Thermal-Recoverable Tough Hydrogels Enhanced by Porphyrin Decorated Graphene Oxide
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Graphene Oxide (GO) Preparation
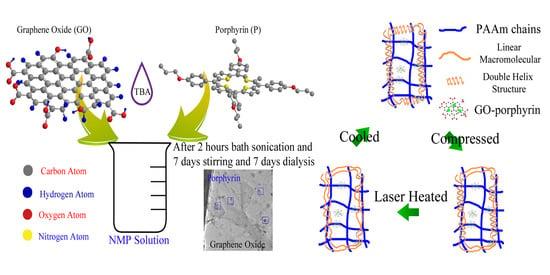
2.3. Porphyrin Decorated Graphene Oxide (PGO) Preparation
2.4. Hydrogels Fabrication
2.5. Mechanical Recovery of Hydrogels
2.6. Materials Characterization
2.7. Compressive Measurement
2.8. Swelling Measurement
3. Results and Discussions
3.1. Porphyrin-Graphene Oxide (PGO)
3.2. PGO Reinforced Hydrogel (PDN)
3.3. Thermal Recovery
3.4. Photothermal Recovery
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Park, H.; Kang, S.W.; Kim, B.S.; Mooney, D.J.; Lee, K.Y. Shear-reversibly Crosslinked Alginate Hydrogels for Tissue Engineering. Macromol. Biosci. 2009, 9, 895–901. [Google Scholar] [CrossRef] [PubMed]
- Guilak, F.; Butler, D.L.; Goldstein, S.A.; Baaijens, F. Biomechanics and mechanobiology in functional tissue engineering. J. Biomech. 2014, 47, 1933–1940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, I.L.; Mauck, R.L.; Burdick, J.A. Hydrogel design for cartilage tissue engineering: A case study with hyaluronic acid. Biomaterials 2011, 32, 8771–8782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Del. Rev. 2001, 53, 321–339. [Google Scholar] [CrossRef]
- Wang, Q.; Cai, J.; Zhang, L.; Xu, M.; Cheng, H.; Han, C.C.; Kuga, S.; Xiao, J.; Xiao, R. A bioplastic with high strength constructed from a cellulose hydrogel by changing the aggregated structure. J. Mater. Chem. A 2013, 1, 6678–6686. [Google Scholar] [CrossRef]
- Kuang, J.; Yuk, K.Y.; Huh, K.M. Polysaccharide-based superporous hydrogels with fast swelling and superabsorbent properties. Carbohydr. Polym. 2011, 83, 284–290. [Google Scholar] [CrossRef]
- Kabiri, K.; Zohuriaan-Mehr, M. Superabsorbent hydrogel composites. Polym. Adv. Technol. 2003, 14, 438–444. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, L.; Zhao, C.; Wang, Q.; Zheng, J. A Robust, One-Pot Synthesis of Highly Mechanical and Recoverable Double Network Hydrogels Using Thermoreversible Sol-Gel Polysaccharide. Adv. Mater. 2013, 25, 4171–4176. [Google Scholar] [CrossRef]
- Altunbas, A.; Lee, S.J.; Rajasekaran, S.A.; Schneider, J.P.; Pochan, D.J. Encapsulation of curcumin in self-assembling peptide hydrogels as injectable drug delivery vehicles. Biomaterials 2011, 32, 5906–5914. [Google Scholar] [CrossRef] [Green Version]
- Martin, J.E.; Patil, A.J.; Butler, M.F.; Mann, S. Guest-Molecule-Directed Assembly of Mesostructured Nanocomposite Polymer/Organoclay Hydrogels. Adv. Funct. Mater. 2011, 21, 674–681. [Google Scholar] [CrossRef]
- Lee, A.L.; Ng, V.W.; Gao, S.; Hedrick, J.L.; Yang, Y.Y. Injectable Hydrogels from Triblock Copolymers of Vitamin E-Functionalized Polycarbonate and Poly (ethylene glycol) for Subcutaneous Delivery of Antibodies for Cancer Therapy. Adv. Funct. Mater. 2014, 24, 1538–1550. [Google Scholar] [CrossRef]
- Tuncaboylu, D.C.; Sari, M.; Oppermann, W.; Okay, O. Tough and self-healing hydrogels formed via hydrophobic interactions. Macromolecules 2011, 44, 4997–5005. [Google Scholar] [CrossRef]
- Kamata, H.; Akagi, Y.; Kayasuga-Kariya, Y.; Chung, U.; Sakai, T. “Nonswellable” Hydrogel Without Mechanical Hysteresis. Science 2014, 343, 873–875. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Jiao, K.; Yang, J.; He, C.; Wang, H. Mechanically strong and thermosensitive macromolecular microsphere composite poly (N-isopropylacrylamide) hydrogels. Polymer 2013, 54, 1596–1602. [Google Scholar]
- Wei, J.H.; Wang, J.L.; Su, S.H.; Wang, S.R.; Qiu, J.J.; Zhang, Z.H.; Christopher, G.; Ning, F.D.; Cong, W.L. 3D printing of an extremely tough hydrogel. RSC Adv. 2015, 5, 81324–81329. [Google Scholar] [CrossRef]
- Wei, J.H.; Wang, J.L.; Su, S.H.; Hasan, M.; Qiu, J.J.; Wang, S.R. A shape healable tough hydrogel. New J. Chem. 2015, 39, 8461–8466. [Google Scholar] [CrossRef]
- Wei, J.H.; Su, S.H.; Wang, J.L.; Qiu, J.J. Imitation proteoglycans improve toughness of double network hydrogels. Mater. Chem. Phys. 2015, 166, 66–72. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.L.; Su, S.H.; Qiu, J.J. Biocompatible swelling graphene oxide reinforced double network hydrogels with high toughness and stiffness. New J. Chem. 2017, 41, 3781–3789. [Google Scholar] [CrossRef]
- Wang, J.L.; Wei, J.H.; Su, S.H.; Qiu, J.J.; Wang, S.R. Ion-linked double-network hydrogel with high toughness and stiffness. J. Mater. Sci. 2015, 50, 5458–5465. [Google Scholar] [CrossRef]
- Zhang, E.; Wang, T.; Zhao, L.; Sun, W.; Liu, X.; Tong, Z. Fast self-healing of graphene oxide-hectorite clay-poly (N, N-dimethylacrylamide) hybrid hydrogels realized by near-infrared irradiation. ACS Appl. Mater. Interfaces 2014, 6, 22855–22861. [Google Scholar] [CrossRef]
- Popa, E.G.; Gomes, M.E.; Reis, R.L. Cell delivery systems using alginate–carrageenan hydrogel beads and fibers for regenerative medicine applications. Biomacromolecules 2011, 12, 3952–3961. [Google Scholar] [CrossRef] [PubMed]
- Daniel-da-Silva, A.L.; Moreira, J.; Neto, R.; Estrada, A.C.; Gil, A.M.; Trindade, T. Impact of magnetic nanofillers in the swelling and release properties of κ-carrageenan hydrogel nanocomposites. Carbohydr. Polym. 2012, 87, 328–335. [Google Scholar] [CrossRef]
- Popa, E.G.; Caridade, S.G.; Mano, J.F.; Reis, R.L.; Gomes, M.E. Chondrogenic potential of injectable κ-carrageenan hydrogel with encapsulated adipose stem cells for cartilage tissue-engineering applications. J. Tissue Eng. Regen. Med. 2013, 9, 550–563. [Google Scholar] [CrossRef] [PubMed]
- Snoeren, T.H.; Payens, T. On the sol-gel transition in solutions of kappa-carrageenan. BBA-Gen. Subj. 1976, 437, 264–272. [Google Scholar] [CrossRef]
- Grasdalen, H.; Smidsroed, O. Cesium-133 NMR in the sol-gel states of aqueous carrageenan.Selective site binding of cesium and potassium ions in. kappa.-carrageenan gels. Macromolecules 1981, 14, 229–231. [Google Scholar]
- Zhu, S.; Zhang, J.; Qiao, C.; Tang, S.; Li, Y.; Yuan, W.; Li, B.; Tian, L.; Liu, F.; Hu, R. Strongly green-photoluminescent graphene quantum dots for bioimaging applications. Chem. Commun. 2011, 47, 6858–6860. [Google Scholar] [CrossRef]
- Wang, J.L.; Wei, J.H.; Su, S.H.; Qiu, J.J. Novel fluorescence resonance energy transfer optical sensors for vitamin B-12 detection using thermally reduced carbon dots. New J. Chem. 2015, 39, 501–507. [Google Scholar] [CrossRef]
- Wang, J.L.; Su, S.H.; Wei, J.H.; Bahgi, R.; Hope-Weeks, L.; Qiu, J.J.; Wang, S.R. Ratio-metric sensor to detect riboflavin via fluorescence resonance energy transfer with ultrahigh sensitivity. Phys. E Low Dimens. Syst. Nanostruct. 2015, 72, 17–24. [Google Scholar] [CrossRef]
- Wang, J.; Qiu, J. A review of carbon dots in biological applications. J. Mater. Sci. 2016, 51, 4728–4738. [Google Scholar] [CrossRef]
- Wang, J.L.; Qiu, J.J. Luminescent Graphene Quantum Dots: As Emerging Fluorescent Materials for Biological Application. Sci. Adv. Mater. 2015, 7, 1979–1989. [Google Scholar] [CrossRef]
- Wei, J.; Qiu, J. Allyl-Functionalization enhanced thermally stable graphene/fluoroelastomer nanocomposites. Polymer 2014, 55, 3818–3824. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Z.; Xu, C.; Li, Y.; Gao, J.; Wang, W.; Liu, Y. High strength graphene oxide/polyvinyl alcohol composite hydrogels. J. Mater. Chem. 2011, 21, 10399–10406. [Google Scholar] [CrossRef]
- Huang, C.; Bai, H.; Li, C.; Shi, G. A graphene oxide/hemoglobin composite hydrogel for enzymatic catalysis in organic solvents. Chem. Commun. 2011, 47, 4962–4964. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Doshi, H.; Srinivasarao, M.; Park, J.O.; Schiraldi, D.A. Fibers from polypropylene/nano carbon fiber composites. Polymer 2002, 43, 1701–1703. [Google Scholar] [CrossRef]
- Wang, K.; Ruan, J.; Song, H.; Zhang, J.; Wo, Y.; Guo, S.; Cui, D. Biocompatibility of graphene oxide. Nanoscale Res. Lett. 2011, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, D.; Zeng, C.; Miao, Z.; Dai, L. Biocompatible graphene oxide-based glucose biosensors. Langmuir 2010, 26, 6158–6160. [Google Scholar] [CrossRef]
- Bai, H.; Li, C.; Wang, X.; Shi, G. A pH-sensitive graphene oxide composite hydrogel. Chem. Commun. 2010, 46, 2376–2378. [Google Scholar] [CrossRef]
- Liu, R.; Liang, S.; Tang, X.-Z.; Yan, D.; Li, X.; Yu, Z.-Z. Tough and highly stretchable graphene oxide/polyacrylamide nanocomposite hydrogels. J. Mater. Chem. 2012, 22, 14160–14167. [Google Scholar] [CrossRef]
- Wang, J.; Qiu, J. A review of organic nanomaterials in photothermal cancer therapy. Cancer Res. Front. 2016, 2, 67–84. [Google Scholar] [CrossRef]
- Su, S.H.; Wang, J.L.; Wei, J.H.; Martinez-Zaguilan, R.; Qiu, J.J.; Wang, S.R. Efficient photothermal therapy of brain cancer through porphyrin functionalized graphene oxide. New J. Chem. 2015, 39, 5743–5749. [Google Scholar] [CrossRef]
- Geng, J.; Kong, B.S.; Yang, S.B.; Jung, H.T. Preparation of graphene relying on porphyrin exfoliation of graphite. Chem. Commun. 2010, 46, 5091–5093. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Chen, W.F.; Yan, L.F. An inorganic-organic double network hydrogel of graphene and polymer. Nanoscale 2013, 5, 6034–6039. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.H.; Wang, J.L.; Su, S.H.; Wang, S.R.; Qiu, J.J. Tough and fully recoverable hydrogels. J. Mater. Chem. B 2015, 3, 5284–5290. [Google Scholar] [CrossRef]
- Du, G.L.; Gao, G.R.; Hou, R.X.; Cheng, Y.J.; Chen, T.; Fu, J.; Fei, B. Tough and Fatigue Resistant Biomimetic Hydrogels of Interlaced Self-Assembled Conjugated Polymer Belts with a Polyelectrolyte Network. Chem. Mater. 2014, 26, 3522–3529. [Google Scholar] [CrossRef]










© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Wei, J.; Su, S.; Qiu, J.; Hu, Z.; Hasan, M.; Vargas, E.; Pantoya, M.; Wang, S. Thermal-Recoverable Tough Hydrogels Enhanced by Porphyrin Decorated Graphene Oxide. Nanomaterials 2019, 9, 1487. https://doi.org/10.3390/nano9101487
Wang J, Wei J, Su S, Qiu J, Hu Z, Hasan M, Vargas E, Pantoya M, Wang S. Thermal-Recoverable Tough Hydrogels Enhanced by Porphyrin Decorated Graphene Oxide. Nanomaterials. 2019; 9(10):1487. https://doi.org/10.3390/nano9101487
Chicago/Turabian StyleWang, Jilong, Junhua Wei, Siheng Su, Jingjing Qiu, Zhonglue Hu, Molla Hasan, Evan Vargas, Michelle Pantoya, and Shiren Wang. 2019. "Thermal-Recoverable Tough Hydrogels Enhanced by Porphyrin Decorated Graphene Oxide" Nanomaterials 9, no. 10: 1487. https://doi.org/10.3390/nano9101487
APA StyleWang, J., Wei, J., Su, S., Qiu, J., Hu, Z., Hasan, M., Vargas, E., Pantoya, M., & Wang, S. (2019). Thermal-Recoverable Tough Hydrogels Enhanced by Porphyrin Decorated Graphene Oxide. Nanomaterials, 9(10), 1487. https://doi.org/10.3390/nano9101487