong, M.; Yi, Y.; Tang, Y.; Wang, C. Nanoporous Ni: Electrodeposition synthesis, morphology, and magnetic property. J. Porous Mater. 2013, 21, 9–14. [Google Scholar] [CrossRef]
- Błaszczyński, T.; Ślosarczyk, A.; Morawski, M. Synthesis of Silica Aerogel by Supercritical Drying Method. Procedia Eng. 2013, 57, 200–206. [Google Scholar] [CrossRef]
- Yu, X.; Tang, Z.; Sun, D.; Ouyang, L.; Zhu, M. Recent advances and remaining challenges of nanostructured materials for hydrogen storage applications. Prog. Mater. Sci. 2017, 88, 1–48. [Google Scholar] [CrossRef]
- Xie, S.Q.; Zhai, Y.C. Electric Field Assisted Hydrogen Adsorption on Porous Nickel Oxide. J. Chin. Soc. Rare Earths 2012, 30, 401–405. [Google Scholar]
- Huang, F.; Wang, R.; Yang, C.; Driss, H.; Chu, W.; Zhang, H. Catalytic performances of Ni/mesoporous SiO2 catalysts for dry reforming of methane to hydrogen. J. Energy Chem. 2016, 25, 709–719. [Google Scholar] [CrossRef]
- Xue, M.; Hu, S.; Chen, H.; Fu, Y.; Shen, J. Preparation of highly loaded and dispersed Ni/SiO2 catalysts. Catal. Commun. 2011, 12, 332–336. [Google Scholar] [CrossRef]
- Zhao, H.-B.; Fu, Z.-B.; Liu, X.-Y.; Zhou, X.-C.; Chen, H.-B.; Zhong, M.-L.; Wang, C.-Y. Magnetic and Conductive Ni/Carbon Aerogels toward High-Performance Microwave Absorption. Ind. Eng. Chem. Res. 2017, 57, 202–211. [Google Scholar] [CrossRef]
- Altınçekiç, T.G.; Boz, İ.; Basaran, A.C.; Aktaş, B.; Kazan, S. Synthesis and Characterization of Ferromagnetic Nickel Nanoparticles. J. Supercond. Nov. Magn. 2011, 25, 2771–2775. [Google Scholar] [CrossRef]
- Tang, S.; Vongehr, S.; Ren, H.; Meng, X. Diameter-controlled synthesis of polycrystalline nickel nanowires and their size dependent magnetic properties. CrystEngComm 2012, 14, 7209–7216. [Google Scholar] [CrossRef]
- Wang, H.; Jiao, X.; Chen, D. Monodispersed Nickel Nanoparticles with Tunable Phase and Size: Synthesis, Characterization, and Magnetic Properties. J. Phys. Chem. C 2008, 112, 18793–18797. [Google Scholar] [CrossRef]
- Ni, X.; Zhao, Q.; Zheng, H.; Li, B.; Song, J.; Zhang, D.; Zhang, X. A Novel Chemical Reduction Route towards the Synthesis of Crystalline Nickel Nanoflowers from a Mixed Source. Eur. J. Inorg. Chem. 2005, 2005, 4788–4793. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, Z.; Zhu, Y. Porous carbon-based materials for hydrogen storage: Advancement and challenges. J. Mater. Chem. A 2013, 1, 9365–9381. [Google Scholar] [CrossRef]
- Guo, Z.P.; Yuan, L.; Konstantinov, K.; Huang, Z.G.; Liu, H.K. Preparation of spherical clusters of metal oxide nanorods and their hydrogen storage behavior. Mater. Lett. 2006, 60, 3891–3894. [Google Scholar] [CrossRef]






| Samples | SBET (m2/g) | Total Volume (cm3/g) | Average Pore Size (nm) |
|---|---|---|---|
| Ni-1 | 76.79 | 0.424 | 22.12 |
| Ni-2 | 120.54 | 0.761 | 25.25 |
| Ni-3 | 13.48 | 0.065 | 19.25 |
| Ni-35 °C | 120.54 | 0.761 | 25.25 |
| Ni-50 °C | 40.48 | 0.347 | 34.29 |
| Samples | Ms (emu/g) | Mr (emu/g) | Hc (Oe) | Resource |
|---|---|---|---|---|
| Ni-1 | 36.52 | 10.5 | 331.11 | This work |
| Ni-2 | 29.11 | 7.8 | 321.78 | This work |
| Ni-3 | 51.76 | 2.7 | 60.62 | This work |
| Bulk Ni | 55 | 2.7 | 100 | [33] |
| Samples | Surface Area (m2/g) | H2 Pressure (MPa) | Hydrogen Storage Capacity (at Room Temperature) (wt %) | Resource |
|---|---|---|---|---|
| Ni-1 | 76.79 | 4.5 | 0.27 | This work |
| Ni-2 | 120.54 | 4.5 | 0.45 | This work |
| Ni-3 | 13.48 | 4.5 | 0.08 | This work |
| (Ni0.347Mn0.346Co0.307)O | -- | 3.1 | 0.42 | [35] |
| (Ni0.924Co0.021Zn0.055)O | -- | 3.1 | 0.71 | [35] |
| carbon aerogel | -- | 6.0 | 0.28 | [18] |
| graphene aerogel | -- | 6.0 | 0.18 | [18] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Zhao, H.; Fu, Z.; Qu, J.; Zhong, M.; Yang, X.; Yi, Y.; Wang, C. Nanoporous Ni with High Surface Area for Potential Hydrogen Storage Application. Nanomaterials 2018, 8, 394. https://doi.org/10.3390/nano8060394
Zhou X, Zhao H, Fu Z, Qu J, Zhong M, Yang X, Yi Y, Wang C. Nanoporous Ni with High Surface Area for Potential Hydrogen Storage Application. Nanomaterials. 2018; 8(6):394. https://doi.org/10.3390/nano8060394
Chicago/Turabian StyleZhou, Xiaocao, Haibo Zhao, Zhibing Fu, Jing Qu, Minglong Zhong, Xi Yang, Yong Yi, and Chaoyang Wang. 2018. "Nanoporous Ni with High Surface Area for Potential Hydrogen Storage Application" Nanomaterials 8, no. 6: 394. https://doi.org/10.3390/nano8060394
APA StyleZhou, X., Zhao, H., Fu, Z., Qu, J., Zhong, M., Yang, X., Yi, Y., & Wang, C. (2018). Nanoporous Ni with High Surface Area for Potential Hydrogen Storage Application. Nanomaterials, 8(6), 394. https://doi.org/10.3390/nano8060394