Electrochemical Deposition of Gold Nanoparticles on Reduced Graphene Oxide by Fast Scan Cyclic Voltammetry for the Sensitive Determination of As(III)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrument
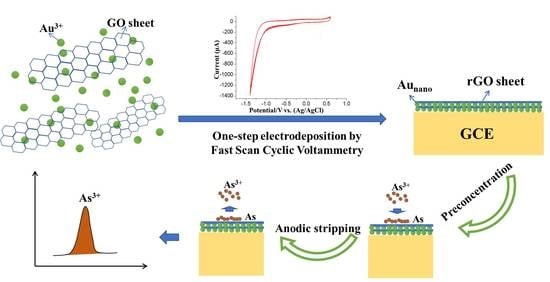
2.3. Synthesis and Modification of the rGO-Aunano Nanocomposite
2.4. Electrochemical Detection of As(III)
2.5. Soil Sample Preparation
3. Results and Discussion
3.1. Electrochemical Deposition of the rGO-Aunano Composite
3.2. Characteristics of the Modified Electrodes
3.3. Optimization of the Experimental Conditions
3.4. Stripping Responses of Different Electrodes
3.5. Analytical Performance of rGO-Aunano/GCE
3.6. Stability Measurements
3.7. Interference Studies
3.8. Application to Real Sample Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ning, Z.; Lobdell, D.T.; Kwok, R.K.; Liu, Z.; Zhang, S.; Ma, C.; Riediker, M.; Mumford, J.L. Residential exposure to drinking water arsenic in Inner Mongolia, China. Toxicol. Appl. Pharmacol. 2007, 222, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Lopipero, P.; Chung, J.; Haque, R.; Hernandez, A.; Moore, L.; Steinmaus, C. Arsenic in drinking water and cancer risks estimated from epidemiological studies in Argentina, Chile, Taiwan and Japan. Epidemiology 2000, 11, 93. [Google Scholar]
- Rodriguez-Lado, L.; Sun, G.; Berg, M.; Zhang, Q.; Xue, H.; Zheng, Q.; Johnson, C.A. Groundwater arsenic contamination throughout China. Science 2013, 341, 866–868. [Google Scholar] [CrossRef] [PubMed]
- Smedley, P.L.; Kinniburgh, D.G. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef]
- Yin, X.B.; Yan, X.P.; Jiang, Y.; He, X.W. On-line coupling of capillary electrophoresis to hydride generation atomic fluorescence spectrometry for arsenic speciation analysis. Anal. Chem. 2002, 74, 3720–3725. [Google Scholar] [CrossRef] [PubMed]
- Aggett, J.; Aspell, A.C. The determination of arsenic(III) and total arsenic by atomic-absorption spectroscopy. Analyst 1976, 101, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.P.; Kerrich, R.; Hendry, M.J. Determination of (ultra) trace amounts of arsenic(III) and arsenic(V) in water by inductively coupled plasma mass spectrometry coupled with flow injection on-line sorption preconcentration and separation in a knotted reactor. Anal. Chem. 1998, 70, 4736–4742. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, H.; Liu, G.; Wang, Z. Optimization of stripping voltammetric sensor by a back propagation artificial neural network for the accurate determination of Pb(II) in the presence of Cd(II). Sensors 2016, 16, 1540. [Google Scholar] [CrossRef]
- Etesami, M.; Karoonian, F.S.; Mohamed, N. Electrochemical deposition of gold nanoparticles on pencil graphite by fast scan cyclic voltammetry. J. Chin. Chem. Soc. 2011, 58, 688–693. [Google Scholar] [CrossRef]
- Xiao, L.; Wildgoose, G.G.; Compton, R.G. Sensitive electrochemical detection of arsenic(III) using gold nanoparticle modified carbon nanotubes via anodic stripping voltammetry. Anal. Chim. Acta 2008, 620, 44–49. [Google Scholar] [CrossRef]
- Bu, L.; Gu, T.; Ma, Y.; Chen, C.; Tan, Y.; Xie, Q.; Yao, S. Enhanced cathodic preconcentration of As(0) at Au and Pt electrodes for anodic stripping voltammetry analysis of As(III) and As(V). J. Phys. Chem. C 2015, 119, 11400–11409. [Google Scholar] [CrossRef]
- Tan, Y.; Li, Y.; Zhu, D. Fabrication of gold nanoparticles using a trithiol (thiocyanuric acid) as the capping agent. Langmuir 2002, 18, 3392–3395. [Google Scholar] [CrossRef]
- Zhang, J.; Oyama, M. Gold nanoparticle arrays directly grown on nanostructured indium tin oxide electrodes: Characterization and electroanalytical application. Anal. Chim. Acta 2005, 540, 299–306. [Google Scholar] [CrossRef]
- Li, W.W.; Kong, F.Y.; Wang, J.Y. Facile one-pot and rapid synthesis of surfactant-free Au-reduced graphene oxide nanocomposite for trace arsenic(III) detection. Electrochim. Acta 2015, 157, 183–190. [Google Scholar] [CrossRef]
- Fukushima, M.; Yanagi, H.; Hayashi, S.; Suganuma, N. Fabrication of gold nanoparticles and their influence on optical properties of dye-doped sol-gel films. Thin Solid Films 2003, 438, 39–43. [Google Scholar] [CrossRef]
- Dai, X.; Nekrassova, O.; Hyde, M.E.; Compton, R.G. Anodic stripping voltammetry of arsenic(III) using gold nanoparticle-modified electrodes. Anal. Chem. 2004, 76, 5924–5929. [Google Scholar] [CrossRef] [PubMed]
- El-Deab, M.S.; Okajima, T.; Ohsaka, T. Electrochemical reduction of oxygen on gold nanoparticle-electrodeposited glassy carbon electrodes. J. Electrochem. Soc. 2003, 150, 851–857. [Google Scholar] [CrossRef]
- Hau, N.Y.; Chang, Y.H.; Huang, Y.T.; Wei, T.C.; Feng, S.P. Direct electroplated metallization on indium tin oxide plastic substrate. Langmuir 2013, 30, 132–139. [Google Scholar] [CrossRef]
- Hau, N.Y.; Chang, Y.H.; Feng, S.P. Kinetics study of silver electrocrystallization on (3-mercaptopropyl) trimethoxysilane-grafted indium tin oxide plastic substrate. Electrochim. Acta 2015, 158, 121–128. [Google Scholar] [CrossRef]
- Feng, H.P.; Paudel, T.; Yu, B.; Chen, S.; Ren, Z.F.; Chen, G. Nanoparticle-Enabled Selective Electrodeposition. Adv. Mater. 2011, 23, 2454–2459. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, H.; Liu, G.; Wang, Z.; Cheng, J. Simultaneous determination of trace Cd(II) and Pb(II) based on Bi/Nafion/reduced graphene oxide-gold nanoparticle nanocomposite film-modified glassy carbon electrode by one-step electrodeposition. Ionics 2016, 23, 767–777. [Google Scholar] [CrossRef]
- Liu, C.; Wang, K.; Luo, S. Direct Electrodeposition of Graphene Enabling the One-Step Synthesis of Graphene–Metal Nanocomposite Films. Small 2011, 7, 1203–1206. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Compton, R.G. Direct electrodeposition of gold nanoparticles onto indium tin oxide film coated glass: Application to the detection of arsenic(III). Anal. Sci. 2006, 22, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Krasnodêbska-Ostrêga, B.; Kowalska, J. Ultrasound-assisted acetic acid extraction of metals from soils. J. Chem. Anal. 2003, 48, 967–974. [Google Scholar]
- Zhou, M.; Wang, Y.; Zhai, Y.; Zhai, J.; Ren, W.; Wang, F.; Dong, S. Controlled synthesis of large-area and patterned electrochemically reduced graphene oxide films. Chem. Eur. J. 2009, 15, 6116–6120. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Wang, J.; Engelhard, M.; Wang, C.; Lin, Y. Facile and controllable electrochemical reduction of graphene oxide and its applications. J. Mater. Chem. C 2010, 20, 743–748. [Google Scholar] [CrossRef]
- Guo, H.L.; Wang, X.F.; Qian, Q.Y.; Wang, F.B.; Xia, X.H. A green approach to the synthesis of graphene nanosheets. ACS Nano 2009, 3, 2653–2659. [Google Scholar] [CrossRef]
- Chen, L.; Tang, Y.; Wang, K.; Liu, C.; Luo, S. Direct electrodeposition of reduced graphene oxide on glassy carbon electrode and its electrochemical application. Electrochem. Commun. 2011, 13, 133–137. [Google Scholar] [CrossRef]
- Jin, R.; Cao, Y.C.; Hao, E.; Métraux, G.S.; Schatz, G.C.; Mirkin, C.A. Controlling anisotropic nanoparticle growth through plasmon excitation. Nature 2003, 425, 487–490. [Google Scholar] [CrossRef]
- Wei, Y.; Kong, L.T.; Yang, R.; Wang, L.; Liu, J.H.; Huang, X.J. Electrochemical impedance determination of polychlorinated biphenyl using a pyrenecyclodextrin-decorated single-walled carbon nanotube hybrid. Chem. Commun. 2011, 47, 5340–5342. [Google Scholar] [CrossRef]
- Chen, D.M.; Gao, Z.F.; Jia, J.; Li, N.B.; Luo, H.Q. A sensitive and selective electrochemical biosensor for detection of mercury(II) ions based on nicking endonuclease-assisted signal amplification. Sens. Actuators B 2015, 210, 290–296. [Google Scholar] [CrossRef]
- Li, S.S.; Zhou, W.Y.; Jiang, M.; Guo, Z.; Liu, J.H.; Zhang, L.; Huang, X.J. Surface Fe(II)/Fe(III) Cycle Promoted Ultra-Highly Sensitive Electrochemical Sensing of Arsenic(III) with Dumbbell-Like Au/Fe3O4 Nanoparticles. Anal. Chem. 2018, 90, 4569–4577. [Google Scholar] [CrossRef] [PubMed]
- Pungjunun, K.; Chaiyo, S.; Jantrahong, I.; Nantaphol, S.; Siangproh, W.; Chailapakul, O. Anodic stripping voltammetric determination of total arsenic using a gold nanoparticle-modified boron-doped diamond electrode on a paper-based device. Microchim. Acta 2018, 185, 324. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Hong, H.G. Anodic stripping voltammetric detection of arsenic(III) at platinum-iron(III) nanoparticle modified carbon nanotube on glassy carbon electrode. Bull. Korean Chem. Soc. 2010, 31, 3077–3083. [Google Scholar] [CrossRef]
- Xie, Z.; Xu, J.; Xie, F.; Xiong, S. Electrochemical Detection of As(III) by a rGO/Fe3O4-modified Screen-Printed Carbon Electrode. Anal. Sci. 2016, 32, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Majid, E.; Hrapovic, S.; Liu, Y.; Male, K.B.; Luong, J.H.T. Electrochemical determination of arsenite using a gold nanoparticle modified glassy carbon electrode and flow analysis. Anal. Chem. 2006, 78, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Nunez-Bajo, E.; Blanco-López, M.C.; Costa-García, A.; Fernández-Abedul, M.T. Electrogeneration of Gold Nanoparticles on Porous-Carbon Paper-Based Electrodes and Application to Inorganic Arsenic Analysis in White Wines by Chronoamperometric Stripping. Anal. Chem. 2017, 89, 6415–6423. [Google Scholar] [CrossRef] [Green Version]
- Devi, P.; Sharma, C.; Kumar, P.; Kumar, M.; Bansod, B.K.S.; Nayak, M.K.; Singla, M.L. Selective electrochemical sensing for arsenite using rGO/Fe3O4 nanocomposites. J. Hazard. Mater. 2017, 322, 85–94. [Google Scholar] [CrossRef]
- Simm, A.O.; Banks, C.E.; Compton, R.G. Sonoelectroanalytical detection of ultra-trace arsenic. Electroanalysis 2005, 17, 335–342. [Google Scholar] [CrossRef]
- Rahman, M.R.; Okajima, T.; Ohsaka, T. Selective detection of As(III) at the Au(111)-like polycrystalline gold electrode. Anal. Chem. 2010, 82, 9169–9176. [Google Scholar] [CrossRef]
- Hossain, M.M.; Islam, M.M.; Ferdousi, S.; Okajima, T.; Ohsaka, T. Anodic Stripping Voltammetric detection of arsenic(III) at gold nanoparticle-modified glassy carbon electrodes prepared by electrodeposition in the presence of various additives. Electroanalysis 2008, 20, 2435–2441. [Google Scholar] [CrossRef]
- Chowdhury, A.N.; Ferdousi, S.; Islam, M.M.; Okajima, T.; Ohsaka, T. Arsenic detection by nanogold/conducting-polymer-modified glassy carbon electrodes. J. Appl. Polym. Sci. 2007, 104, 1306–1311. [Google Scholar] [CrossRef]











| Electrodes | Technique | Linear Range (μg/L) | Detection Limit (μg/L) | Reference |
|---|---|---|---|---|
| Au-RGO/GCE | ASLSV | 0.3–20 | 0.1 | [14] |
| AuNP/BDD-modified electrode | SWASV | 100–1500 | 20 | [33] |
| nanoPt-Fe(III)/MWCNT/GCE | ASV | 0–225 | 0.75 | [34] |
| rGO-Fe3O4/SPCE | SWASV | 2–20 | 0.3 | [35] |
| Gold nanoparticle/GCE | ASV | 0–15 | 0.25 | [36] |
| AuNP-PCWEs | SWASV | 2–50 | 2.2 | [37] |
| rGO/Fe3O4/GCE | SWASV | 0.1–20 | 0.12 | [38] |
| Gold disk | SWASV | 225–1800 | 3.7 | [39] |
| Sub-BT/Au | DPASV | 0–11.25 | 0.28 | [40] |
| Nano-Au/GCE | LSV | 3.675–87.075 | 1.8 | [41] |
| I−-nano-Au/PANI/GCE | SWV | 610–3050 | 0.4 | [42] |
| rGO-Aunano/GCE | SWASV | 1–60 | 0.08 | This work |
| Sample No. | Added (μg/L) | Detected by SWASV a (μg/L) | Detected by HG-AFS a (μg/L) | tcalculated | Recovery (%) |
|---|---|---|---|---|---|
| 1 | - | 13.57 ± 0.58 b | 13.69 ± 0.22 b | 1.95 | - |
| 5.00 | 18.63 ± 0.74 | 101.20 | |||
| 10.00 | 23.42 ± 0.60 | 98.50 | |||
| 2 | - | 18.62 ± 0.52 | 18.73 ± 0.23 | 1.73 | - |
| 10.00 | 28.43 ± 0.49 | 98.10 | |||
| 15.00 | 33.49 ± 0.62 | 99.13 | |||
| 3 | - | 15.38 ± 0.63 | 15.44 ± 0.41 | 1.62 | - |
| 15.00 | 30.13 ± 0.41 | 98.33 | |||
| 20.00 | 35.09 ± 0.73 | 98.55 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, G.; Liu, G. Electrochemical Deposition of Gold Nanoparticles on Reduced Graphene Oxide by Fast Scan Cyclic Voltammetry for the Sensitive Determination of As(III). Nanomaterials 2019, 9, 41. https://doi.org/10.3390/nano9010041
Zhao G, Liu G. Electrochemical Deposition of Gold Nanoparticles on Reduced Graphene Oxide by Fast Scan Cyclic Voltammetry for the Sensitive Determination of As(III). Nanomaterials. 2019; 9(1):41. https://doi.org/10.3390/nano9010041
Chicago/Turabian StyleZhao, Guo, and Gang Liu. 2019. "Electrochemical Deposition of Gold Nanoparticles on Reduced Graphene Oxide by Fast Scan Cyclic Voltammetry for the Sensitive Determination of As(III)" Nanomaterials 9, no. 1: 41. https://doi.org/10.3390/nano9010041
APA StyleZhao, G., & Liu, G. (2019). Electrochemical Deposition of Gold Nanoparticles on Reduced Graphene Oxide by Fast Scan Cyclic Voltammetry for the Sensitive Determination of As(III). Nanomaterials, 9(1), 41. https://doi.org/10.3390/nano9010041