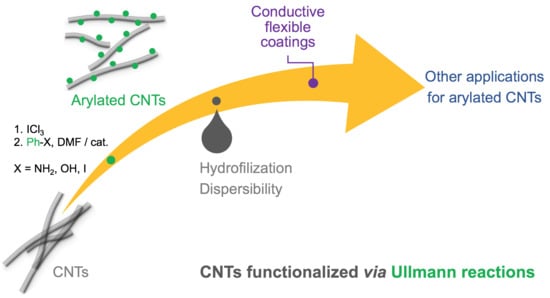
Ullmann Reactions of Carbon Nanotubes—Advantageous and Unexplored Functionalization toward Tunable Surface Chemistry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of Chlorinated CNTs (CNT-Cl)
2.2.2. Reaction of MWCNTs with Iodine Monochloride
2.2.3. Ullmann-Type Reactions of CNT-Cl
2.2.4. Preparation of MWCNT-Based Electroconductive Pastes
2.3. Characterization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schnorr, J.M.; Swager, T.M. Emerging Applications of Carbon Nanotubes. Chem. Mater. 2011, 23, 646–657. [Google Scholar] [CrossRef]
- Alshehri, R.; Ilyas, M.; Hasan, A.; Arnaout, A.; Ahmed, F.; Memic, A. Carbon Nanotubes in Biomedical Applications: Factors, Mechanisms, and Remedies of Toxicity. J. Med. Chem. 2016, 59, 8149–8167. [Google Scholar] [CrossRef] [PubMed]
- Zhbanov, A.I.; Pogorelov, E.G.; Chang, Y.C. Van der Waals Interaction between Two Crossed Carbon Nanotubes. ACS Nano 2010, 4, 5937–5945. [Google Scholar] [CrossRef] [PubMed]
- Treacy, M.M.J.; Ebbesen, T.W.; Gibson, J.M. Exceptionally high Young’s modulus observed for individual carbon nanotubes. Nature 1996, 381, 678–680. [Google Scholar] [CrossRef]
- Khandoker, N.; Hawkins, S.C.; Ibrahim, R.; Huynh, C.P.; Deng, F. Tensile Strength of Spinnable Multiwall Carbon Nanotubes. Procedia Eng. 2011, 10, 2572–2578. [Google Scholar] [CrossRef]
- Lekawa-Raus, A.; Patmore, J.; Kurzepa, L.; Bulmer, J.; Koziol, K. Electrical Properties of Carbon Nanotube Based Fibers and Their Future Use in Electrical Wiring. Adv. Funct. Mater. 2014, 24, 3661–3682. [Google Scholar] [CrossRef]
- Han, Z.; Fina, A. Thermal conductivity of carbon nanotubes and their polymer nanocomposites: A review. Prog. Polym. Sci. 2011, 36, 914–944. [Google Scholar] [CrossRef]
- Aberefa, O.A.; Daramola, M.O.; Lyuke, S.E. Production and functionalization of carbon nanotubes for application in membrane synthesis for natural gas separation. Microporous Mesoporous Mater. 2019, 280, 26–36. [Google Scholar] [CrossRef]
- Marchesan, S.; Kostarelos, K.; Bianco, A.; Prato, M. The winding road for carbon nanotubes in nanomedicine. Mater. Today 2015, 18, 12–19. [Google Scholar] [CrossRef]
- Anson-Casaos, A.; Gonzalez-Dominguez, J.M.; Terrado, E.; Martinez, M.T. Surfactant-free assembling of functionalized single-walled carbon nanotube buckypapers. Carbon 2010, 48, 1480–1488. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Xu, X.C. Nondestructive covalent functionalization of carbon nanotubes byselective oxidation of the original defects with K2FeO4. Appl. Surface Sci. 2015, 346, 520–527. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, G.; Liu, H.; Wu, J.; Qiu, Y.; Gu, B.L.; Duan, W. Chemical Functionalization of Carbon Nanotubes by Carboxyl Groups on Stone-Wales Defects: A Density Functional Theory Study. J. Phys. Chem. B 2006, 110, 10266–10271. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, F.G.; Cotta, A.A.C.; Gorgulho, H.F.; Santos, A.P.; Macedo, W.A.A.; Furtado, C.A. Comparative temporal analysis of multiwalled carbon nanotube oxidation reactions: Evaluating chemical modifications on true nanotube surface. Appl. Surface Sci. 2015, 357, 1015–1023. [Google Scholar] [CrossRef]
- Simmons, J.M.; Nichols, B.M.; Baker, S.E.; Marcus, M.S.; Castellini, O.M.; Lee, C.S.; Hamers, R.J.; Eriksson, M.A. Effect of Ozone Oxidation on Single-Walled Carbon Nanotubes. J. Phys. Chem. B 2006, 110, 7113–7118. [Google Scholar] [CrossRef] [PubMed]
- Tchoul, M.N.; Ford, W.T.; Lolli, G.; Resesco, D.E.; Arepalli, S. Effect of Mild Nitric Acid Oxidation on Dispersability, Size, and Structure of Single-Walled Carbon Nanotubes. Chem. Mater. 2008, 19, 5765–5772. [Google Scholar] [CrossRef]
- Malikov, E.; Akperov, O.H.; Muradov, M.B.; Eyvazova, G.; Maharramov, A.M.; Kukovecz, A.; Konya, Z. Oxidation of multiwalled carbon nanotubes using different oxidation agents like nitric acid and potassium permanganate. News Baku Univ. 2014, 4, 49–59. [Google Scholar]
- Ovejero, G.; Sotelo, J.L.; Romero, M.D.; Rodriguez, A.; Ocana, M.A.; Rodriguez, G.; Garcia, J. Multiwalled Carbon Nanotubes for Liquid-Phase Oxidation. Functionalization, Characterization, and Catalytic Activity. Ind. Eng. Chem. Res. 2006, 45, 2206–2212. [Google Scholar] [CrossRef]
- Bortolamiol, T.; Lukanov, P.; Galibert, A.M.; Soula, B.; Lonchambon, P.; Datas, L.; Flahaut, E. Double-walled carbon nanotubes: Quantitative purification assessment, balance between purification and degradation and solution filling as an evidence of opening. Carbon 2014, 78, 79–90. [Google Scholar] [CrossRef]
- Ziegler, K.J.; Gu, Z.; Peng, H.; Flor, E.L.; Hauge, R.H.; Smalley, R.E. Controlled Oxidative Cutting of Single-Walled Carbon Nanotubes. J. Am. Chem. Soc. 2005, 127, 1541–1547. [Google Scholar] [CrossRef]
- Li, W.; Cheng, R.M.; Xu, X.C.; Chen, Y.; Sun, M.L. Effect of hydroxyl radical on the surface and structure of multi-walled carbon nanotubes. Chin. J. Inorg. Chem. 2005, 21, 186–190. [Google Scholar]
- Wepasnick, K.A.; Smith, B.A.; Schrote, K.E.; Wilson, H.K.; Diegelmann, S.R.; Fairbrother, D.H. Surface and structural characterization of multi-walled carbon nanotubes following different oxidative treatments. Carbon 2011, 49, 24–36. [Google Scholar] [CrossRef]
- Menard-Moyon, C.; Dumas, F.; Doris, E.; Mioskowski, C. Functionalization of Single-Wall Carbon Nanotubes by Tandem High-Pressure/Cr(CO)6 Activation of Diels−Alder Cycloaddition. J. Am. Chem. Soc. 2006, 128, 14764–14765. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, F.G.; Herrero, M.A.; De M Munoz, J.; Giordani, S.; Diaz-Ortiz, A.; Filippone, S.; Ruaro, G.; Meneghetti, M.; Prato, M.; Vazguez, E. Reversible Microwave-Assisted Cycloaddition of Aziridines to Carbon Nanotubes. J. Am. Chem. Soc. 2007, 129, 14580–14581. [Google Scholar] [CrossRef] [PubMed]
- Kolanowska, A.; Kuziel, A.; Li, Y.; Jurczyk, S.; Boncel, S. Rieche formylation of carbon nanotubes—One-step and versatile functionalization route. RSC Adv. 2017, 7, 51347–51381. [Google Scholar] [CrossRef]
- Janas, D.; Herman, A.P.; Boncel, S.; Koziol, K.K.K. Iodine monochloride as a powerful enhancer of electrical conductivity of carbon nanotube wire. Carbon 2014, 73, 225–233. [Google Scholar] [CrossRef]
- Janas, D.; Boncel, S.; Koziol, K.K.K. Electrothermal halogenation of carbon nanotube films. Carbon 2014, 73, 259–266. [Google Scholar] [CrossRef]
- Gershoni-Poranne, R.; Pappo, D.; Solel, E.; Keinan, E. Corannulene ethers via Ullmann condensation. Org. Lett. 2009, 11, 5146–5149. [Google Scholar] [CrossRef]
- Akhavan, M.; Hemmati, S.; Hekmati, M.; Veisi, H. CuCl heterogenized on metformine-modified multi walled carbon nanotubes as recyclable nanocatalyst for Ullmann-type C-O and C-N coupling reactions. N. J. Chem. 2018, 42, 2782–2789. [Google Scholar] [CrossRef]
- Veisi, H.; Metghalchi, Y.; Hekmati, M.; Samadzadeh, S. CuI heterogenized on thiosemicarbazide modified-multi walled carbon nanotubes (thiosemicarbazide-MWCNTs-CuI): Novel heterogeneous and reusable nanocatalyst in the C-N Ullmann coupling reactions. Appl. Organomet. Chem. 2017, 31, e3676. [Google Scholar] [CrossRef]
- Saberi, D.; Sheykhan, M.; Niknam, K.; Heydari, A. Preparation of carbon nanotube-supported α-Fe2O3@CuO nanocomposite: A highly efficient and magnetically separable catalyst in cross-coupling of aryl halides with phenols. Catal. Sci. Technol. 2013, 3, 2025–2031. [Google Scholar] [CrossRef]
- White, C.M.; Banks, R.; Hamerton, I.; Watts, J.F. Characterisation of commercially CVD grown multi-walled carbon nanotubes for paint applications. Progr. Organic Coat. 2016, 90, 44–53. [Google Scholar] [CrossRef]
- Armare, W.L.F.; Perrin, D.D. Purification of Laboratory Chemicals, 4th ed.; Butterworth-Heinemann: Oxford, UK, 1996; pp. 63–360. [Google Scholar]
- Abdelkader, V.K.; Domingo-Garcia, M.; Gutierrez-Valero, M.; Lopez-Garzon, R.; Melguizo, M.; Garcia-Gallarin, C.; Lopez-Garzon, J.; Perez-Mendoza, M.J. Sidewall Chlorination of Carbon Nanotubes by Iodine Trichloride. J. Phys. Chem. C 2014, 116, 2641–2649. [Google Scholar] [CrossRef]
- Terzyk, A.P.; Bryk, P.; Korczeniewski, E.; Kowalczyk, P.; Zawadzka, A.; Płóciennik, P.; Wiśniewski, M.; Wesołowski, R. Water Nanodroplet on a Hydrocarbon “Carpet”-the Mechanism of Water Contact Angle Stabilization by Airborne Contaminations on Graphene, Au, and PTFE Surfaces. Langmuir 2019, 35, 420–422. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, P.; Deditius, A.; Ela, W.P.; Wiśniewski, M.; Gauden, P.A.; Terzyk, A.P.; Furmaniak, S.; Włoch, J.; Kaneko, K.; Neimark, A.V. Super-Sieving Effect in Phenol Adsorption from Aqueous Solutions on Nanoporous Carbon Beads. Carbon 2018, 135, 12–20. [Google Scholar] [CrossRef]
- Pasternak, M.; Sonnino, T. Studies of IBr, ICl, and I2Cl6 Crystal Properties by Means of the Mössbauer Effect in 129I. I. Chemical Bonds. J. Chem. Phys. 1968, 48, 1997–2003. [Google Scholar] [CrossRef]
- Boswijk, K.H.; Wiebenga, E.H. The crystal structure of I2Cl6 (ICl3). Acta Crystallographica 1954, 7, 417–423. [Google Scholar] [CrossRef]
- Monnier, F.; Taillefer, M. Catalytic C-C, C-N, and C-O Ullmann-type coupling reactions. Angew. Chem. Int. Ed. 2009, 48, 6954–6971. [Google Scholar] [CrossRef]
- Panda, N.; Jena, A.K. Cu/Fe-Catalyzed Carbon-Carbon and Carbon-Heteroatom Cross-Coupling Reactions. Organic Chem. Curr. Res. 2015, 4, 1000130. [Google Scholar] [CrossRef] [Green Version]
- Sperotto, E.; Van Klink, G.P.M.; Van Koten, G.; De Vries, J.G. The Mechanism of the Modified Ullmann Reaction. Dalton Trans. 2010, 39, 10338–10351. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Q.; Debnath, S.; Gregan, E.; Byrne, H.J. Effect of Solvent Solubility Parameters on the Dispersion of Single-Walled Carbon Nanotubes. J. Phys. Chem. C 2008, 112, 20154–20158. [Google Scholar] [CrossRef]
- Bergin, S.D.; Sun, Z.; Rickard, D.; Streich, P.V.; Hamilton, J.P.; Coleman, J.N. Multicomponent Solubility Parameters for Single-Walled Carbon Nanotube−Solvent Mixtures. ACS Nano 2009, 3, 2340–2350. [Google Scholar] [CrossRef] [PubMed]
- Osuna, S.; Torrent-Sucarrat, M.; Sola, M.; Geerlings, P.; Ewels, C.P.; Van Lier, G. Reaction Mechanisms for Graphene and Carbon Nanotube Fluorination. J. Phys. Chem. C 2010, 114, 3340–3345. [Google Scholar] [CrossRef]
- Pełech, I.; Narkiewicz, U.; Moszyński, D.; Pełech, R. Simultaneous Purification and Functionalization of Carbon Nanotubes using Chlorination. J. Mater. Res. 2012, 27, 2368–2374. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Jorio, A.; Souza Filho, A.G.; Saito, R. Defect characterization in graphene and carbon nanotubes using Raman spectroscopy. Philos. Trans. R. Soc. A 2010, 368, 5355–5377. [Google Scholar] [CrossRef] [PubMed]
- Dresselhaus, M.S.; Dresselhaus, G.; Saito, R.; Jorio, A. Raman spectroscopy of carbon nanotubes. Phys. Rep. 2005, 409, 47–99. [Google Scholar] [CrossRef]
- Kolanowska, A.; Kuziel, A.W.; Herman, A.P.; Jędrysiak, R.G.; Giżewski, T.; Boncel, S. Electroconductive textile coatings from pastes based on individualized multi-wall carbon nanotubes - synergy of surfactant and nanotube aspect ratio. Progr. Org. Coat. 2019, 130, 260–269. [Google Scholar] [CrossRef]
- Qiao, S.; Xie, K.; Qi, J. Copper-Catalyzed Coupling of Thiourea with Aryl Iodides: The Direct Synthesis of Aryl Thiols. Chin. J. Chem. 2010, 28, 1441–1443. [Google Scholar] [CrossRef]
- Xu, H.-J.; Zhao, X.-Y.; Deng, J.; Fu, Y.; Feng, Y.-S. Efficient C–S cross coupling catalyzed by Cu2O. Tetrahedron Lett. 2009, 50, 434–437. [Google Scholar] [CrossRef]
- Prasad, D.J.C.; Naidu, A.B.; Sekar, G. An efficient intermolecular C(aryl)–S bond forming reaction catalyzed by BINAM–copper(II) complex. Tetrahedron Lett. 2009, 50, 1411–1415. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, H.; Sun, F.; Li, Y.; Fu, Z.; Jin, K. A highly efficient and widely functional-group-tolerant catalyst system for copper(I)-catalyzed S-arylation of thiols with aryl halides. Tetrahedron 2009, 65, 9737–9741. [Google Scholar] [CrossRef]
- Ramaswamy, G.K.; Mohanasundaram, T.; Velayutham, M.; Kuppuswamy, B.K. Ortho-carboxylate Effects in Copper-mediated Nucleophilic Substitution of Halothiophenecarboxylic Acids and Halobenzoic Acids with Sodium Bisulphite. J. Chin. Chem. Soc. 2011, 58, 884–892. [Google Scholar] [CrossRef]










| Characteristics | Nanocyl™ NC7000 | In-House MWCNTs (Aligned Array = ‘Carpet’/’Forest’) | TUBALL™ SWCNTs |
|---|---|---|---|
| Average outer diameter, nm | 9.5 | 60–70 | 1.6 |
| Average length, µm | 1.5 | 200 | >5 |
| Aspect ratio | 150 | 3000 | 3000 |
| Carbon purity, wt.% | 90 | 98 | 85 |
| Fe-base catalyst residue, wt.% | <1 | 5.4 | <1.5 |
| Modification | Functionalization Degree [mmol g−1 CNTs] | |||
|---|---|---|---|---|
| Nanocyl NC7000™ MWCNTs | In-House MWCNTs | TUBALL™ SWCNTs | ||
| Chlorination by ICl3 | 3.2 | 2.6 | 5.0 | |
| Halogenation by ICl | 0.6 | 0.4 | - | |
| O-Arylation | DMF | 2.5 | 1.1 | 3.5 |
| Toluene | 1.3 | 0.4 | - | |
| Acetonitrile | 1.2 | 0.4 | - | |
| DMSO | 0.9 | 0.3 | - | |
| C-Arylation | DMF | 2.6 | 1.3 | 2.6 |
| Toluene | 1.0 | 0.8 | - | |
| Acetonitrile | 1.2 | 0.6 | - | |
| DMSO | 0.9 | 0.9 | - | |
| N-Arylation | DMF | 2.7 | 1.5 | 2.7 |
| Toluene | 1.3 | 0.5 | - | |
| Acetonitrile | 1.3 | 0.5 | - | |
| DMSO | 1.0 | 0.5 | - | |
| Nanocyl NC7000™ MWCNTs | Element | Atomic Concentration [%] |
|---|---|---|
| Chlorinated with ICl3 | C | 95.2 ± 0.5 |
| Cl | 4.6 ± 0.4 | |
| I | 0.2 ± 0.05 | |
| Chlorinated with ICl | C | 93.9 ± 0.1 * |
| Cl | 3.1 ± 0.3 | |
| I | 1.6 ± 0.2 | |
| O-arylated | C | 84.3 ± 0.6 |
| O | 12.6 ± 0.5 | |
| Cl | 3.1 ± 0.4 | |
| N-arylated | C | 86.3 ± 0.2 |
| N | 12.5 ± 0.4 | |
| Cl | 1.2 ± 0.1 | |
| C-arylated | C | 99.5 ± 0.1 |
| Cl | 0.3 ± 0.1 | |
| I | 0.2 ± 0.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolanowska, A.; Kuziel, A.W.; Jędrysiak, R.G.; Krzywiecki, M.; Korczeniewski, E.; Wiśniewski, M.; Terzyk, A.P.; Boncel, S. Ullmann Reactions of Carbon Nanotubes—Advantageous and Unexplored Functionalization toward Tunable Surface Chemistry. Nanomaterials 2019, 9, 1619. https://doi.org/10.3390/nano9111619
Kolanowska A, Kuziel AW, Jędrysiak RG, Krzywiecki M, Korczeniewski E, Wiśniewski M, Terzyk AP, Boncel S. Ullmann Reactions of Carbon Nanotubes—Advantageous and Unexplored Functionalization toward Tunable Surface Chemistry. Nanomaterials. 2019; 9(11):1619. https://doi.org/10.3390/nano9111619
Chicago/Turabian StyleKolanowska, Anna, Anna Wioleta Kuziel, Rafał Grzegorz Jędrysiak, Maciej Krzywiecki, Emil Korczeniewski, Marek Wiśniewski, Artur Piotr Terzyk, and Sławomir Boncel. 2019. "Ullmann Reactions of Carbon Nanotubes—Advantageous and Unexplored Functionalization toward Tunable Surface Chemistry" Nanomaterials 9, no. 11: 1619. https://doi.org/10.3390/nano9111619
APA StyleKolanowska, A., Kuziel, A. W., Jędrysiak, R. G., Krzywiecki, M., Korczeniewski, E., Wiśniewski, M., Terzyk, A. P., & Boncel, S. (2019). Ullmann Reactions of Carbon Nanotubes—Advantageous and Unexplored Functionalization toward Tunable Surface Chemistry. Nanomaterials, 9(11), 1619. https://doi.org/10.3390/nano9111619