Pulsed Laser Deposition of Nanostructured MoS3/np-Mo//WO3−y Hybrid Catalyst for Enhanced (Photo) Electrochemical Hydrogen Evolution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Methods and Materials
2.2. Computational Methods
3. Results
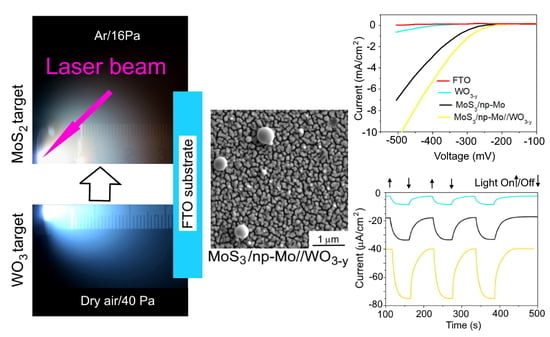
3.1. Characterization of Pulsed Laser Plumes Formed during Ablation of WO3 and MoS2 Targets in Background Gases
3.2. Composition and Structure of WO3−y and MoS3/np-Mo Films
3.3. Electrocatalytic and Photoelectrocatalytic Properites of WO3−y, MoS3/np-Mo and MoS3/np-Mo// WO3−y Films
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A


References
- Yang, L.; Xie, C.; Jin, J.; Ali, R.N.; Feng, C.; Liu, P.; Xiang, B. Properties, preparation and applications of low dimensional transition metal dichalcogenides. Nanomaterials 2018, 8, 463. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Mi, Z.; Shen, M. Silicon based photoelectrodes for photoelectrochemical water splitting. Opt. Express 2019, 27, A51–A80. [Google Scholar] [CrossRef] [PubMed]
- Joe, J.; Yang, H.; Bae, C.; Shin, H. Metal chalcogenides on silicon photocathodes for efficient water splitting: A mini overview. Catalysts 2019, 9, 149. [Google Scholar] [CrossRef]
- Peter, C.K.; Vesborg, P.C.K.; Seger, B.; Chorkendorff, I. Recent Development in Hydrogen Evolution Reaction Catalysts and Their Practical Implementation. J. Phys. Chem. Lett. 2015, 6, 951–957. [Google Scholar] [CrossRef]
- Benck, J.D.; Hellstern, T.R.; Kibsgaard, J.; Chakthranont, P.; Jaramillo, T.F. Catalyzing the Hydrogen Evolution Reaction (HER) with Molybdenum Sulfide Nanomaterials. ACS Catal. 2014, 4, 3957–3971. [Google Scholar] [CrossRef]
- Hellstern, T.R.; Palm, D.W.; Carter, J.; DeAngelis, A.D.; Horsley, K.; Weinhardt, L.; Yang, W.; Blum, M.; Gaillard, N.; Heske, C.; et al. Molybdenum Disulfide Catalytic Coatings via Atomic Layer Deposition for Solar Hydrogen Production from Copper Gallium Diselenide Photocathodes. ACS Appl. Energy Mater. 2019, 22, 1060–1066. [Google Scholar] [CrossRef]
- Laursen, A.B.; Kegnæs, S.; Dahl, S.; Chorkendorff, I. Molybdenum sulfides—efficient and viable materials for electro–and photoelectrocatalytic hydrogen evolution. Energy Environ. Sci. 2012, 5, 5577–5591. [Google Scholar] [CrossRef]
- Wang, Y.; Kong, B.; Zhao, D.; Wang, H.; Selomulya, C. Strategies for developing transition metal phosphides as heterogeneous electrocatalysts for water splitting. Nano Today 2017, 15, 26–55. [Google Scholar] [CrossRef]
- Huang, Z.-F.; Song, J.; Pan, L.; Zhang, X.; Wang, L.; Zou, J.-J. Tungsten oxides for photocatalysis, electrochemistry, and phototherapy. Adv. Mater. 2015, 27, 5309–5327. [Google Scholar] [CrossRef]
- Zhong, X.; Tang, J.; Wang, J.; Shao, M.; Chai, J.; Wang, S.; Yang, M.; Yang, Y.; Wang, N.; Wang, S.; et al. 3D heterostructured pure and N-Doped Ni3S2/VS2 nanosheets for high efficient overall water splitting. Electrochim. Acta 2018, 269, 55–61. [Google Scholar] [CrossRef]
- Pan, L.F.; Li, Y.H.; Yang, S.; Liu, P.F.; Yu, M.Q.; Yang, H.G. Molybdenum carbide stabilized on graphene with high electrocatalytic activity for hydrogen evolution reaction. Chem. Commun. 2014, 50, 13135–13137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, H.; Tian, C.; Wang, L.; Wu, A.; Meng, M.; Zhao, L.; Fu, H. Phosphorus-modified tungsten nitride/reduced graphene oxide as a high-performance, non-noble-metal electrocatalyst for the hydrogen evolution reaction. Angew. Chem. 2015, 54, 6325–6329. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, S.; Salamone, M.M.; Robertson, A.W.; Nayak, S.; Kim, H.; Tsang, S.C.E.; Pasta, M.; Warner, J.H. Edge-enriched 2D MoS2 thin films grown by chemical vapor deposition for enhanced catalytic performance. ACS Catal. 2017, 7, 877–886. [Google Scholar] [CrossRef]
- Meng, C.; Lin, M.-C.; Du, X.-W.; Zhou, Y. Molybdenum disulfide modified by laser irradiation for catalyzing hydrogen evolution. ACS Sustain. Chem. Eng. 2019, 7, 6999–7003. [Google Scholar] [CrossRef]
- Li, Y.H.; Liu, P.F.; Pan, L.F.; Wang, H.F.; Yang, Z.Z.; Zheng, L.R.; Hu, P.; Zhao, H.J.; Gu, L.; Yang, H.G. Local atomic structure modulations activate metal oxide as electrocatalyst for hydrogen evolution in acidic water. Nat. Commun. 2015, 6, 8064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Zhu, X.; Xiong, S.; Wu, X.; Shan, Y.; Chu, P.K. Synergistic WO3·2H2O nanoplates/WS2 hybrid catalysts for high-efficiency hydrogen evolution. ACS Appl. Mater. Interfaces 2016, 8, 13966–13972. [Google Scholar] [CrossRef]
- Jiang, X.; Sun, B.; Song, Y.; Dou, M.; Ji, J.; Wang, F. One-pot synthesis of MoS2/WS2 ultrathin nanoflakes with vertically aligned structure on indium tin oxide as a photocathode for enhanced photo-assistant electrochemical hydrogen evolution reaction. RSC Adv. 2017, 7, 49309–49319. [Google Scholar] [CrossRef]
- Bozheyev, F.; Xi, F.; Plate, P.; Dittrich, T.; Fiechter, S.; Ellmer, K. Efficient charge transfer at a homogeneously distributed (NH4)2Mo3S13/WSe2 heterojunction for solar hydrogen evolution. J. Mater. Chem. A 2019, 7, 10769–10780. [Google Scholar] [CrossRef]
- Pumera, M.; Sofer, Z.; Ambrosi, A. Layered transition metal dichalcogenides for electrochemical energy generation and storage. J. Mater. Chem. A 2014, 2, 8981–8987. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, K.; Lin, H.; Li, X.; Chan, H.C.; Yang, L.; Gao, Q. Heteronanorods of MoS2-Ni3S2 as efficient and stable bi-functional electrocatalysts for overall water splitting. ACS Catal. 2017, 74, 2357–2366. [Google Scholar] [CrossRef]
- Merki, D.; Fierro, S.; Vrubel, H.; Hu, X. Amorphous molybdenum sulfide films as catalysts for electrochemical hydrogen production in water. Chem. Sci. 2011, 2, 1262–1267. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.-W.; Liu, C.-J.; Lin, J.-Y. Facile synthesis of MoS3/carbon nanotube nanocomposite with high catalytic activity toward hydrogen evolution reaction. Appl. Catal. B-Environ. 2013, 134, 75–82. [Google Scholar] [CrossRef]
- Escalera-López, D.; Lou, Z.; Rees, N.V. Benchmarking the activity, stability, and inherent electrochemistry of amorphous molybdenum sulfide for hydrogen production. Adv. Energy Mater. 2019, 9, 1802614. [Google Scholar] [CrossRef]
- Mabayoje, O.; Wygant, B.R.; Wang, M.; Liu, Y.; Mullins, C.B. Sulfur-rich MoS6 as an electrocatalyst for the hydrogen evolution reaction. ACS Appl. Energy Mater. 2018, 1, 4453–4458. [Google Scholar] [CrossRef]
- Xi, F.; Bogdanoff, P.; Harbauer, K.; Plate, P.; Höhn, C.; Rappich, J.; Wang, B.; Han, X.; van de Krol, R.; Fiechter, S. Structural transformation identification of sputtered amorphous MoSx as efficient hydrogen evolving catalyst during electrochemical activation. ACS Catal. 2019, 9, 2368–2380. [Google Scholar] [CrossRef]
- Xie, Y.P.; Liu, G.; Yin, L.; Cheng, H.-M. Crystal facet-dependent photocatalytic oxidation and reduction reactivity of monoclinic WO3 for solar energy conversion. J. Mater. Chem. 2012, 22, 6746–6751. [Google Scholar] [CrossRef]
- Chen, Z.; Cummins, D.; Reinecke, B.N.; Clark, E.; Sunkara, M.K.; Jaramillo, T.F. Core-shell MoO3-MoS2 nanowires for hydrogen evolution: A functional design for electrocatalytic materials. Nano Lett. 2011, 11, 4168–4175. [Google Scholar] [CrossRef]
- Fominski, V.Y.; Grigoriev, S.N.; Romanov, R.I.; Volosova, M.A.; Grunin, A.I.; Teterina, G.D. The formation of a hybrid structure from tungsten selenide and oxide plates for a hydrogen-evolution electrocatalyst. Technol. Phys. Lett. 2016, 42, 553–556. [Google Scholar] [CrossRef]
- Zhang, X.; Du, Z.; Luo, X.; Sun, A.; Wu, Z.; Wang, D. Template-free fabrication of hierarchical MoS2/MoO2 nanostructures as efficient catalysts for hydrogen production. Appl. Surf. Sci. 2018, 433, 723–729. [Google Scholar] [CrossRef]
- Donley, M.S.; Murray, P.T.; Barber, S.A.; Haas, T.W. Deposition and properties of MoS2 thin films grown by pulsed laser evaporation. Surf. Coat. Technol. 1988, 36, 329–340. [Google Scholar] [CrossRef]
- Walck, S.D.; Zabinski, J.S.; Donley, M.S.; Bultman, J.E. Evolution of surface topography in pulsed-laser-deposited thin films of MoS2. Surf. Coat. Technol. 1993, 62, 412–416. [Google Scholar] [CrossRef]
- Fominski, V.Y.; Romanov, R.I.; Fominski, D.V.; Dzhumaev, P.S.; Troyan, I.A. Normal and grazing incidence pulsed laser deposition of nanostructured MoSx hydrogen evolution catalysts from a MoS2 target. Opt. Laser Technol. 2018, 102, 74–84. [Google Scholar] [CrossRef]
- Fominski, V.Y.; Markeev, A.M.; Nevolin, V.N.; Prokopenko, V.B.; Vrublevski, A.R. Pulsed laser deposition of MoSx films in a buffer gas atmosphere. Thin Solid Films 1994, 248, 240–246. [Google Scholar] [CrossRef]
- Wang, R.; Sun, P.; Wang, H.; Wang, X. Pulsed laser deposition of amorphous molybdenum disulfide films for efficient hydrogen evolution reaction. Electrochim. Acta 2017, 258, 876–882. [Google Scholar] [CrossRef]
- Fominski, V.Y.; Nevolin, V.N.; Romanov, R.I.; Smurov, I. Ion-assisted deposition of MoSxfilms from laser-generated plume under pulsed electric field. J. Appl. Phys. 2001, 89, 1449–1457. [Google Scholar] [CrossRef]
- Fominski, V.Y.; Romanov, R.I.; Fominski, D.V.; Shelyakov, A.V. Preparation of MoSex>3/Mo-NPs catalytic films for enhanced hydrogen evolution by pulsed laser ablation of MoSe2 target. Nucl. Instrm. Methods Phys. Res. B 2018, 416, 30–40. [Google Scholar] [CrossRef]
- Fominski, V.Y.; Romanov, R.I.; Fominski, D.V.; Shelyakov, A.V. Regulated growth of quasi-amorphous MoSx thin-film hydrogen evolution catalysts by pulsed laser deposition of Mo in reactive H2S gas. Thin Solid Films 2017, 642, 58–68. [Google Scholar] [CrossRef]
- Filipescu, M.; Orlando, S.; Russo, V.; Lamperti, A.; Purice, A.; Moldovan, A.; Dinescu, M. Morphological and structural studies of WOx thin films deposited by laser ablation. Appl. Surf. Sci. 2007, 253, 8258–8262. [Google Scholar] [CrossRef]
- Bailini, A.; Fonzo, F.D.; Fusi, M.; Casari, C.S.; Bassi, A.L.; Russo, V.; Baserga, A.; Bottani, C.E. Pulsed laser deposition of tungsten and tungsten oxide thin films with tailored structure at the nano- and mesoscale. Appl. Surf. Sci. 2007, 253, 8130–8135. [Google Scholar] [CrossRef]
- Lethy, K.J.; Beena, D.; Kumar, R.V.; Pillai, V.P.M.; Ganesan, V.; Sathe, V. Structural, optical and morphological studies on laser ablated nanostructured WO3 thin films. Appl. Surf. Sci. 2008, 254, 2369–2376. [Google Scholar] [CrossRef]
- Fominski, V.; Romanov, R.; Zuev, V.; Soloviev, A.; Goikhman, A.; Maksimova, K.; Shvets, P.; Demin, M. Comparison of hydrogen detection by WOx/SiC and Pt/WOx/SiC structures using amperometric and potentiometric modes of measurement. Thin Sold Films 2019, 669, 461–470. [Google Scholar] [CrossRef]
- Ossi, P.M.; Bailini, A.; Geszti, O.; Radnóczi, G. Morphology and growth mechanism of WOx films prepared by laser ablation of W in different atmospheres. EPL 2008, 83, 68005. [Google Scholar] [CrossRef]
- Fominski, V.Y.; Grigoriev, S.N.; Romanov, R.I.; Volosova, M.A.; Fominski, D.V.; Irzhak, A.V. Structure, morphology and electrocatalytic properties of WOx thin films prepared by reactive pulsed laser deposition. J. Phys. Conf. Ser. 2018, 941, 012062. [Google Scholar] [CrossRef]
- Mayer, M. SIMNRA User’s Guide, Report IPP 9/113; Max-Planck-Institut für Plasmaphysik: Garching, Germany, 1997. [Google Scholar]
- Sutherland, D.N. Comments on Vold’s simulation of floc formation. J. Colloid Interface Sci. 1966, 22, 300–302. [Google Scholar] [CrossRef]
- Witten, T.A., Jr.; Sander, L.M. Diffusion-limited aggregation, a kinetic critical phenomenon. Phys. Rev. Lett. 1981, 47, 1400–1403. [Google Scholar] [CrossRef]
- Gnedovets, A.G.; Fominski, V.Y.; Nevolin, V.N.; Romanov, R.I.; Fominski, D.V.; Soloviev, A.A. Models of WOx films growth during pulsed laser deposition at elevated pressures of reactive gas. J. Phys. Conf. Ser. 2017, 941, 012064. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. Quantum Espresso: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef]
- Xia, X.; Wu, W.; Wang, Z.; Bao, Y.; Huang, Z.; Gao, Y. A hydrogen sensor based on orientation aligned TiO2 thin films with low concentration detecting limit and short response time. Sens. Actuator B-Chem. 2016, 234, 192–200. [Google Scholar] [CrossRef]
- Kuzmin, A.; Purans, J.; Cazzanelli, E.; Vinegoni, C.; Mariotto, G. X-ray diffraction, extended X-ray absorption fine structure and Raman spectroscopy studies of WO3 powders and (1−x)WO3−y·xReO2 mixtures. J. Appl. Phys. 1998, 84, 5515–5524. [Google Scholar] [CrossRef]
- Lu, D.Y.; Chen, J.; Zhou, J.; Deng, S.Z.; Xu, N.S.; Xu, J.B. Raman spectroscopic study of oxidation and phase transition in W18O49 nanowires. J. Raman Spectrosc. 2007, 38, 176–180. [Google Scholar] [CrossRef]
- Vrubel, H.; Merki, D.; Hu, X. Hydrogen evolution catalyzed by MoS3 and MoS2 particles. Energy Environ. Sci. 2012, 5, 6136–6144. [Google Scholar] [CrossRef]
- Voevodin, A.A.; Jones, J.G.; Zabinski, J.S. Characterization of ZrO2/Y2O3 laser ablation plasma in vacuum, oxygen, and argon environments. Appl. Phys. Lett. 2001, 78, 730–739. [Google Scholar] [CrossRef]
- He, Z.; Que, W. Molybdenum disulfide nanomaterials: Structures, properties, synthesis and recent progress on hydrogen evolution reaction. Appl. Mater. Today 2016, 3, 23–56. [Google Scholar] [CrossRef]
- Xiao, Y.-H.; Zhang, W.-D. MoS2 quantum dots interspersed WO3 nanoplatelet arrays with enhanced photoelectrochemical activity. Electrochim. Acta 2017, 252, 416–423. [Google Scholar] [CrossRef]
- Guo, M.; Xing, Z.; Zhao, T.; Li, Z.; Yang, S.; Zhou, W. WS2 quantum dots/MoS2@WO3-x core-shell hierarchical dual Z-scheme tandem heterojunctions with wide-spectrum response and enhanced photocatalytic performance. Appl. Catal. B-Environ. 2019, 257, 117913. [Google Scholar] [CrossRef]
- Mai, M.; Ma, X.; Zhou, H.; Ye, M.; Li, T.; Ke, S.; Lin, P.; Zeng, X. Effect of oxygen pressure on pulsed laser deposited WO3 thin films for photoelectrochemical water splitting. J. Alloy. Compd. 2017, 722, 913–919. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, H.; Jiang, C.; Yuan, J.; Huang, X.; Liu, P.; Feng, W. Z-scheme system of WO3@MoS2/CdS for photocatalytic evolution H2: MoS2 as the charge transfer mode switcher, electron-hole mediator and cocatalyst. Appl. Catal. B-Environ. 2019, 259, 118073. [Google Scholar] [CrossRef]
- Meng, A.; Zhu, B.; Zhong, B.; Zhang, L.; Cheng, B. Direct Z-scheme TiO2/CdS hierarchical photocatalyst for enhanced photocatalytic H2-production activity. Appl. Surf. Sci. 2017, 422, 518–527. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Y.; Zhu, D.; Xin, Y.; Zhang, B. Mediator-free Z-scheme photocatalytic system based on ultrathin CdS nanosheets for efficient hydrogen evolution. J. Mater. Chem. A 2016, 4, 13626–13635. [Google Scholar] [CrossRef]
- Pazhamalai, P.; Krishnamoorthy, K.; Sahoo, S.; Mariappan, V.K.; Kim, S. Supercapacitive properties of amorphous MoS3 and crystalline MoS2 nanosheets in organic electrolyte. Inorg. Chem. Front. 2019, in press. [Google Scholar] [CrossRef]
- Pan, Y.; Guan, W. Prediction of new stable structure, promising electronic and thermodynamic properties of MoS3: Ab initio calculations. J. Power Sources 2016, 325, 246–251. [Google Scholar] [CrossRef]
- Banyamin, Z.Y.; Kelly, P.J.; West, G.; Boardman, J. Electrical and optical properties of fluorine doped tin oxide thin films prepared by magnetron sputtering. Coatings 2014, 4, 732–746. [Google Scholar] [CrossRef]
- Charlie, T.; Abild-Pedersen, F.; Nørskov, J.K. Tuning the MoS2 Edge-Site Activity for Hydrogen Evolution via Support Interactions. Nano Lett. 2014, 14, 1381–1387. [Google Scholar] [CrossRef]
- Ye, H.; Wang, L.; Deng, S.; Zeng, X.; Nie, K.; Duchesne, P.N.; Wang, B.; Liu, S.; Zhou, J.; Zhao, F.; et al. Amorphous MoS3 Infiltrated with Carbon Nanotubes as an Advanced Anode Material of Sodium-Ion Batteries with Large Gravimetric, Areal, and Volumetric Capacities. Adv. Energy Mater. 2017, 7, 1601602. [Google Scholar] [CrossRef]












© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fominski, V.; Gnedovets, A.; Fominski, D.; Romanov, R.; Kartsev, P.; Rubinkovskaya, O.; Novikov, S. Pulsed Laser Deposition of Nanostructured MoS3/np-Mo//WO3−y Hybrid Catalyst for Enhanced (Photo) Electrochemical Hydrogen Evolution. Nanomaterials 2019, 9, 1395. https://doi.org/10.3390/nano9101395
Fominski V, Gnedovets A, Fominski D, Romanov R, Kartsev P, Rubinkovskaya O, Novikov S. Pulsed Laser Deposition of Nanostructured MoS3/np-Mo//WO3−y Hybrid Catalyst for Enhanced (Photo) Electrochemical Hydrogen Evolution. Nanomaterials. 2019; 9(10):1395. https://doi.org/10.3390/nano9101395
Chicago/Turabian StyleFominski, Vyacheslav, Alexey Gnedovets, Dmitry Fominski, Roman Romanov, Petr Kartsev, Oxana Rubinkovskaya, and Sergey Novikov. 2019. "Pulsed Laser Deposition of Nanostructured MoS3/np-Mo//WO3−y Hybrid Catalyst for Enhanced (Photo) Electrochemical Hydrogen Evolution" Nanomaterials 9, no. 10: 1395. https://doi.org/10.3390/nano9101395
APA StyleFominski, V., Gnedovets, A., Fominski, D., Romanov, R., Kartsev, P., Rubinkovskaya, O., & Novikov, S. (2019). Pulsed Laser Deposition of Nanostructured MoS3/np-Mo//WO3−y Hybrid Catalyst for Enhanced (Photo) Electrochemical Hydrogen Evolution. Nanomaterials, 9(10), 1395. https://doi.org/10.3390/nano9101395