Effectiveness of Calcium Phosphate Desensitising Agents in Dental Hypersensitivity Over 24 Weeks of Clinical Evaluation
Abstract
:1. Introduction
2. Materials and Methods
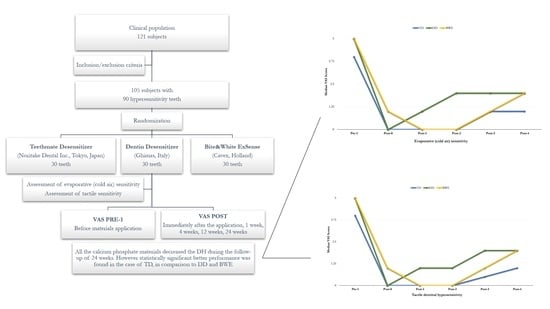
2.1. Study Design and Ethical Aspects
2.2. Participants
2.3. Clinical Procedure
2.4. Sample Size Estimation and Statistical Analysis
3. Results
3.1. Evaporative (Cold Air) Sensitivity
3.2. Tactile Dentinal Hypersensitivity
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brännström, M. Sensitivity of dentine. Oral Surg. Oral Med. Oral Pathol. 1966, 21, 517–526. [Google Scholar] [CrossRef]
- Brännström, M.; Linden, L.A.; Johnson, G. Movement of dentinal and pulpal fluid caused by clinical procedures. J. Dent. Res. 1968, 47, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Orchardson, R.; Gillam, D.G. Managing dentin hypersensitivity. J. Am. Dent. Assoc. 2006, 137, 990–998. [Google Scholar] [CrossRef]
- Splieth, C.H.; Tachou, A. Epidemiology of dentin hypersensitivity. Clin. Oral Investig. 2013, 17 (Suppl. S1), 3–8. [Google Scholar] [CrossRef] [Green Version]
- Browning, W.D.; Chan, D.C.N.; Frazier, K.B.; Callan, R.S.; Blalock, J.S. Safety and efficacy of a nightguard bleaching agent containing sodium fluoride and potassium nitrate. Quintessence Int. 2004, 35, 693–698. [Google Scholar]
- Wang, Y.; Gao, J.; Jiang, T.; Liang, S.; Zhou, Y.; Matis, B.A. Evaluation of the efficacy of potassium nitrate and sodium fluoride as desensitizing agents during tooth bleaching treatment—A systematic review and meta-analysis. J. Dent. 2015, 43, 913–923. [Google Scholar] [CrossRef]
- Bekes, K.; Hirsch, C. What is known about the influence of dentine hypersensitivity on oral health-related quality of life? Clin. Oral Investig. 2013, 17, S45–S51. [Google Scholar] [CrossRef] [Green Version]
- West, N.X.; Sanz, M.; Lussi, A.; Bartlett, D.; Bouchard, P.; Bourgeois, D. Prevalence of dentine hypersensitivity and study of associated factors: A European population- based cross-sectional study. J. Dent. 2013, 41, 841–851. [Google Scholar] [CrossRef]
- Pashley, D.H. Dentine permeability, dentine sensitivity and treatment through tubule occlusion. J. Endod. 1986, 12, 465–474. [Google Scholar] [CrossRef]
- Pashley, D.H. How can sensitive dentine become hypersensitive and can it be reversed. J. Dent. 2013, 41, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Duran, I.; Sengun, A. The long-term effectiveness of five current desensitizing products on cervical dentine sensitivity. J. Oral Rehabil. 2004, 31, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Pamir, T.; Özyazici, M.; Baloglu, E.; Önal, B. The efficacy of three desensitizing agents in treatment of dentine hypersensitivity. J. Clin. Pharm. Ther. 2005, 30, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Polderman, R.N.; Frencken, J.E. Comparison between effective- ness of a low-viscosity glass ionomer and a resin-based glutaraldehyde containing primer in treating dentine hypersensitivity: A 25.2-month evaluation. J. Dent. 2007, 35, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Kara, C.; Orbak, R. Comparative evaluation of Nd:YAG laser and fluoride varnish for the treatment of dentinal hypersensitivity. J. Endod. 2009, 35, 971–974. [Google Scholar] [CrossRef] [PubMed]
- Milia, E.; Castelli, G.; Bortone, A.; Sotgiu, G.; Manunta, A.; Pinna, R.; Gallina, G. Short-term response of three resin-based materials as desensitizing agents under oral environmental exposure. Acta Odontol. Scand. 2012, 71, 599–609. [Google Scholar] [CrossRef]
- Pinna, R.; Bortone, A.; Sotgiu, G.; Dore, S.; Usai, P.; Milia, E. Clinical evaluation of the efficacy of one self- adhesive composite in dental hypersensitivity. Clin. Oral Investig. 2015, 19, 1663–1672. [Google Scholar] [CrossRef]
- Cianconi, L.; Palopoli, P.; Campanella, V.; Mancini, M. Composition and microstructure of MTA and Aureoseal Plus: XRF, EDS, XRD and FESEM evaluation. Eur. J. Paediatr. Dent. 2016, 17, 281–285. [Google Scholar]
- Chieruzzi, M.; Pagano, S.; Moretti, S.; Pinna, R.; Milia, E.; Torre, L.; Eramo, S. Nanomaterials for tissue engineering in dentistry. Nanomaterials 2016, 6, 134. [Google Scholar] [CrossRef]
- Pinna, R.; Milia, E.; Usai, P.; Crivelli, P.; Pagano, S.; Sotgiu, G.; Schmalz, G. Efficiency of desensitizing materials in xerostomic patients with head and neck cancer: A comparative clinical study. Clin. Oral Investig. 2019. [Google Scholar] [CrossRef]
- Thanatvarakorn, O.; Nakashima, S.; Sadr, A.; Prasansuttiporn, T.; Ikeda, M.; Tagami, J. In vitro evaluation of dentinal hydraulic conductance and tubule sealing by a novel calcium-phosphate desensitizer. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 303–309. [Google Scholar] [CrossRef]
- Vano, M.; Derchi, G.; Barone, A.; Pinna, R.; Usai, P.; Covani, U. Reducing dentine hypersensitivity with nano- hydroxyapatite toothpaste: A double-blind randomized controlled trial. Clin. Oral Investig. 2017, 22, 303–320. [Google Scholar] [CrossRef] [PubMed]
- Hanning, M.; Hanning, C. Nanomaterials in preventive dentistry. Nat. Nanotechnol. 2010, 5, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.C. Next generation calcium phosphate-based biomaterials. Dent. Mater. J. 2009, 28, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Chiba, A.; Scheffel, D.L.; Hebling, J.; Agee, K.; Niu, L.N.; Tay, F.R.; Pashley, D.H. Effects of a Dicalcium and Tetracalcium Phosphate-Based Desensitizer on In Vitro Dentin Permeability. PLoS ONE 2016, 11, e0158400. [Google Scholar] [CrossRef] [Green Version]
- Takagi, S.; Chow, L.C.; Hirayama, S.; Sugawara, A. Premixed calcium phosphate cement pastes. J. Biomed. Mater. Res. Part B Biomater. 2003, 67, 689–696. [Google Scholar] [CrossRef]
- Mitchell, J.C.; Musanjel, L.; Ferracane, J.L. Biomimetic dentin desensitizer based on nano-structured bioactive glass. Dent. Mater. 2011, 27, 386–393. [Google Scholar] [CrossRef]
- Plagmann, H.C.; König, J.; Bernimoulin, J.P.; Rudhart, A.C.; Deschner, J. A clinical study comparing two high-fluoride dentifrices for the treatment of dentinal hypersensitivity. Quintessence Int. 1997, 28, 403–408. [Google Scholar]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMJ Med. 2010, 8, 18. [Google Scholar]
- Pinna, R.; Usai, P.; Filigheddu, E.; Garcia-Godoy, F.; Milia, E. The role of adhesive materials and oral biofilm in the failure of adhesive resin restorations. Am. J. Dent. 2017, 30, 285–292. [Google Scholar]
- Machado, A.C.; Rabelo, F.E.M.; Maximiano, V.; Lopes, R.M.; Aranha, A.C.C.; Scaramucci, T. Effect of in-office desensitizers containing calcium and phosphate on dentin permeability and tubule occlusion. J. Dent. 2019, 86, 53–59. [Google Scholar] [CrossRef]
- Hirayama, S.; Takagi, S.; Markovic, M.; Chow, L.C. Properties of Calcium Phosphate Cements With Different Tetracalcium Phosphate and Dicalcium Phosphate Anhydrous Molar Ratios. J. Res. Natl. Inst. Stand. Technol. 2008, 113, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Takagi, S.; Chow, L.C.; Suzuki, K. Reaction of calcium phosphate cements with different amounts of tetracalcium phosphate and dicalcium phosphate anhydrous. J. Biomed. Mater. Res. 1999, 46, 504–510. [Google Scholar] [CrossRef]
- Mason, S.; Hughes, N.; Sufi, F.; Bannon, L.; May, B.; North, M.; Holt, J. A comparative clinical study investigating the efficacy of a dentifrice containing 8% strontium acetate and 1040 ppm fluoride in a silica base and a control dentifrice containing 1450 ppm fluorine in silica base to provide immediate relief of dentine hyper- sensitivity. J. Clin. Dent. 2010, 21, 42–48. [Google Scholar] [PubMed]
- Huang, S.; Gao, S.; Yu, H. Remineralization potential of nano-hydroxyapatite on initial enamel lesions: An in vitro study. Caries Res. 2011, 45, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Najibfard, K.; Ramalingam, K.; Chedjieu, I.; Amaechi, B.T. Remineralization of early caries by a nano- hydroxyapatite dentice. J. Clin. Dent. 2011, 22, 139–143. [Google Scholar] [PubMed]
- Yuan, P.; Shen, X.; Liu, J.; Hou, Y.; Zhu, M.; Huang, J.; Xu, P. Effects of dentifrice containing hydroxyapatite on dentinal tubule occlusion and aqueous hexavalent chromium cations sorption: A preliminary study. PLoS ONE 2012, 7, e45283. [Google Scholar] [CrossRef] [PubMed]



| Material | Manufacturer | Main Components | Batch No. | Mode of Application |
|---|---|---|---|---|
| Teethmate™ Desensitizer (TD) | Kuraray Noritake Dental Inc., Tokyo, Japan | Powder: Tetra-calcium phosphate, dicalcium phosphate anhydrous. Liquid: Water, preservative | 041,118 | 1. Mix the powder with the liquid for 30 s, then rub the obtained slurry on the dried affected dentine for 30 s. Rinse the excess of the slurry with water spray or by having the patient rinse. |
| Dentin Desensitizer (DD) | Ghimas, Casalecchio di Reno, Bologna, Italy | A proprietary gel phase of n-HAP in an alcohol vehicle | 2015-001 | 1. After cleaning, apply the paste on the saliva-wetted dentine surface using a brush for 45 s. 2. Repeat the procedure for at least 3 times; 3. Rinse with water spray. |
| Bite&White ExSense (BWE) | Cavex Holland, Haarlem, Netherlands | A proprietary gel phase of nano-HAP in a water vehicle | 150,702 | 1. Dispense a small amount of the paste onto a clean finger. Gently apply the gel on all the surfaces of the tooth allowing to remain for 10 min. 2. Spit out any excess of the gel and rinse the mouth with water. |
| TD | DD | BWE | p-Value | |
|---|---|---|---|---|
| Pre-1, median (IQR) | 4 (2–6) | 5 (4–7) | 5 (2–6) | 0.01 1 |
| Post-0, median (IQR) | 0 (0–1) | 0 (0–2) | 1 (0–2) | 0.08 |
| Post-1, median (IQR) | 0 (0–0) | 1 (0–3) | 0 (0–0) | 0.0001 2 |
| Post-2, median (IQR) | 0 (0–2) | 2 (0–3) | 0 (0–0) | 0.0001 3 |
| Post-3, median (IQR) | 1 (0–2) | 2 (1–4) | 1 (0–3) | 0.0007 4 |
| Post-4, median (IQR) | 1 (0–2) | 2 (1–5) | 2 (2–4) | 0.0002 5 |
| p-value | <0.0001 | <0.0001 | <0.0001 |
| TD | DD | BWE | p-Value | |
|---|---|---|---|---|
| Pre-1, median (IQR) | 4 (2–6) | 5 (4–7) | 5 (2–6) | 0.01 1 |
| Post-0, median (IQR) | 0 (0–1) | 0 (0–2) | 1 (0–2) | 0.08 |
| Post-1, median (IQR) | 0 (0–0) | 1 (0–2) | 0 (0–0) | 0.0001 2 |
| Post-2, median (IQR) | 0 (0–2) | 1 (0–3) | 0 (0–0) | 0.0001 3 |
| Post-3, median (IQR) | 0.5 (0–2) | 2 (1–3) | 1 (0–2) | 0.0007 4 |
| Post-4, median (IQR) | 1 (0–2) | 2 (1–5) | 2 (1–3) | 0.0002 5 |
| p-value | <0.0001 | <0.0001 | <0.0001 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usai, P.; Campanella, V.; Sotgiu, G.; Spano, G.; Pinna, R.; Eramo, S.; Saderi, L.; Garcia-Godoy, F.; Derchi, G.; Mastandrea, G.; et al. Effectiveness of Calcium Phosphate Desensitising Agents in Dental Hypersensitivity Over 24 Weeks of Clinical Evaluation. Nanomaterials 2019, 9, 1748. https://doi.org/10.3390/nano9121748
Usai P, Campanella V, Sotgiu G, Spano G, Pinna R, Eramo S, Saderi L, Garcia-Godoy F, Derchi G, Mastandrea G, et al. Effectiveness of Calcium Phosphate Desensitising Agents in Dental Hypersensitivity Over 24 Weeks of Clinical Evaluation. Nanomaterials. 2019; 9(12):1748. https://doi.org/10.3390/nano9121748
Chicago/Turabian StyleUsai, Paolo, Vincenzo Campanella, Giovanni Sotgiu, Giovanni Spano, Roberto Pinna, Stefano Eramo, Laura Saderi, Franklin Garcia-Godoy, Giacomo Derchi, Giorgio Mastandrea, and et al. 2019. "Effectiveness of Calcium Phosphate Desensitising Agents in Dental Hypersensitivity Over 24 Weeks of Clinical Evaluation" Nanomaterials 9, no. 12: 1748. https://doi.org/10.3390/nano9121748
APA StyleUsai, P., Campanella, V., Sotgiu, G., Spano, G., Pinna, R., Eramo, S., Saderi, L., Garcia-Godoy, F., Derchi, G., Mastandrea, G., & Milia, E. (2019). Effectiveness of Calcium Phosphate Desensitising Agents in Dental Hypersensitivity Over 24 Weeks of Clinical Evaluation. Nanomaterials, 9(12), 1748. https://doi.org/10.3390/nano9121748