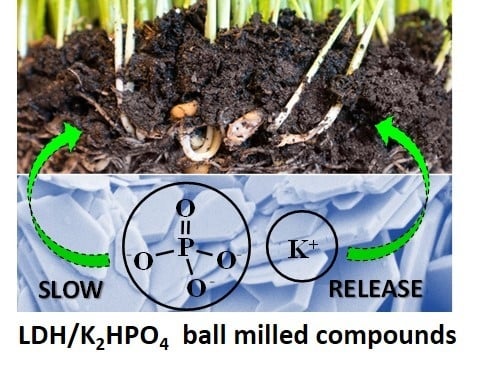
Potential Sustainable Slow-Release Fertilizers Obtained by Mechanochemical Activation of MgAl and MgFe Layered Double Hydroxides and K2HPO4
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Slow-Release Fertilizers
2.2. Mechanochemical Treatment
2.3. Release Experiments
2.4. Characterization Techniques
3. Results
3.1. Influence of LDH Thermal Treatment on Milled Compounds and Their Release Properties
3.2. Detailed Kinetic Study
3.3. Structural and Textural Characterizations of Residues upon Release
3.4. Characterization Results of Assays Involving the Incorporation of Carboxymethylcellulose (CMC)
3.5. Kinetics of Nutrients Release for MgAl200/K2HPO4 and MgFe200/K2HPO4 Formulated with CMC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lubkowski, K. Environmental impact of fertilizer use and slow release of mineral nutrients as a response to this challenge. Pol. J. Chem. Technol. 2016, 18, 72–79. [Google Scholar] [CrossRef] [Green Version]
- An, D.; Liu, B.; Yang, L.; Wang, T.-J.; Kan, C. Fabrication of graphene oxide/polymer latex composite film coated on KNO3 fertilizer to extend its release duration. Chem. Eng. J. 2017, 311, 318–325. [Google Scholar] [CrossRef]
- Naz, M.Y.; Sulaiman, S.A. Slow release coating remedy for nitrogen loss from conventional urea: A review. J. Control. Release 2016, 225, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Qiao, D.; Liu, H.; Yu, L.; Bao, X.; Simon, G.P.; Petinakis, E.; Chen, L. Preparation and Characterization of Slow-Release Fertilizer Encapsulated by Starch-Based Superabsorbent Polymer. Carbohydr. Polym. 2016, 147, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Boontung, W.; Moonmangmee, S.; Chaiyasat, A.; Chaiyasat, P. Preparation of Poly(l-lactic acid) Capsule Encapsulating Fertilizer. Adv. Mater. Res. 2012, 506, 303–306. [Google Scholar] [CrossRef]
- Tang, J.; Hong, J.; Liu, Y.; Wang, B.; Hua, Q.; Liu, L.; Ying, D. Urea Controlled-Release Fertilizer Based on Gelatin Microspheres. J. Polym. Environ. 2018, 26, 1930–1939. [Google Scholar] [CrossRef]
- Bernardo, M.P.; Guimarães, G.G.F.; Majaron, V.F.; Ribeiro, C. Controlled Release of Phosphate from Layered Double Hydroxide Structures: Dynamics in Soil and Application as Smart Fertilizer. ACS Sustain. Chem. Eng. 2018, 6, 5152–5161. [Google Scholar] [CrossRef]
- Woo, M.A.; Woo Kim, T.; Paek, M.-J.; Ha, H.-W.; Choy, J.-H.; Hwang, S.-J. Phosphate-intercalated Ca–Fe-layered double hydroxides: Crystal structure, bonding character, and release kinetics of phosphate. J. Solid State Chem. 2011, 184, 171–176. [Google Scholar] [CrossRef]
- Zhang, Q.; Tongamp, W.; Saito, F. Mechanochemical synthesis of kaolin–KH2PO4 and kaolin–NH4H2PO4 complexes for application as slow release fertilizer. Powder Technol. 2011, 212, 354–358. [Google Scholar] [CrossRef]
- Borges, R.; Brunatto, S.F.; Leitão, A.A.; de Carvalho, G.S.G.; Wypych, F. Solid-state mechanochemical activation of clay minerals and soluble phosphate mixtures to obtain slow-release fertilizers. Clay Miner. 2015, 50, 153–162. [Google Scholar] [CrossRef]
- Borges, R.; Dutra, L.M.; Barison, A.; Wypych, F. MAS NMR and EPR study of structural changes in talc and montmorillonite induced by grinding. Clay Miner. 2018, 51, 69–80. [Google Scholar] [CrossRef]
- Borges, R.; Prevot, V.; Forano, C.; Wypych, F. Design and Kinetic Study of Sustainable Potential Slow-Release Fertilizer Obtained by Mechanochemical Activation of Clay Minerals and Potassium Monohydrogen Phosphate. Ind. Eng. Chem. Res. 2017, 56, 708–716. [Google Scholar] [CrossRef]
- Borges, R.; Baika, L.M.; Grassi, M.T.; Wypych, F. Mechanochemical conversion of chrysotile/K2HPO4 mixtures into potential sustainable and environmentally friendly slow-release fertilizers. J. Environ. Manag. 2018, 206, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Forano, C.; Costantino, U.; Prévot, V.; Gueho, C.T. Layered Double Hydroxides (LDH). In Developments in Clay Science; Bergaya, F., Lagaly, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 5, pp. 745–782. [Google Scholar]
- Rives, V. Layered Double Hydroxides: Present and Future; Vicente, R., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2001; Volume 22. [Google Scholar]
- Qu, J.; Zhang, Q.; Li, X.; He, X.; Song, S. Mechanochemical approaches to synthesize layered double hydroxides: A review. Appl. Clay Sci. 2016, 119, 185–192. [Google Scholar] [CrossRef]
- Intasa-ard, S.; Imwiset, K.; Bureekaew, S.; Ogawa, M. Mechanochemical methods for the preparation of intercalation compounds, from intercalation to the formation of layered double hydroxides. Dalton Trans. 2018, 47, 2896–2916. [Google Scholar] [CrossRef] [PubMed]
- Forano, C. Environmental Remediation Involving Layered Double Hydroxides; Elsevier: Amsterdam, The Netherlands, 2004; Volume 1, pp. 425–458. [Google Scholar]
- Jaiswal, A.; Gautam, R.K.; Chattopadhyaya, M.C. Layered Double Hydroxides and the Environment: An Overview. In Advanced Materials for Agriculture, Food, and Environmental Safety; Wiley: Salem, VA, USA, 2014. [Google Scholar]
- Luengo, C.V.; Volpe, M.A.; Avena, M.J. High sorption of phosphate on Mg-Al layered double hydroxides: Kinetics and equilibrium. J. Environ. Chem. Eng. 2017, 5, 4656–4662. [Google Scholar] [CrossRef]
- Benício, L.P.F.; Constantino, V.R.L.; Pinto, F.G.; Vergütz, L.; Tronto, J.; da Costa, L.M. Layered Double Hydroxides: New Technology in Phosphate Fertilizers Based on Nanostructured Materials. ACS Sustain. Chem. Eng. 2017, 5, 399–409. [Google Scholar] [CrossRef]
- Everaert, M.; da Silva, R.C.; Degryse, F.; McLaughlin, M.J.; Smolders, E. Limited Dissolved Phosphorus Runoff Losses from Layered Double Hydroxide and Struvite Fertilizers in a Rainfall Simulation Study. J. Environ. Qual. 2018, 47, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Everaert, M.; Warrinnier, R.; Baken, S.; Gustafsson, J.-P.; De Vos, D.; Smolders, E. Phosphate-Exchanged Mg–Al Layered Double Hydroxides: A New Slow Release Phosphate Fertilizer. ACS Sustain. Chem. Eng. 2016, 4, 4280–4287. [Google Scholar] [CrossRef]
- Hatami, H.; Fotovat, A.; Halajnia, A. Comparison of adsorption and desorption of phosphate on synthesized Zn-Al LDH by two methods in a simulated soil solution. Appl. Clay Sci. 2018, 152, 333–341. [Google Scholar] [CrossRef]
- Donato, R.K.; Luza, L.; da Silva, R.F.; Moro, C.C.; Guzatto, R.; Samios, D.; Matějka, L.; Dimzoski, B.; Amico, S.C.; Schrekker, H.S. The role of oleate-functionalized layered double hydroxide in the melt compounding of polypropylene nanocomposites. Mater. Sci. Eng. C 2012, 32, 2396–2403. [Google Scholar] [CrossRef]
- Das, J.; Patra, B.S.; Baliarsingh, N.; Parida, K.M. Adsorption of phosphate by layered double hydroxides in aqueous solutions. Appl. Clay Sci. 2006, 32, 252–260. [Google Scholar] [CrossRef]
- Graeser, S.; Postl, W.; Bojar, H.-P.; Berlepsch, P.; Armbruster, T.; Raber, T.; Ettinger, K.; Walter, F. Struvite-(K), KMgPO4·6H2O, the potassium equivalent of struvite—A new mineral. Eur. J. Mineral. 2008, 20, 629–633. [Google Scholar] [CrossRef]
- Johnson, C.A.; Glasser, F.P. Hydrotalcite-like minerals (M2Al(OH)6(CO3)0.5.XH2O, where m = Mg, Zn, Co, Ni) in the environment: synthesis, characterization and thermodynamic stability. Clays Clay Miner. 2003, 51, 1–8. [Google Scholar] [CrossRef]
- Chen, M.; Li, Z.; Huang, P.; Li, X.; Qu, J.; Yuan, W.; Zhang, Q. Mechanochemical transformation of apatite to phosphoric slow-release fertilizer and soluble phosphate. Process Saf. Environ. Prot. 2018, 114, 91–96. [Google Scholar] [CrossRef]
- Vyalikh, A.; Massiot, D.; Scheler, U. Structural characterisation of aluminium layered double hydroxides by 27Al solid-state NMR. Solid State Nucl. Magn. Reson. 2009, 36, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Zhou, Y.; Yang, R.; Wang, M.; Shen, L.; Li, Y.; Xue, N.; Guo, X.; Ding, W.; Peng, L. Dehydration and Dehydroxylation of Layered Double Hydroxides: New Insights from Solid-State NMR and FT-IR Studies of Deuterated Samples. J. Phys. Chem. C 2015, 119, 12325–12334. [Google Scholar] [CrossRef]
- Pushparaj, S.S.C.; Forano, C.; Prevot, V.; Lipton, A.S.; Rees, G.J.; Hanna, J.V.; Nielsen, U.G. How the Method of Synthesis Governs the Local and Global Structure of Zinc Aluminum Layered Double Hydroxides. J. Phys. Chem. C 2015, 119, 27695–27707. [Google Scholar] [CrossRef]
- Tkáč, I.; Komadel, P.; Müller, D. Acid-Treated Montmorillonites—A Study by 29Si and 27Al MAS NMR. Clay Miner. 1994, 29, 11–19. [Google Scholar] [CrossRef]
- Li, M.; Mazzei, P.; Cozzolino, V.; Monda, H.; Hu, Z.; Piccolo, A. Optimized procedure for the determination of P species in soil by liquid-state 31P-NMR spectroscopy. Chem. Biol. Technol. Agric. 2015, 2, 7. [Google Scholar] [CrossRef]
- Bak, M.; Thomsen, J.K.; Jakobsen, H.J.; Petersen, S.E.; Petersen, T.E.; Nielsen, N.C. Solid-state 13C and 31P NMR analysis of urinary stones. J. Urol. 2000, 164, 856–863. [Google Scholar] [CrossRef]
- Halajnia, A.; Oustan, S.; Najafi, N.; Khataee, A.R.; Lakzian, A. Adsorption–desorption characteristics of nitrate, phosphate and sulfate on Mg–Al layered double hydroxide. Appl. Clay Sci. 2013, 80–81, 305–312. [Google Scholar] [CrossRef]
- Neupane, G.; Donahoe, R.J.; Arai, Y. Kinetics of competitive adsorption/desorption of arsenate and phosphate at the ferrihydrite–water interface. Chem. Geol. 2014, 368, 31–38. [Google Scholar] [CrossRef]
- Rout, P.R.; Bhunia, P.; Dash, R.R. Effective utilization of a sponge iron industry by-product for phosphate removal from aqueous solution: A statistical and kinetic modelling approach. J. Taiwan Inst. Chem. Eng. 2015, 46, 98–108. [Google Scholar] [CrossRef]
- Yoon, S.-Y.; Lee, C.-G.; Park, J.-A.; Kim, J.-H.; Kim, S.-B.; Lee, S.-H.; Choi, J.-W. Kinetic, equilibrium and thermodynamic studies for phosphate adsorption to magnetic iron oxide nanoparticles. Chem. Eng. J. 2014, 236, 341–347. [Google Scholar] [CrossRef]
- Hanhoun, M.; Montastruc, L.; Azzaro-Pantel, C.; Biscans, B.; Frèche, M.; Pibouleau, L. Temperature impact assessment on struvite solubility product: A thermodynamic modeling approach. Chem. Eng. J. 2011, 167, 50–58. [Google Scholar] [CrossRef] [Green Version]
- Babic-Ivancic, V.; Kontrec, J.; Kralj, D.; Brecevic, L. Precipitation diagrams of struvite and dissolution kinetics of different struvite morphologies. Croat. Chem. Acta 2002, 75, 89–106. [Google Scholar]
- Hemvichian, K.; Chanthawong, A.; Suwanmala, P. Synthesis and characterization of superabsorbent polymer prepared by radiation-induced graft copolymerization of acrylamide onto carboxymethyl cellulose for controlled release of agrochemicals. Radiat. Phys. Chem. 2014, 103, 167–171. [Google Scholar] [CrossRef]
- Kenawy, E.-R.; Azaam, M.M.; El-nshar, E.M. Preparation of carboxymethyl cellulose-g-poly (acrylamide)/montmorillonite superabsorbent composite as a slow-release urea fertilizer. Polym. Adv. Technol. 2018, 29, 2072–2079. [Google Scholar] [CrossRef]
- Olad, A.; Zebhi, H.; Salari, D.; Mirmohseni, A.; Reyhani Tabar, A. Slow-release NPK fertilizer encapsulated by carboxymethyl cellulose-based nanocomposite with the function of water retention in soil. Mater. Sci. Eng. C 2018, 90, 333–340. [Google Scholar] [CrossRef]
- Biswal, D.R.; Singh, R.P. Characterisation of carboxymethyl cellulose and polyacrylamide graft copolymer. Carbohydr. Polym. 2004, 57, 379–387. [Google Scholar] [CrossRef]











| System | Method | Time of Milling (h) | LDH/K2HPO4/CMC Weight Ratio |
|---|---|---|---|
| MgAl/K2HPO4/CMC | Method 1 | 3 | 1.00:1.65:0.53 |
| Method 2 | 3 | 1.00:1.65:0.53 | |
| Method 3 | 9 | 1.00:1.65:0.53 | |
| MgFe/K2HPO4/CMC | Method 1 | 3 | 1.00:0.82:0.40 |
| Method 2 | 3 | 1.00:0.82:0.40 | |
| Method 3 | 9 | 1.00:0.82:0.40 |
| Nutrient | Temperature (°C) | Kinetic Parameters | |||
|---|---|---|---|---|---|
| K | Pseudo-second order | ||||
| kII (min−1) | qe (mg g−1) | R2 | Exp. qe (mg g−1) | ||
| 10 | 0.070 | 1.32 | 0.997 | 1.31 | |
| 25 | 0.134 | 1.46 | 0.999 | 1.44 | |
| 50 | 0.519 | 1.43 | 0.999 | 1.44 | |
| P | 10 | 0.085 | 0.722 | 0.995 | 0.717 |
| 25 | 0.342 | 0.462 | 0.999 | 0.453 | |
| 50 | 0.481 | 0.738 | 0.999 | 0.733 | |
| Nutrient | Temperature (°C) | Kinetic Parameters | ||||||
|---|---|---|---|---|---|---|---|---|
| K | Pseudo-second order | |||||||
| kII (min−1) | qe (mg g−1) | R2 | Exp. qe (mg g−1) | |||||
| 10 | 0.31 | 0.447 | 0.998 | 0.443 | ||||
| 25 | 1.06 | 0.594 | 0.999 | 0.592 | ||||
| 50 | 0.631 | 0.542 | 0.999 | 0.541 | ||||
| P | Intraparticular diffusion | |||||||
| Kd (mg g−1 min−0.5) | Intercept | R2 | ||||||
| 10 | 0.001 | 0.002 | 0.993 | |||||
| 25 | 0.003 | 0.001 | 0.995 | |||||
| Pseudo-second order | ||||||||
| kII (min−1) | qe (mg g−1) | R2 | Exp. qe (mg g−1) | |||||
| 50 | 1.79 | 0.0826 | 0.997 | 0.0807 | ||||
| Release time | CMC/ MgAl200/K2HPO4 | CMC/MgFe200/K2HPO4 | |||
|---|---|---|---|---|---|
| P (%) | K (%) | P (%) | K (%) | ||
| 1 h | Method 1 | 6.6 | 16.3 | 2.4 | 12.9 |
| Method 2 | 8.0 | 23.0 | 6.2 | 25.4 | |
| Method 3 | 11.1 | 21.7 | 5.9 | 17.1 | |
| 168 h | Method 1 | 73.3 | 100.0 | 33.4 | 82.7 |
| Method 2 | 62.3 | 92.6 | 28.2 | 80.6 | |
| Method 3 | 69.5 | 92.4 | 32.0 | 76.4 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borges, R.; Wypych, F.; Petit, E.; Forano, C.; Prevot, V. Potential Sustainable Slow-Release Fertilizers Obtained by Mechanochemical Activation of MgAl and MgFe Layered Double Hydroxides and K2HPO4. Nanomaterials 2019, 9, 183. https://doi.org/10.3390/nano9020183
Borges R, Wypych F, Petit E, Forano C, Prevot V. Potential Sustainable Slow-Release Fertilizers Obtained by Mechanochemical Activation of MgAl and MgFe Layered Double Hydroxides and K2HPO4. Nanomaterials. 2019; 9(2):183. https://doi.org/10.3390/nano9020183
Chicago/Turabian StyleBorges, Roger, Fernando Wypych, Elodie Petit, Claude Forano, and Vanessa Prevot. 2019. "Potential Sustainable Slow-Release Fertilizers Obtained by Mechanochemical Activation of MgAl and MgFe Layered Double Hydroxides and K2HPO4" Nanomaterials 9, no. 2: 183. https://doi.org/10.3390/nano9020183
APA StyleBorges, R., Wypych, F., Petit, E., Forano, C., & Prevot, V. (2019). Potential Sustainable Slow-Release Fertilizers Obtained by Mechanochemical Activation of MgAl and MgFe Layered Double Hydroxides and K2HPO4. Nanomaterials, 9(2), 183. https://doi.org/10.3390/nano9020183