3.1. Galactose Sensitivity
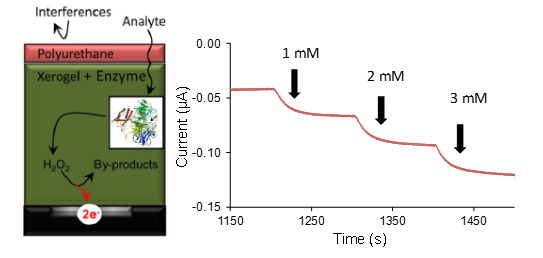
The general strategy of our biosensor design is illustrated in
Scheme 1 and features layer-by-layer (LbL) construction at a platinum electrode with a sol-gel layer embedded with enzyme and capped with a semi-permeable membrane of blended polyurethane (see Experimental Section).
This general scheme has been successfully employed in previous studies on a glucose model system [
2] as well as for uric acid sensing [
13]. The scheme was first constructed, as described in the Experimental Section, using an OTMS xerogel layer capped with the blended PU at a platinum electrode. As in other work studying similar materials [
2,
9,
13], the successful deposition of these layers was independently verified with cyclic voltammetry probing experiments of ferricyanide at each interface to observe the subsequent blocking of solution redox species at modified electrodes. Both the successful initial deposition of the xerogel layer and subsequent capping of the PU layer at the platinum electrode are confirmed with the depression of the ferricyande probe molecule cyclic voltammetry at these modified electrodes (
Figure 1). Upon immersion into buffer and subsequent injections of galactose while under potential control (+0.65 V), amperometric responses in the form of a stair-case current-time (
I-t) curve were clearly observed (
Figure 2A).
In these results, the injection of the galactose substrate resulted in the apparent production of peroxide via an enzymatic reaction. The peroxide was then subsequently and immediately oxidized at the electrode interface to yield a sharp increase in anodic current and the expected stair-step response. As a control, injections at xerogel-modified electrodes without immobilized GaOx exhibited no current response.
I-t curves can be translated into calibration curves (
Figure 2B) which show a linear response to galactose concentrations that easily span the relevant clinical diagnosis range (0.056 to 0.56 mM). The presence of peroxide was verified by repeating the experiment but adding catalase, a peroxide-consuming enzyme, to the test solution after multiple injections of galactose. Upon injection of the catalase, the step response immediately reverses with lower current observed indicating a decreased concentration of peroxide in the solution (
Supplementary Materials, Figure SM-1). Similar results were achieved using platinum electrodes modified with xerogel layers of either PTMS or IBTMS and capped with PU (
Supplementary Materials, Figures SM-2–4).
With other schemes of this nature, the addition of any material to an electrode, but particularly the outer PU membrane, typically results in more defined stair-step current responses at the expense of lower sensitivity (i.e., smaller step currents and lower slope of the corresponding calibration curve) [
2,
13]. This type of signal attenuation is not unexpected when additional materials are used to modify—a result typically observed when one modifies an electrode with additional material. Interestingly, in the galactose oxidase system, the addition of a PU outer layer generally resulted in less noise (a more defined amperometric response and a substantially higher sensitivity (slope) when compared to electrodes without PU (
Figure 2B). This result was highly repeatable and was also observed at the systems using PTMS and IBTMS as well (
Supplementary Materials, Figures SM-3 and SM-4). This result, the enhancement of signal with the addition of the PU ad-layer, stands in stark contrast to prior work on xerogel-based biosensor schemes of this nature and is explored further in a later section to understand the factors contributing to this effect.
The amperometric response of the PU-capped sensors using GaOx-doped OTMS, IBTMS, and PTMS was very robust. Stability studies examined the system’s ability to maintain sensitivity levels after multiple uses of modified electrodes. PU-capped enzyme-doped OTMS, PTMS, and IBTMS xerogel modified electrodes were stored for 24 h in PBS (pH = 7.0) at 5–7 °C and subsequently measured for galactose sensitivity. After each measurement, the modified electrodes were stored under the aforementioned conditions for an additional 24 h. Biosensor performance after multiple uses was determined by calculating the sensor’s percent function after each run according to Equation (1), where
Sn is the sensitivity of a given run,
n, and
Si is the sensitivity of the initial run:
Overall, the results of these stability experiments, shown in
Supplementary Materials (Figure SM-5), suggested an initial decay of sensitivity before stabilizing between 20% and 60% of the original function depending on the type of xerogel. This retention is acceptable considering the relevant physiological concentration of galactose to be detected is so low (0.056 to 0.55 mM). While the sensors remained effective after nearly 10 days, the most notable decrease in sensitivity seemed to occur after 5 days of usage. It is possible that the initial decay and eventual plateau is representing experimental evidence of the continued cross-linking that occurs with the silane precursors and consequential changes to the porosity of the films.
3.2. Polyurethane Enhancement Effect
Within the general scheme, the effects of individual variables (silane precursor, buffer pH, drying time, and enzyme concentration) were systematically examined to understand the unique enhancement effect observed with the addition of PU to these systems. First, the effect of buffer pH was studied given its influence on enzyme function and its importance in using the sensors in biological fluid (blood, for example, exhibits a typical pH of 7.4). Considering pH effects, prior work has suggested that GaOx exhibits optimum catalytic efficiency as pH increases from 6.5 to 7.5, with decreases in activity observed above pH 7.5 [
17]. This research found that for electrodes modified with both OTMS and PTMS, xerogels that both exhibited increased sensitivity with the addition of PU, adjusting the pH from 6.5 to 7.0 did not result in significant changes in sensitivity (
Supplementary Materials, Figure SM-6). The results suggest that small adjustments in pH within the enzyme’s optimal range are not having a significant impact.
Enzyme concentration and drying time, the latter affecting film porosity/permeability, have been previously shown to influence the effectiveness of similar xerogel-based biosensing schemes [
2,
18]. For drying (i.e., aging), GaOx-doped xerogels were investigated after being dried and aged for both 24 and 48 h. The results for the OTMS and PTMS suggest that there was an inconsequential difference in galactose sensitivity for the two different aging times (
Supplementary Materials, Figure SM-6). Similarly, GaOx enzyme concentration was explored to see if sensitivity would be enhanced at higher concentrations. Doubling the enzyme concentration (from 5.4 mg/mL to 10.8 mg/mL) used in modification of the PTMS and OTMS systems also did not result in significant improvement in galactose sensitivity (
Supplementary Materials, Figure SM-6).
A variety of silane precursors (
Scheme 2) were studied for their viability as immobilization matrices for GaOx and to see if the type of xerogel affected the PU signal enhancement. Of the silane precursors tested, only electrodes modified with APTMS (
5) failed to detect galactose at all. This PU effect of increasing sensitivity was observed only with specific silane precursors: OTMS (
1c), PTMS (
1b), IBTMS (
3), and PhTMS (
4). It is notable that these particular silane precursors all possess hydrocarbon-based R-groups. In contrast, other silane precursors used to form GaOx-doped xerogels, including MTMS (
1a), MPTMS (
2), and ODTMS (
1d), did not exhibit an enhancement effect with the addition of the PU capping layer. It should be noted that xerogels formed from representative silane precursors from both these groups were also examined for pH, GaOx concentration, and drying time dependence, including ODTMS, MTMS, and PhTMS xerogel systems. As with the other systems, the results suggest that none of these factors have significant impact on galactose sensitivity (
Supplementary Materials, Figure SM-7). A summary analysis of galactose sensitivity as a function of silane precursor used to create the xerogel layer with and without PU is shown in
Figure 3. For each system without and with PU, a Welch’s
t-test was performed; significant differences were observed for IBTMS, OTMS, PTMS, PhTMS (
p = 0.0076, 0.0045, 0.0055, 0.0043, respectively) while no significant differences were observed for MTMS, MPTMS, and ODTMS (
p = 0.9182, 0.5778, and 0.2138, respectively). The number of ODTMS films tested were limited in this study with the results displaying high variability that resulted in a
p value that, while lower than MTMS and MPTMS, still showed no statistically significant difference. The ODTMS results are not entirely unexpected based on prior work where such films were found to have low porosity/high hydrophobicity and, ultimately, lower sensor sensitivity—all results likely from the significantly longer octadecyl hydrocarbon chain. Overall, the data support the conclusion that xerogels formed from silanes containing medium, aliphatic side chains result in sensors with higher sensitivity upon inclusion of a PU outer layer. A summary of galactose sensitivity parameters for the PU-capped systems OTMS, PTMS, IBTMS, and Ph-TMS is provided as
Table 1.
While the PU enhancement effect was clearly established for certain systems, the source of the effect was not well-understood. It should be noted that adjusting the ratio of HPU to TPU for outer blended PU layer did not yield significant enhancement for any of the systems (results not shown), suggesting that the hydrophobic/hydrophilic nature of the outer layer is less significant than its physical interaction with the enzyme-doped xerogel layer and the testing environment. It was hypothesized that the significant sensitivity enhancement provided by the PU outer layer in these systems may be related to the layer’s ability to mitigate GaOx enzyme leaching, particularly given the smaller size of GaOx compared to other oxidase enzymes that have been embedded in xerogels [
2,
13,
16]. In that prior work with other enzymes (e.g., glucose oxidase, uricase), dramatic increases in sensitivity with the addition of PU capping layers were notably not observed. To test this hypothesis, representative films of GaOx-embedded xerogels that exhibited either significant (OTMS) or insignificant (MTMS) enhancement with PU capping were spectroscopically studied by forming the films on glass slides and observing their UV-Vis spectra to see the effects of exposure to PBS over ~30 min. Briefly, xerogel films comprised of these two silanes, each doped with GaOx, were formed on glass slides under the same conditions as the modified electrodes used in the biosensors. The slides were then immersed in PBS and periodically removed, dried, and measured with a UV-Vis spectrophotometer. Additional experimental details on these specific procedures are provided in the
Supplementary Materials. Because the films are formed and soaked without a PU layer, they are potentially susceptible to GaOx leaching from the film, an effect that would result in decreasing absorbance over time. As shown in
Figure 4, the spectra for GaOx-doped MTMS upon exposure to PBS are relatively consistent over time suggesting that the enzyme content of the film remains relatively constant (minimal leaching). The OTMS xerogel, on the other hand, shows a significant decrease in absorbance almost immediately upon exposure to solution (
Figure 4B), a trend that continues more slowly after 5 min of exposure up to 30 min of exposure (
Figure 4B, inset). This result suggests that, without the PU layer, there is extensive leaching of the enzyme from the OTMS matrix. Analogous films of MTMS and OTMS that are capped with PU do not show this effect (
Figure 4C,D).
Protein quantification assays (Bicinchoninic acid (BCA) assays) were conducted to provide more quantitative data in regards to the enzyme leaching. Experimental details for the assay experiment and analysis is provided in the
Supplementary Materials. Briefly, xerogel films comprised of representative silane precursors that either exhibit sensitivity enhancement (OTMS and IBTMS), or do not enhance sensitivity (MPTMS and MTMS), were deposited on electrodes and half of them were capped with PU. The films with and without the PU capping layer were then exposed to PBS for one hour before aliquots of the soak solution were transferred to an assay plate. The amount of leached GaOx in the solutions contacting the films was quantified by comparing to a standard curve as shown in
Figure 5. Analysis using a 2-sample Welch’s
t-test determined that the difference associated with the addition of PU was more significant for IBTMS and OTMS than for MPTMS and MTMS (
p = 0.0592 < 0.1477 < 0.2198 < 0.2862, respectively). The statistical analysis is not as robust with this method due to the experimental set up and smaller sample size; however, the trend of enzyme leaching is consistent with the electrochemical data. That is, the addition of PU to the IBTMS and OTMS xerogels seems to aid with the retention of GaOx, which is leached to a greater extent from these xerogels than MPTMS and MTMS gels, effectively resulting in the observed elevation in the sensitivity of the sensor due to increased metabolic activity at the electrode surface. Taken collectively, the BCA assay and UV-Vis spectroscopy results suggest that the xerogels of OTMS and IBTMS, where the sensitivity enhancement is observed, are more porous than the xerogels where the PU effect is not observed (MPTMS and MTMS)—a result that is comparable to prior characterization of these films. The greater porosity of the films allows for more substantial leaching, particularly for a smaller enzyme like GaOx, and necessitates the PU capping layer. The larger porosity of the more sensitive films may also contribute to a film structure environment more conducive to substrate/peroxide diffusion (
Scheme 1) [
8]. These results collectively point out the critical nature of selecting an appropriate xerogel matrix in order to gain sensitivity toward an analyte.
3.3. Galactose Biosensing Performance Using Nanomaterials and Polymer Layers
Sensitivity toward galactose was achieved with the GaOx xerogel layer and enhanced with the addition of the PU capping layer but effective biosensor design promotes both sensitivity and selectivity toward the targeted analyte. Even though selectivity is critical for biosensing performance, it is not uncommon for reported biosensor designs in the literature to either insufficiently address interferent response [
26,
27,
31]. For the xerogel-based, LbL-constructed 1st generation biosensor design in this study, some selectivity is achieved with the enzymatic reaction, the choice of xerogel (i.e., silane precursor), and the PU blend comprising the capping layer. However, more effective selectivity is often achieved in such systems with the addition of a semi-permeable membrane, often an electropolymer, to achieve discrimination against specific interferent species [
2,
13,
16]. The PU-capped OTMS and PTMS xerogel systems in this study, while sensitive toward galactose, were also found to be susceptible to specific interferents as observed in the amperometric
I-t curves of these systems (
Supplementary Materials, Figure SM-9), with and without the PU capping layer, during injections of both galactose as well as common endogenous interferent species (e.g., ascorbic acid (AA), sodium nitrite, UA, oxalic acid, and glucose). In these results, the well-established, traditional effect of the PU capping layer is easily observed—a significant improvement in the noise associated with the recorded amperometric signal [
2,
13]. However, the results also illustrate that the PU-capping layer and different xerogels are insufficient for selectivity against both AA and UA. These species are often problematic interferents as both are electroactive and undergo oxidation at the applied potential (+0.65 vs. Ag/AgCl, satd. KCl). Both species are anions at the pH of the working solution (~7) suggesting that any additional layers added to the system for selectivity should increase the anionic character of the film to discriminate against urate and ascorbate.
While many polymeric layers are available for this type of application [
33], a prior study with galactose suggested that the incorporation of anionic Nafion and a copolymer of diaminobenzene and resorcinol was effective for both AA and UA discrimination while allowing for substantial H
2O
2 diffusion [
24]. As described in the Experimental Details Section, procedures were developed to apply the copolymer of diaminobenzene and resorcinol (DBR). However, as seen in prior work [
2,
13], the application of additional layers, such as a semi-permeable polymer layer, often leads to significant signal attenuation and sacrifices sensitivity. The LbL construction of these xerogel-based films, however, allows for the use of nanomaterials such as nanoparticles [
9,
18] or carbon nanotubes (CNTs) [
19,
34] that can significantly enhance the targeted analyte signal in the presence of the selective polymeric membranes.
In designing an effective galactose biosensor based off of the materials and strategy in this study, GaOx-doped xerogels of IBTMS were selected as they showed the highest sensitivity in the PU capped systems (
Table 1). Platinum electrodes were first modified with a DBR electropolymer layer (See Experimental Details) before COOH-SWCNTs were dispersed with and without 1% Nafion in ethanol solutions. Both films were subsequently modified with IBTMS xerogels doped with GaOx and an outer PU layer.
Figure 6A shows the
I-t and calibration curve collected during galactose injections for the system constructed using the Nafion membrane to disperse the CNTs. Comparing this result to the same experiments conducted without employing the Nafion layer (i.e., COOH-SWCNTs dispersed without Nafion—
Supplementary Materials, Figure SM-10), it reveals the expected effects of incorporating both the Nafion and the COOH-SWCNTs; the incorporation of COOH-SWCNTs produces the highest sensitivity of any system tested (0.245 μA/mM) but the addition of the Nafion layer dampens that enhancement (0.036 μA/mM), though still maintaining effective sensitivity. The limit of detection (LOD) is 78 μM and the lower limit of quantification is 260 μM, both of which are well below the diagnostic levels for galactosemia (≥0.5 mM) [
21].
While the decrease in sensitivity with the addition of Nafion is an undesired effect, it is justified if greater selectivity can be achieved. In
Figure 6B,
I-t curves during injections of common interferent species at systems using Nafion used to disperse the COOH-SWCNTs show the substantial impact the polymer layer has on certain interferent response, particularly discrimination against ascorbic acid. As in previous studies [
9,
16,
35,
36], selectivity coefficients (
Kjamp) were calculated to provide conservative quantitative assessment of selectivity as has been done in previous studies using the following equation:
where ∆
Ij and ∆
Ig are the measured currents for a specific interferent species (
j) and galactose and
Cj and
Cg are concentrations of the interferent species and galactose, respectively. Negative values of
Kamp indicate that the interferent is inconsequential (i.e., no significant response compared to analyte response) whereas species with near zero or slightly positive values are selected for by the sensor. The selectivity coefficient graph (
Figure 6B, inset) reiterates the effective discrimination of the common interferents with the addition of the Nafion when compared to analogous systems not employing the Nafion layer (
Supplementary Materials, Figure SM-11). While the system, as presented, is not optimized toward uric acid discrimination, the demonstrated effectiveness of employing the anionic Nafion layer to eliminate ascorbate suggests that minor modifications to the LbL approach, including further incorporation of Nafion or other anionic polymers or nanomaterials [
37], perhaps in the PU layer itself would be effective. Preliminary results of the system where citrate-stabilized gold nanoparticles (CS-NPs) were infused into an additional PU layer to add anionic character at the solution interface showed more effective discrimination of urate (
Supplementary Materials, Figure SM-12), though the use of gold NPs in this manner requires additional study.
A critical aspect of any potential biomedical device of this nature is its effective operation in bodily fluids. In the case of galactosemia diagnostic tests, the media most likely to be screened is blood or blood serum.
Figure 7 shows representative
I-t curve and calibration curve for an optimized film in blood serum samples and shows that galactose sensitivity, linear range, LOD (112 μM) and the lower limit of quantification (373 μM) are maintained for effective diagnostic galactose concentrations. Calibrated sensor constructs were used on a limited number of blood serum samples spiked with 2 mM galactose (
Supplementary Materials, Figure SM-13) and showed an average percent recovery of nearly 90% (relative average error −12 (±3)%;
n = 3).
The performance of the sensors designed in this study equal or exceed published reports on amperometric galactose biosensors [
23,
24,
25,
26,
27,
28,
29,
30,
31], though parameters and measurements are often difficult to compare across studies which involve different media, testing procedures, and often do not define how a parameter was measured (e.g., few report selectivity coefficients). A table of comparison is provided in
Supplementary Materials (Table SM-1). The most problematic issue with previously reported galactose biosensors of this nature appears to be that the linear range is often misaligned with the most useful diagnostic concentrations (e.g., 0.5 mM to 10 mM), with reports showing galactose detection at either too high [
26,
29] or too low [
23,
28,
30] of a concentration range. A number of the reports also do not report galactose sensitivity [
23,
24,
25,
26,
27,
28,
29,
30,
31,
32,
33,
34,
35,
36], test in blood media [
26,
27,
29,
31] or test for discrimination of common intereferents [
23,
27,
28,
29,
31]. While some sensors are comparable to the system described here, our study benefits from fundamental exploration trying to understand the functionality of the xerogel enzyme immobilization matrix and semi-permeable membranes as well as the incorporation of nanomaterials for signal enhancement—all of which, if understood, could allow for adapting the strategy and materials of this nature to other targets of interest.