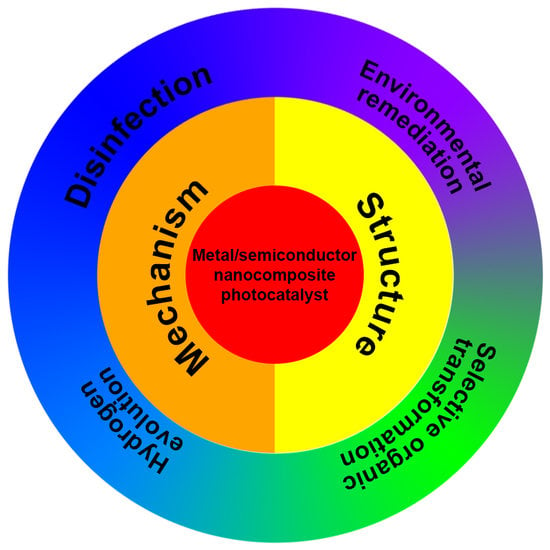
Metal/Semiconductor Nanocomposites for Photocatalysis: Fundamentals, Structures, Applications and Properties
Abstract
:1. Introduction
2. Fundamentals
2.1. Principles of Photocatalysis
- (1)
- If the redox potential of the substrate is lower than the CB edge of the semiconductor photocatalyst, then the substrate can undergo reductive reactions.
- (2)
- If the redox potential of the substrate is higher than the VB edge of the semiconductor photocatalyst, then the substrate can undergo oxidative reactions.
- (3)
- If the redox potential of the substrate is higher than the CB edge or lower than the VB of the semiconductor photocatalyst, then the substrate can undergo neither reductive nor oxidative reactions.
- (4)
- If the redox potential of the substrate is lower than the CB edge and higher than the VB of the semiconductor photocatalyst, then the substrate can undergo either reductive or oxidative reactions.
2.2. Mechanisms for the Enhanced Properties of Metal/Semiconductor (M/S) Nanocomposite Photocatalysts
2.2.1. Enhanced Charge Separation
2.2.2. Enhanced Visible Light Absorption
Surface Plasmon Resonance (SPR)-Induced Electron Injection
Charge Separation Induced by Near-Field Electric Field (NFEF)
Scattering-Enhanced Light Absorption
3. Structures of M/S Nanocomposite Photocatalysts
3.1. Conventional Structure
3.1.1. Photoreduction
3.1.2. Impregnation
3.1.3. Deposition-Precipitation
3.1.4. Chemical Vapor Deposition (CVD)
3.2. Core-Shell Structure
3.3. Yolk-Shell Structure
3.4. Janus Structure
3.5. Array Structure
3.6. Multi-Junction Structure
4. Applications and Properties of M/S Nanocomposite Photocatalysts
4.1. Environmental Remediation
4.2. Selective Organic Transformation
4.3. Hydrogen Evolution
4.4. Disinfection
5. Conclusions and Perspectives
- (1)
- To date, most of metals utilized to combine with semiconductor photocatalysts are noble metals, which are scarce in nature and expensive. To save the use of noble metals, coupling semiconductors with alloys composed of noble and non-noble metals is highly recommended. In addition, due to the non-linear relationship between the properties of M/S nanocomposite photocatalysts and metal loading, precise control over the metal loading in the M/S nanocomposite photocatalysts deserve further research.
- (2)
- For more efficient utilization of SPR to enhance the properties of M/S nanocomposite photocatalysts, the SPR excitation wavelength of the metal nanoparticles should overlap the absorption edge of the semiconductor nanoparticles, which could be achieved by changing the shape and particle size of the metal nanoparticles and modulating the band structure of the semiconductor nanoparticles.
- (3)
- Synergistic utilization of enhanced charge separation at the metal-semiconductor interface and SPR of metals might endow the M/S nanocomposite photocatalysts with even better properties under visible light illumination, because the SPR-induced charge separation in M/S nanocomposite photocatalysts could be further enhanced by introducing another metal co-catalyst with large work function to the M/S nanocomposite photocatalysts.
- (4)
- Due to their intrinsic ability to prohibit particle agglomeration, the core-shell, yolk-shell and array structures (especially the array structure) might be the ideal structures for M/S nanocomposite photocatalysts. Therefore, there exists a strong demand for more facile synthesis of these structures.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Sun, F.; Wang, H.; He, Y.; Li, L.; Huang, Z.; Wu, Q.; Yu, J.C. Photochemical growth of cadmium-rich CdS nanotubes at the air–water interface and their use in photocatalysis. J. Mater. Chem. 2009, 19, 6901–6906. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X. Photocatalytic CO2 reduction by CdS promoted with a zeolitic imidazolate framework. Appl. Catal. B Environ. 2015, 162, 494–500. [Google Scholar] [CrossRef]
- An, X.; Yu, X.; Yu, J.C.; Zhang, G. CdS nanorods/reduced graphene oxide nanocomposites for photocatalysis and electrochemical sensing. J. Mater. Chem. A 2013, 1, 5158–5164. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, R.; Lin, J.; Zhu, Y. Enhancement of photocurrent and photocatalytic activity of ZnO hybridized with graphite-like C3N4. Energy Environ. Sci. 2011, 4, 2922–2929. [Google Scholar] [CrossRef]
- McLaren, A.; Valdes-Solis, T.; Li, G.; Tsang, S.C. Shape and Size Effects of ZnO Nanocrystals on Photocatalytic Activity. J. Am. Chem. Soc. 2009, 131, 12540–12541. [Google Scholar] [CrossRef] [PubMed]
- Elmolla, E.S.; Chaudhuri, M. Degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution by the UV/ZnO photocatalytic process. J. Hazard. Mater. 2010, 173, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Miyauchi, M.; Takashio, M.; Tobimatsu, H. Photocatalytic Activity of SrTiO3 Codoped with Nitrogen and Lanthanum under Visible Light Illumination. Langmuir 2004, 20, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Konta, R.; Ishii, T.; Kato, H.; Kudo, A. Photocatalytic Activities of Noble Metal Ion Doped SrTiO3 under Visible Light Irradiation. J. Phys. Chem. B 2004, 108, 8992–8995. [Google Scholar] [CrossRef]
- Ye, S.; Wang, R.; Wu, M.-Z.; Yuan, Y.-P. A review on g-C3N4 for photocatalytic water splitting and CO2 reduction. Appl. Surf. Sci. 2015, 358, 15–27. [Google Scholar] [CrossRef]
- Lam, S.-M.; Sin, J.-C.; Mohamed, A.R. A review on photocatalytic application of g-C3N4/semiconductor (CNS) nanocomposites towards the erasure of dyeing wastewater. Mater. Sci. Semicond. Process. 2016, 47, 62–84. [Google Scholar] [CrossRef]
- Li, J.; Wu, N. Semiconductor-based photocatalysts and photoelectrochemical cells for solar fuel generation: A review. Catal. Sci. Technol. 2015, 5, 1360–1384. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Zhan, X.; Wang, F.; Safdar, M.; He, J. Visible light driven type II heterostructures and their enhanced photocatalysis properties: A review. Nanoscale 2013, 5, 8326–8339. [Google Scholar] [CrossRef] [PubMed]
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and Catalytic Applications of CeO2-Based Materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.M.; Cai, L.; Liu, C.; Cho, I.S.; Lee, C.H.; Weisse, J.M.; Yang, P.; Zheng, X. Simultaneously Efficient Light Absorption and Charge Separation in WO3/BiVO4 Core/Shell Nanowire Photoanode for Photoelectrochemical Water Oxidation. Nano Lett. 2014, 14, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef] [PubMed]
- Daghrir, R.; Drogui, P.; Robert, D. Modified TiO2 For Environmental Photocatalytic Applications: A Review. Ind. Eng. Chem. Res. 2013, 52, 3581–3599. [Google Scholar] [CrossRef]
- Dong, S.; Feng, J.; Fan, M.; Pi, Y.; Hu, L.; Han, X.; Liu, M.; Sun, J.; Sun, J. Recent developments in heterogeneous photocatalytic water treatment using visible light-responsive photocatalysts: A review. RSC Adv. 2015, 5, 14610–14630. [Google Scholar] [CrossRef]
- Burda, C.; Lou, Y.; Chen, X.; Samia, A.C.S.; Stout, J.; Gole, J.L. Enhanced Nitrogen Doping in TiO2 Nanoparticles. Nano Lett. 2003, 3, 1049–1051. [Google Scholar] [CrossRef]
- Yu, J.C.; Yu, J.; Ho, W.; Zhang, J. Effects of F- Doping on the Photocatalytic Activity and Microstructures of Nanocrystalline TiO2 Powders. Chem. Mater. 2002, 14, 3808–3816. [Google Scholar] [CrossRef]
- Liu, L.; Ouyang, S.; Ye, J. Gold-Nanorod-Photosensitized Titanium Dioxide with Wide-Range Visible-Light Harvesting Based on Localized Surface Plasmon Resonance. Angew. Chem. 2013, 125, 6821–6825. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, C.; Ma, W.; Zhao, J. Visible-Light-Induced Aerobic Oxidation of Alcohols in a Coupled Photocatalytic System of Dye-Sensitized TiO2 and TEMPO. Angew. Chem. Int. Ed. 2008, 47, 9730–9733. [Google Scholar] [CrossRef] [PubMed]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-Light Photocatalysis in Nitrogen-Doped Titanium Oxides. Science 2001, 293, 269. [Google Scholar] [CrossRef] [PubMed]
- Dhanalakshmi, K.B.; Latha, S.; Anandan, S.; Maruthamuthu, P. Dye sensitized hydrogen evolution from water. Int. J. Hydrogen Energy 2001, 26, 669–674. [Google Scholar] [CrossRef]
- Youngblood, W.J.; Lee, S.-H.A.; Maeda, K.; Mallouk, T.E. Visible Light Water Splitting Using Dye-Sensitized Oxide Semiconductors. Acc. Chem. Res. 2009, 42, 1966–1973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Peng, T.; Song, S. Recent advances in dye-sensitized semiconductor systems for photocatalytic hydrogen production. J. Mater. Chem. A 2016, 4, 2365–2402. [Google Scholar] [CrossRef]
- Khaki, M.R.D.; Shafeeyan, M.S.; Raman, A.A.A.; Daud, W.M.A.W. Application of doped photocatalysts for organic pollutant degradation—A review. J. Environ. Manag. 2017, 198, 78–94. [Google Scholar] [CrossRef] [PubMed]
- Wu, N. Plasmonic metal–semiconductor photocatalysts and photoelectrochemical cells: A review. Nanoscale 2018, 10, 2679–2696. [Google Scholar] [CrossRef] [PubMed]
- Linic, S.; Christopher, P.; Ingram, D.B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat. Mater. 2011, 10, 911. [Google Scholar] [CrossRef] [PubMed]
- Marschall, R. Semiconductor Composites: Strategies for Enhancing Charge Carrier Separation to Improve Photocatalytic Activity. Adv. Funct. Mater. 2014, 24, 2421–2440. [Google Scholar] [CrossRef]
- Qu, Y.; Duan, X. Progress, challenge and perspective of heterogeneous photocatalysts. Chem. Soc. Rev. 2013, 42, 2568–2580. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Bahnemann, D.; Ye, J.; Li, G. Photocatalysis: Fundamentals and Perspectives; Royal Society of Chemistry: London, UK, 2016. [Google Scholar] [CrossRef]
- Castellote, M.; Bengtsson, N. Principles of TiO2 Photocatalysis. In Applications of Titanium Dioxide Photocatalysis to Construction Materials: State-of-the-Art Report of the RILEM Technical Committee 194-TDP; Ohama, Y., Van Gemert, D., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 5–10. [Google Scholar] [CrossRef]
- Peter, L.M. CHAPTER 1 Photoelectrochemistry: From Basic Principles to Photocatalysis. In Photocatalysis: Fundamentals and Perspectives; The Royal Society of Chemistry: London, UK, 2016; pp. 1–28. [Google Scholar] [CrossRef]
- Mills, A.; Le Hunte, S. An overview of semiconductor photocatalysis. J. Photochem. Photobiol. A Chem. 1997, 108, 1–35. [Google Scholar] [CrossRef]
- Chen, S.; Wang, L.-W. Thermodynamic Oxidation and Reduction Potentials of Photocatalytic Semiconductors in Aqueous Solution. Chem. Mater. 2012, 24, 3659–3666. [Google Scholar] [CrossRef]
- Ohtani, B. Revisiting the fundamental physical chemistry in heterogeneous photocatalysis: Its thermodynamics and kinetics. Phys. Chem. Chem. Phys. 2014, 16, 1788–1797. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhao, X.; Terashima, C.; Fujishima, A.; Nakata, K. Thermodynamic and kinetic analysis of heterogeneous photocatalysis for semiconductor systems. Phys. Chem. Chem. Phys. 2014, 16, 8751–8760. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yates, J.T. Band Bending in Semiconductors: Chemical and Physical Consequences at Surfaces and Interfaces. Chem. Rev. 2012, 112, 5520–5551. [Google Scholar] [CrossRef] [PubMed]
- Kamat, P.V. Manipulation of Charge Transfer Across Semiconductor Interface. A Criterion That Cannot Be Ignored in Photocatalyst Design. J. Phys. Chem. Lett. 2012, 3, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.-Y.; Li, X.-H.; Zhang, Y.-N.; Wei, X.; Wang, K.-X.; Chen, J.-S. Highly Efficient Dehydrogenation of Formic Acid over a Palladium-Nanoparticle-Based Mott–Schottky Photocatalyst. Angew. Chem. 2013, 125, 12038–12041. [Google Scholar] [CrossRef]
- Liu, Y.; Gu, X.; Qi, W.; Zhu, H.; Shan, H.; Chen, W.L.; Tao, P.; Song, C.Y.; Shang, W.; Deng, T.; et al. Enhancing the Photocatalytic Hydrogen Evolution Performance of a Metal/Semiconductor Catalyst through Modulation of the Schottky Barrier Height by Controlling the Orientation of the Interface. ACS Appl. Mater. Interfaces 2017, 9, 12494–12500. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Wang, D.G.; Han, H.X.; Li, C. Roles of Cocatalysts in Photocatalysis and Photoelectrocatalysis. Accounts Chem. Res. 2013, 46, 1900–1909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubacka, A.; Fernández-García, M.; Colón, G. Advanced Nanoarchitectures for Solar Photocatalytic Applications. Chem. Rev. 2012, 112, 1555–1614. [Google Scholar] [CrossRef] [PubMed]
- Teoh, W.Y.; Scott, J.A.; Amal, R. Progress in Heterogeneous Photocatalysis: From Classical Radical Chemistry to Engineering Nanomaterials and Solar Reactors. J. Phys. Chem. Lett. 2012, 3, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Cronin, S.B. A Review of Surface Plasmon Resonance-Enhanced Photocatalysis. Adv. Funct. Mater. 2013, 23, 1612–1619. [Google Scholar] [CrossRef]
- Warren, S.C.; Thimsen, E. Plasmonic solar water splitting. Energy Environ. Sci. 2012, 5, 5133–5146. [Google Scholar] [CrossRef]
- Yu, J.; Qi, L.; Jaroniec, M. Hydrogen Production by Photocatalytic Water Splitting over Pt/TiO2 Nanosheets with Exposed (001) Facets. J. Phys. Chem. C 2010, 114, 13118–13125. [Google Scholar] [CrossRef]
- Lin, C.-H.; Chao, J.-H.; Liu, C.-H.; Chang, J.-C.; Wang, F.-C. Effect of Calcination Temperature on the Structure of a Pt/TiO2 (B) Nanofiber and Its Photocatalytic Activity in Generating H2. Langmuir 2008, 24, 9907–9915. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Chen, T.; Su, W.; Zhou, G.; Zong, X.; Lei, Z.; Li, C. H-2 production with ultra-low CO selectivity via photocatalytic reforming of methanol on Au/TiO2 catalyst. Int. J. Hydrogen Energy 2008, 33, 1243–1251. [Google Scholar] [CrossRef]
- Shi, H.; Wang, X.; Zheng, M.; Wu, X.; Chen, Y.; Yang, Z.; Zhang, G.; Duan, H. Hot-Electrons Mediated Efficient Visible-Light Photocatalysis of Hierarchical Black Au-TiO2 Nanorod Arrays on Flexible Substrate. Adv. Mater. Interfaces 2016, 3, 1600588. [Google Scholar] [CrossRef]
- Ghosh Chaudhuri, R.; Paria, S. Core/Shell Nanoparticles: Classes, Properties, Synthesis Mechanisms, Characterization, and Applications. Chem. Rev. 2012, 112, 2373–2433. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Liu, S.Q.; Xu, Y.J. Recent progress on metal core@semiconductor shell nanocomposites as a promising type of photocatalyst. Nanoscale 2012, 4, 2227–2238. [Google Scholar] [CrossRef] [PubMed]
- Sudeep, P.K.; Takechi, K.; Kamat, P.V. Harvesting photons in the infrared. Electron injection from excited tricarbocyanine dye (IR-125) into TiO2 and Ag@TiO2 core-shell nanoparticles. J. Phys. Chem. C 2007, 111, 488–494. [Google Scholar] [CrossRef]
- Kamata, K.; Lu, Y.; Xia, Y.N. Synthesis and characterization of monodispersed core-shell spherical colloids with movable cores. J. Am. Chem. Soc. 2003, 125, 2384–2385. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qiao, S.Z.; Chen, J.S.; Lou, X.W.; Xing, X.R.; Lu, G.Q. Yolk/shell nanoparticles: New platforms for nanoreactors, drug delivery and lithium-ion batteries. Chem. Commun. 2011, 47, 12578–12591. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Zhang, P.; Chang, X.; Cai, W.; Wang, T.; Gong, J. Gold Nanorod@TiO2 Yolk-Shell Nanostructures for Visible-Light-Driven Photocatalytic Oxidation of Benzyl Alcohol. Small 2015, 11, 1892–1899. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Zhou, Y.; Li, H.; Li, P.; Zou, Z. Au@TiO2 yolk-shell hollow spheres for plasmon-induced photocatalytic reduction of CO2 to solar fuel via a local electromagnetic field. Nanoscale 2015, 7, 14232–14236. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Han, J.; Xiong, H.; Guo, R. Yolk@Shell Nanoarchitecture of Au@r-GO/TiO2 Hybrids as Powerful Visible Light Photocatalysts. Langmuir 2015, 31, 6220–6228. [Google Scholar] [CrossRef] [PubMed]
- de Gennes, P.-G. Soft Matter (Nobel Lecture). Angew. Chem. Int. Ed. 1992, 31, 842–845. [Google Scholar] [CrossRef]
- Lattuada, M.; Hatton, T.A. Synthesis, properties and applications of Janus nanoparticles. Nano Today 2011, 6, 286–308. [Google Scholar] [CrossRef]
- Walther, A.; Müller, A.H.E. Janus Particles: Synthesis, Self-Assembly, Physical Properties, and Applications. Chem. Rev. 2013, 113, 5194–5261. [Google Scholar] [CrossRef] [PubMed]
- Seh, Z.W.; Liu, S.; Low, M.; Zhang, S.-Y.; Liu, Z.; Mlayah, A.; Han, M.-Y. Janus Au-TiO2 Photocatalysts with Strong Localization of Plasmonic Near-Fields for Efficient Visible-Light Hydrogen Generation. Adv. Mater. 2012, 24, 2310–2314. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L.; Song, J. Piezoelectric Nanogenerators Based on Zinc Oxide Nanowire Arrays. Science 2006, 312, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Neale, N.R.; Miedaner, A.; Frank, A.J. Enhanced Charge-Collection Efficiencies and Light Scattering in Dye-Sensitized Solar Cells Using Oriented TiO2 Nanotubes Arrays. Nano Lett. 2007, 7, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-W.; Lu, Y.-J.; Chen, H.-Y.; Lee, H.-M.; Gwo, S. InGaN/GaN nanorod array white light-emitting diode. Appl. Phys. Lett. 2010, 97, 073101. [Google Scholar]
- Wang, X.; Liow, C.; Qi, D.; Zhu, B.; Leow, W.R.; Wang, H.; Xue, C.; Chen, X.; Li, S. Programmable Photo-Electrochemical Hydrogen Evolution Based on Multi-Segmented CdS-Au Nanorod Arrays. Adv. Mater. 2014, 26, 3506–3512. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yu, J.G.; Jaroniec, M. All-Solid-State Z-Scheme Photocatalytic Systems. Adv. Mater. 2014, 26, 4920–4935. [Google Scholar] [CrossRef] [PubMed]
- Low, J.; Jiang, C.; Cheng, B.; Wageh, S.; Al-Ghamdi, A.A.; Yu, J. A Review of Direct Z-Scheme Photocatalysts. Small Methods 2017, 1, 1700080. [Google Scholar] [CrossRef]
- Qi, K.; Cheng, B.; Yu, J.; Ho, W. A review on TiO2-based Z-scheme photocatalysts. Chin. J. Catal. 2017, 38, 1936–1955. [Google Scholar] [CrossRef]
- Tada, H.; Mitsui, T.; Kiyonaga, T.; Akita, T.; Tanaka, K. All-solid-state Z-scheme in CdS–Au–TiO2 three-component nanojunction system. Nat. Mater. 2006, 5, 782. [Google Scholar] [CrossRef] [PubMed]
- McCullagh, C.; Skillen, N.; Adams, M.; Robertson, P.K.J. Photocatalytic reactors for environmental remediation: A review. J. Chem. Technol. Biotechnol. 2011, 86, 1002–1017. [Google Scholar] [CrossRef]
- Khin, M.M.; Nair, A.S.; Babu, V.J.; Murugan, R.; Ramakrishna, S. A review on nanomaterials for environmental remediation. Energy Environ. Sci. 2012, 5, 8075–8109. [Google Scholar] [CrossRef]
- Singh, S.; Mahalingam, H.; Singh, P.K. Polymer-supported titanium dioxide photocatalysts for environmental remediation: A review. Appl. Catal. A Gen. 2013, 462–463, 178–195. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Tahir, M.N.; Natalio, F.; Cambaz, M.A.; Panthöfer, M.; Branscheid, R.; Kolb, U.; Tremel, W. Controlled synthesis of linear and branched Au@ZnO hybrid nanocrystals and their photocatalytic properties. Nanoscale 2013, 5, 9944–9949. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zeng, D.; Zhang, K.; Lu, A.; Wang, L.; Peng, D.-L. Au–ZnO hybrid nanoflowers, nanomultipods and nanopyramids: One-pot reaction synthesis and photocatalytic properties. Nanoscale 2014, 6, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Yang, B.; Wu, M.; Xu, J.; Fu, Z.; Lv, Y.; Guo, T.; Zhao, Y.; Zhu, C. Synthesis of Ag/ZnO nanorods array with enhanced photocatalytic performance. J. Hazard. Mater. 2010, 182, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Wu, H.; Zhang, R.; Pan, W. Enhanced Photocatalysis of Electrospun Ag−ZnO Heterostructured Nanofibers. Chem. Mater. 2009, 21, 3479–3484. [Google Scholar] [CrossRef]
- Georgekutty, R.; Seery, M.K.; Pillai, S.C. A highly efficient Ag-ZnO photocatalyst: Synthesis, properties, and mechanism. J. Phys. Chem. C 2008, 112, 13563–13570. [Google Scholar] [CrossRef]
- Kochuveedu, S.T.; Kim, D.-P.; Kim, D.H. Surface-Plasmon-Induced Visible Light Photocatalytic Activity of TiO2 Nanospheres Decorated by Au Nanoparticles with Controlled Configuration. J. Phys. Chem. C 2012, 116, 2500–2506. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, S.; Gao, W. Ag/ZnO heterostructures and their photocatalytic activity under visible light: Effect of reducing medium. J. Hazard. Mater. 2015, 287, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, V.; Wolf, E.; Kamat, P.V. Semiconductor−Metal Composite Nanostructures. To What Extent Do Metal Nanoparticles Improve the Photocatalytic Activity of TiO2 Films? J. Phys. Chem. B 2001, 105, 11439–11446. [Google Scholar] [CrossRef]
- Jiang, Z.; Wei, W.; Mao, D.; Chen, C.; Shi, Y.; Lv, X.; Xie, J. Silver-loaded nitrogen-doped yolk-shell mesoporous TiO2 hollow microspheres with enhanced visible light photocatalytic activity. Nanoscale 2015, 7, 784–797. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Tu, T.; Wen, M.; Wu, Q. Assembly synthesis of Cu2O-on-Cu nanowires with visible-light-enhanced photocatalytic activity. Dalton Trans. 2015, 44, 15645–15652. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, Y.; Xiong, T.; Jiang, G.; Zhang, Y.; Wu, Z.; Dong, F. Activation of amorphous bismuth oxide via plasmonic Bi metal for efficient visible-light photocatalysis. J. Catal. 2017, 352, 102–112. [Google Scholar] [CrossRef]
- Dong, F.; Li, Q.; Sun, Y.; Ho, W.-K. Noble Metal-Like Behavior of Plasmonic Bi Particles as a Cocatalyst Deposited on (BiO)2CO3 Microspheres for Efficient Visible Light Photocatalysis. Acs Catal. 2014, 4, 4341–4350. [Google Scholar] [CrossRef]
- Dong, F.; Zhao, Z.; Sun, Y.; Zhang, Y.; Yan, S.; Wu, Z. An Advanced Semimetal–Organic Bi Spheres–g-C3N4 Nanohybrid with SPR-Enhanced Visible-Light Photocatalytic Performance for NO Purification. Environ. Sci. Technol. 2015, 49, 12432–12440. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Huang, Y.; Li, Y.; Zhang, Q.; Cao, J.-j.; Ho, W.; Lee, S.C. Plasmonic Bi/ZnWO4 Microspheres with Improved Photocatalytic Activity on NO Removal under Visible Light. ACS Sustain. Chem. Eng. 2016, 4, 6912–6920. [Google Scholar] [CrossRef]
- Dong, F.; Xiong, T.; Yan, S.; Wang, H.; Sun, Y.; Zhang, Y.; Huang, H.; Wu, Z. Facets and defects cooperatively promote visible light plasmonic photocatalysis with Bi nanowires@BiOCl nanosheets. J. Catal. 2016, 344, 401–410. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, S.; Fu, X.; Xu, Y.-J. Synthesis of M@TiO2 (M = Au, Pd, Pt) Core–Shell Nanocomposites with Tunable Photoreactivity. J. Phys. Chem. C 2011, 115, 9136–9145. [Google Scholar] [CrossRef]
- Cushing, S.K.; Li, J.; Meng, F.; Senty, T.R.; Suri, S.; Zhi, M.; Li, M.; Bristow, A.D.; Wu, N. Photocatalytic Activity Enhanced by Plasmonic Resonant Energy Transfer from Metal to Semiconductor. J. Am. Chem. Soc. 2012, 134, 15033–15041. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Chen, W.; Ma, D.; Yang, Y.; Liu, S.; Huang, S. Size control of Au@Cu2O octahedra for excellent photocatalytic performance. J. Mater. Chem. 2012, 22, 719–724. [Google Scholar] [CrossRef]
- Yang, T.-T.; Chen, W.-T.; Hsu, Y.-J.; Wei, K.-H.; Lin, T.-Y.; Lin, T.-W. Interfacial Charge Carrier Dynamics in Core-Shell Au-CdS Nanocrystals. J. Phys. Chem. C 2010, 114, 11414–11420. [Google Scholar] [CrossRef]
- Wu, X.-F.; Song, H.-Y.; Yoon, J.-M.; Yu, Y.-T.; Chen, Y.-F. Synthesis of Core-Shell Au@TiO2 Nanoparticles with Truncated Wedge-Shaped Morphology and Their Photocatalytic Properties. Langmuir 2009, 25, 6438–6447. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, Y.; Wang, Z.; Gao, Y.; Huang, Y.; Cao, J.; Ho, W.; Lee, S. Controllable Synthesis of Core–Shell Bi@Amorphous Bi2O3 Nanospheres with Tunable Optical and Photocatalytic Activity for NO Removal. Ind. Eng. Chem. Res. 2017, 56, 10251–10258. [Google Scholar] [CrossRef]
- Li, J.T.; Cushing, S.K.; Bright, J.; Meng, F.K.; Senty, T.R.; Zheng, P.; Bristow, A.D.; Wu, N.Q. Ag@Cu2O Core-Shell Nanoparticles as Visible-Light Plasmonic Photocatalysts. ACS Catal. 2013, 3, 47–51. [Google Scholar] [CrossRef]
- Li, Q.; Wang, F.; Sun, L.; Jiang, Z.; Ye, T.; Chen, M.; Bai, Q.; Wang, C.; Han, X. Design and Synthesis of Cu@CuS Yolk–Shell Structures with Enhanced Photocatalytic Activity. Nano-Micro Lett. 2017, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wei, Z.; Wu, T.; Peng, Q.; Li, Y. Au−ZnO Hybrid Nanopyramids and Their Photocatalytic Properties. J. Am. Chem. Soc. 2011, 133, 5660–5663. [Google Scholar] [CrossRef] [PubMed]
- Yao, K.X.; Liu, X.; Zhao, L.; Zeng, H.C.; Han, Y. Site-specific growth of Au particles on ZnO nanopyramids under ultraviolet illumination. Nanoscale 2011, 3, 4195–4200. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Batista, M.J.; Ballari, M.M.; Kubacka, A.; Alfano, O.M.; Fernández-García, M. Braiding kinetics and spectroscopy in photo-catalysis: The spectro-kinetic approach. Chem. Soc. Rev. 2019, 48, 637–682. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J. Detection and characterisation of radicals using electron paramagnetic resonance (EPR) spin trapping and related methods. Methods 2016, 109, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, Y.; Hirai, T. Selective organic transformations on titanium oxide-based photocatalysts. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 157–170. [Google Scholar] [CrossRef]
- Lang, X.; Chen, X.; Zhao, J. Heterogeneous visible light photocatalysis for selective organic transformations. Chem. Soc. Rev. 2014, 43, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Kisch, H. Semiconductor photocatalysis for organic synthesis. Adv. Photochem. 2001, 26, 93–143. [Google Scholar]
- Park, H.; Choi, W. Photocatalytic conversion of benzene to phenol using modified TiO2 and polyoxometalates. Catal. Today 2005, 101, 291–297. [Google Scholar] [CrossRef]
- Ide, Y.; Nakamura, N.; Hattori, H.; Ogino, R.; Ogawa, M.; Sadakane, M.; Sano, T. Sunlight-induced efficient and selective photocatalytic benzene oxidation on TiO2-supported gold nanoparticles under CO2 atmosphere. Chem. Commun. 2011, 47, 11531–11533. [Google Scholar] [CrossRef] [PubMed]
- Yuzawa, H.; Kumagai, J.; Yoshida, H. Reaction Mechanism of Aromatic Ring Amination of Benzene and Substituted Benzenes by Aqueous Ammonia over Platinum-Loaded Titanium Oxide Photocatalyst. J. Phys. Chem. C 2013, 117, 11047–11058. [Google Scholar] [CrossRef]
- Yuzawa, H.; Yoshida, H. Direct aromatic-ring amination by aqueous ammonia with a platinum loaded titanium oxide photocatalyst. Chem. Commun. 2010, 46, 8854–8856. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Hashimoto, K.; Kominami, H. Preparation of Au/CeO2 Exhibiting Strong Surface Plasmon Resonance Effective for Selective or Chemoselective Oxidation of Alcohols to Aldehydes or Ketones in Aqueous Suspensions under Irradiation by Green Light. J. Am. Chem. Soc. 2012, 134, 14526–14533. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Fu, X.; Xu, Y.-J. A facile and green approach to synthesize Pt@CeO2 nanocomposite with tunable core-shell and yolk-shell structure and its application as a visible light photocatalyst. J. Mater. Chem. 2011, 21, 8152–8158. [Google Scholar] [CrossRef]
- Pradhan, S.; Ghosh, D.; Chen, S. Janus Nanostructures Based on Au−TiO2 Heterodimers and Their Photocatalytic Activity in the Oxidation of Methanol. ACS Appl. Mater. Interfaces 2009, 1, 2060–2065. [Google Scholar] [CrossRef] [PubMed]
- Tada, H.; Ishida, T.; Takao, A.; Ito, S. Drastic Enhancement of TiO2-Photocatalyzed Reduction of Nitrobenzene by Loading Ag Clusters. Langmuir 2004, 20, 7898–7900. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Wang, Y.; Zhang, Q.; Deng, W.; Wang, Y. MgO- and Pt-Promoted TiO2 as an Efficient Photocatalyst for the Preferential Reduction of Carbon Dioxide in the Presence of Water. ACS Catal. 2014, 4, 3644–3653. [Google Scholar] [CrossRef]
- Zhai, Q.; Xie, S.; Fan, W.; Zhang, Q.; Wang, Y.; Deng, W.; Wang, Y. Photocatalytic Conversion of Carbon Dioxide with Water into Methane: Platinum and Copper(I) Oxide Co-catalysts with a Core-Shell Structure. Angew. Chem. Int. Ed. 2013, 52, 5776–5779. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Pitts, D.T.; Zhao, H.; Zhao, C.; Li, Y. Silver-incorporated bicrystalline (anatase/brookite) TiO2 microspheres for CO2 photoreduction with water in the presence of methanol. Appl. Catal. A Gen. 2013, 467, 474–482. [Google Scholar] [CrossRef]
- Wang, Z.; Teramura, K.; Hosokawa, S.; Tanaka, T. Photocatalytic conversion of CO2 in water over Ag-modified La2Ti2O7. Appl. Catal. B Environ. 2015, 163, 241–247. [Google Scholar] [CrossRef]
- Feng, X.; Sloppy, J.D.; LaTemp, T.J.; Paulose, M.; Komarneni, S.; Bao, N.; Grimes, C.A. Synthesis and deposition of ultrafine Pt nanoparticles within high aspect ratio TiO2 nanotube arrays: Application to the photocatalytic reduction of carbon dioxide. J. Mater. Chem. 2011, 21, 13429–13433. [Google Scholar] [CrossRef]
- Sarina, S.; Zhu, H.; Jaatinen, E.; Xiao, Q.; Liu, H.; Jia, J.; Chen, C.; Zhao, J. Enhancing Catalytic Performance of Palladium in Gold and Palladium Alloy Nanoparticles for Organic Synthesis Reactions through Visible Light Irradiation at Ambient Temperatures. J. Am. Chem. Soc. 2013, 135, 5793–5801. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Z.; Zhai, Z.; Guo, X.; Guo, X.-Y. Visible-Light-Driven Photocatalytic Suzuki–Miyaura Coupling Reaction on Mott–Schottky-type Pd/SiC Catalyst. J. Phys. Chem. C 2015, 119, 3238–3243. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Domen, K. Photocatalytic Water Splitting: Recent Progress and Future Challenges. J. Phys. Chem. Lett. 2010, 1, 2655–2661. [Google Scholar] [CrossRef]
- Ahmad, H.; Kamarudin, S.K.; Minggu, L.J.; Kassim, M. Hydrogen from photo-catalytic water splitting process: A review. Renew. Sustain. Energy Rev. 2015, 43, 599–610. [Google Scholar] [CrossRef]
- Bi, L.; Gao, X.; Ma, Z.; Zhang, L.; Wang, D.; Xie, T. Enhanced Separation Efficiency of PtNix/g-C3N4 for Photocatalytic Hydrogen Production. ChemCatChem 2017, 9, 3779–3785. [Google Scholar] [CrossRef]
- Ingram, D.B.; Linic, S. Water Splitting on Composite Plasmonic-Metal/Semiconductor Photoelectrodes: Evidence for Selective Plasmon-Induced Formation of Charge Carriers near the Semiconductor Surface. J. Am. Chem. Soc. 2011, 133, 5202–5205. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Batista, M.J.; Meira, D.M.; Colon, G.; Kubacka, A.; Fernandez-Garcia, M. Phase-Contact Engineering in Mono- and Bimetallic Cu-Ni Co-catalysts for Hydrogen Photocatalytic Materials. Angew. Chem. -Int. Ed. 2018, 57, 1199–1203. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Yu, J.C. Pt3Co-loaded CdS and TiO2 for photocatalytic hydrogen evolution from water. J. Mater. Chem. A 2013, 1, 12221–12228. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Kang, Z. 3D Branched ZnO Nanowire Arrays Decorated with Plasmonic Au Nanoparticles for High-Performance Photoelectrochemical Water Splitting. ACS Appl. Mater. Interfaces 2014, 6, 4480–4489. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Chen, B.; Bosman, M.; Cao, X.; Chen, J.; Zheng, B.; Zhang, H. Au Nanoparticle-Modified MoS2 Nanosheet-Based Photoelectrochemical Cells for Water Splitting. Small 2014, 10, 3537–3543. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shao, M.; Ning, F.; Xu, S.; Li, Z.; Wei, M.; Evans, D.G.; Duan, X. Au nanoparticles sensitized ZnO nanorod@nanoplatelet core-shell arrays for enhanced photoelectrochemical water splitting. Nano Energy 2015, 12, 231–239. [Google Scholar] [CrossRef]
- Sheeney-Haj-Ichia, L.; Pogorelova, S.; Gofer, Y.; Willner, I. Enhanced Photoelectrochemistry in CdS/Au Nanoparticle Bilayers. Adv. Funct. Mater. 2004, 14, 416–424. [Google Scholar] [CrossRef]
- Zhukovskyi, M.; Tongying, P.; Yashan, H.; Wang, Y.; Kuno, M. Efficient Photocatalytic Hydrogen Generation from Ni Nanoparticle Decorated CdS Nanosheets. ACS Catal. 2015, 5, 6615–6623. [Google Scholar] [CrossRef]
- Rosseler, O.; Shankar, M.V.; Du, M.K.-L.; Schmidlin, L.; Keller, N.; Keller, V. Solar light photocatalytic hydrogen production from water over Pt and Au/TiO2(anatase/rutile) photocatalysts: Influence of noble metal and porogen promotion. J. Catal. 2010, 269, 179–190. [Google Scholar] [CrossRef]
- Gu, Q.; Long, J.; Fan, L.; Chen, L.; Zhao, L.; Lin, H.; Wang, X. Single-site Sn-grafted Ru/TiO2 photocatalysts for biomass reforming: Synergistic effect of dual co-catalysts and molecular mechanism. J. Catal. 2013, 303, 141–155. [Google Scholar] [CrossRef]
- Hong, J.W.; Wi, D.H.; Lee, S.-U.; Han, S.W. Metal–Semiconductor Heteronanocrystals with Desired Configurations for Plasmonic Photocatalysis. J. Am. Chem. Soc. 2016, 138, 15766–15773. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhao, K.; Tang, H.; Chen, Y.; Lu, C.; Liu, W.; Gao, Y.; Zhao, H.; Tang, Z. New Insight into the Role of Gold Nanoparticles in Au@CdS Core–Shell Nanostructures for Hydrogen Evolution. Small 2014, 10, 4664–4670. [Google Scholar] [CrossRef] [PubMed]
- Ngaw, C.K.; Xu, Q.; Tan, T.T.Y.; Hu, P.; Cao, S.; Loo, J.S.C. A strategy for in-situ synthesis of well-defined core–shell Au@TiO2 hollow spheres for enhanced photocatalytic hydrogen evolution. Chem. Eng. J. 2014, 257, 112–121. [Google Scholar] [CrossRef]
- Lee, Y.J.; Joo, J.B.; Yin, Y.; Zaera, F. Evaluation of the Effective Photoexcitation Distances in the Photocatalytic Production of H2 from Water using Au@Void@TiO2 Yolk–Shell Nanostructures. ACS Energy Lett. 2016, 1, 52–56. [Google Scholar] [CrossRef]
- Yu, Z.B.; Xie, Y.P.; Liu, G.; Lu, G.Q.; Ma, X.L.; Cheng, H.-M. Self-assembled CdS/Au/ZnO heterostructure induced by surface polar charges for efficient photocatalytic hydrogen evolution. J. Mater. Chem. A 2013, 1, 2773–2776. [Google Scholar] [CrossRef]
- Chava, R.K.; Do, J.Y.; Kang, M. Smart Hybridization of Au Coupled CdS Nanorods with Few Layered MoS2 Nanosheets for High Performance Photocatalytic Hydrogen Evolution Reaction. ACS Sustain. Chem. Eng. 2018, 6, 6445–6457. [Google Scholar] [CrossRef]
- Kim, Y.G.; Jo, W.-K. Photodeposited-metal/CdS/ZnO heterostructures for solar photocatalytic hydrogen production under different conditions. Int. J. Hydrogen Energy 2017, 42, 11356–11363. [Google Scholar] [CrossRef]
- Zhou, H.; Pan, J.; Ding, L.; Tang, Y.; Ding, J.; Guo, Q.; Fan, T.; Zhang, D. Biomass-derived hierarchical porous CdS/M/TiO2 (M = Au, Ag, pt, pd) ternary heterojunctions for photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2014, 39, 16293–16301. [Google Scholar] [CrossRef]
- Wang, Q.; Hisatomi, T.; Ma, S.S.K.; Li, Y.; Domen, K. Core/Shell Structured La- and Rh-Codoped SrTiO3 as a Hydrogen Evolution Photocatalyst in Z-Scheme Overall Water Splitting under Visible Light Irradiation. Chem. Mater. 2014, 26, 4144–4150. [Google Scholar] [CrossRef]
- Maeda, K.; Teramura, K.; Lu, D.; Saito, N.; Inoue, Y.; Domen, K. Noble-metal/Cr2O3 core/shell nanoparticles as a cocatalyst for photocatalytic overall water splitting. Angew. Chem. Int. Ed. 2006, 45, 7806–7809. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, T.; Tomoda, R.; Nakajima, T.; Wake, H. Photoelectrochemical sterilization of microbial cells by semiconductor powders. FEMS Microbiol. Lett. 1985, 29, 211–214. [Google Scholar] [CrossRef] [Green Version]
- Gamage, J.; Zhang, Z. Applications of Photocatalytic Disinfection. Int. J. Photoenergy 2010, 2010, 764870. [Google Scholar] [CrossRef]
- Malato, S.; Fernández-Ibáñez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Byrne, J.A.; Fernandez-Ibañez, P.A.; Dunlop, P.S.M.; Alrousan, D.M.A.; Hamilton, J.W.J. Photocatalytic Enhancement for Solar Disinfection of Water: A Review. Int. J. Photoenergy 2011, 2011, 798051. [Google Scholar] [CrossRef]
- Sarria, V.; Parra, S.; Adler, N.; Péringer, P.; Benitez, N.; Pulgarin, C. Recent developments in the coupling of photoassisted and aerobic biological processes for the treatment of biorecalcitrant compounds. Catal. Today 2002, 76, 301–315. [Google Scholar] [CrossRef]
- Dalrymple, O.K.; Stefanakos, E.; Trotz, M.A.; Goswami, D.Y. A review of the mechanisms and modeling of photocatalytic disinfection. Appl. Catal. B Environ. 2010, 98, 27–38. [Google Scholar] [CrossRef]
- Muñoz-Batista, M.J.; Fontelles-Carceller, O.; Ferrer, M.; Fernández-García, M.; Kubacka, A. Disinfection capability of Ag/g-C3N4 composite photocatalysts under UV and visible light illumination. Appl. Catal. B Environ. 2016, 183, 86–95. [Google Scholar] [CrossRef]
- Pratap Reddy, M.; Venugopal, A.; Subrahmanyam, M. Hydroxyapatite-supported Ag–TiO2 as Escherichia coli disinfection photocatalyst. Water Res. 2007, 41, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.V.; Raza, G. Photocatalytic disinfection of water with Ag–TiO2 nanocrystalline composite. Ionics 2009, 15, 579–587. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Z.; Bai, H.; Sun, D.D. Concurrent filtration and solar photocatalytic disinfection/degradation using high-performance Ag/TiO2 nanofiber membrane. Water Res. 2012, 46, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Rtimi, S.; Giannakis, S.; Sanjines, R.; Pulgarin, C.; Bensimon, M.; Kiwi, J. Insight on the photocatalytic bacterial inactivation by co-sputtered TiO2–Cu in aerobic and anaerobic conditions. Appl. Catal. B Environ. 2016, 182, 277–285. [Google Scholar] [CrossRef]
- Zhu, L.; He, C.; Huang, Y.; Chen, Z.; Xia, D.; Su, M.; Xiong, Y.; Li, S.; Shu, D. Enhanced photocatalytic disinfection of E. coli 8099 using Ag/BiOI composite under visible light irradiation. Sep. Purif. Technol. 2012, 91, 59–66. [Google Scholar] [CrossRef]
- Shi, H.; Li, G.; Sun, H.; An, T.; Zhao, H.; Wong, P.-K. Visible-light-driven photocatalytic inactivation of E. coli by Ag/AgX-CNTs (X=Cl, Br, I) plasmonic photocatalysts: Bacterial performance and deactivation mechanism. Appl. Catal. B Environ. 2014, 158–159, 301–307. [Google Scholar] [CrossRef]
- Gao, P.; Ng, K.; Sun, D.D. Sulfonated graphene oxide–ZnO–Ag photocatalyst for fast photodegradation and disinfection under visible light. J. Hazard. Mater. 2013, 262, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Sinha, S.; Suar, M.; Yun, S.-I.; Mishra, A.; Tripathy, S.K. Solar-photocatalytic disinfection of Vibrio cholerae by using Ag@ZnO core–shell structure nanocomposites. J. Photochem. Photobiol. B Biol. 2015, 142, 68–76. [Google Scholar] [CrossRef] [PubMed]














| Photocatalyst | Structure | Pollutant | Light source | Reference |
|---|---|---|---|---|
| Au/TiO2 | Conventional structure | RhB | Visible light | [51] |
| Au/ZnO | Conventional structure | RhB, phenol red, procion red | Ultraviolet (UV) light | [76] |
| Au/ZnO | Conventional structure | RhB | UV light | [77] |
| Ag/ZnO | Conventional structure | MB | UV light | [78] |
| Ag/ZnO | Conventional structure | RhB | UV light | [79] |
| Ag/ZnO | Conventional structure | RhB | Simulated sunlight | [80] |
| Au/TiO2 | Conventional structure | MB, MO, p-nitrophenol | Visible light | [81] |
| Ag/ZnO | Conventional structure | RhB | Visible light | [82] |
| Au/TiO2, Pt/TiO2, Ir/TiO2 | Conventional structure | Azo dye | UV light | [83] |
| Ag/TiO2 | Conventional structure | RhB, ciprofloxacin | Visible light | [84] |
| Cu/Cu2O | Conventional structure | RhB, MO, MB | Visible light | [85] |
| Bi/amorphous bismuth oxide (A-BO) | Conventional structure | NO | Visible light | [86] |
| Bi/(BiO)2CO3 | Conventional structure | NO | Visible light | [87] |
| Bi/g-C3N4 | Conventional structure | NO | Visible light | [88] |
| Bi/ZnWO4 | Conventional structure | NO | Visible light | [89] |
| Bi/BiOCl | Conventional structure | NO | Visible light | [90] |
| M@TiO2 (M = Au, Pd, Pt) | Core–shell structure | RhB | UV light, visible light | [91] |
| Au@Cu2O | Core–shell structure | MO | Visible light | [92] |
| Au@Cu2O | Core–shell structure | MO | Visible light | [93] |
| Au@CdS | Core–shell structure | RhB | Visible light | [94] |
| Au@TiO2 | Core–shell structure | Acetaldehyde | UV light, visible light | [95] |
| Bi@Bi2O3 | Core–shell structure | NO | Visible light | [96] |
| Ag@Cu2O | Core–shell structure | MO | Visible light | [97] |
| Au@TiO2 | Yolk–shell structure | RhB | Visible light, simulated sunlight | [59] |
| Cu@CuS | Yolk–shell structure | MB | Simulated sunlight | [98] |
| Au/ZnO | Janus structure | RhB | UV light | [99] |
| Au/ZnO | Janus structure | MO | UV light | [100] |
| CdS/Au/TiO2 | Multi-junction structure | Methyl viologen (MV) | UV light | [71] |
| Photocatalyst | Structure | Reaction Type | Reactant | Light Source | Reference |
|---|---|---|---|---|---|
| Pt/TiO2, Pd/TiO2 | Conventional structure | Oxidation reaction | Benzene | UV light | [106] |
| Au/TiO2 | Conventional structure | Oxidation reaction | Benzene | Simulated sunlight | [107] |
| Pt/TiO2 | Conventional structure | Oxidation reaction | Benzene | UV light | [108] |
| Pt/TiO2 | Conventional structure | Oxidation reaction | (substituted) Benzene | UV light | [109] |
| Au/CeO2 | Conventional structure | Oxidation reaction | Aromatic alcohols | Visible light | [110] |
| Pt@CeO2 | Core-shell, yolk-shell structure | Oxidation reaction | Benzyl alcohol | Visible light | [111] |
| Au/TiO2 | Janus structure | Oxidation reaction | Methanol | UV light | [112] |
| Ag/TiO2 | Conventional structure | Reduction reaction | Nitrobenzene | UV light | [113] |
| M/TiO2 (M = Pt, Pd, Rh, Ag and Au) | Conventional structure | Reduction reaction | Carbon dioxide (CO2) | UV light | [114] |
| Pt-Cu/TiO2 | Conventional structure | Reduction reaction | CO2 | Visible light | [115] |
| Ag/TiO2 | Conventional structure | Reduction reaction | CO2 | Simulated sunlight | [116] |
| Ag/La2Ti2O7 | Conventional structure | Reduction reaction | CO2 | UV light | [117] |
| Pt/TiO2 | Conventional structure | Reduction reaction | CO2 | Simulated sunlight | [118] |
| Au-Pd/ZrO2 | Conventional structure | Oxidation, coupling reaction | Benzylamine, benzyl alcohol etc. | Visible light | [119] |
| Pd/SiC | Conventional structure | Coupling reaction | Iodobenzene, phenylboronic acid | Visible light | [120] |
| Photocatalyst | Structure | Light Souce | Reference |
|---|---|---|---|
| Pt/TiO2 | Conventional structure | UV light | [27] |
| PtNix/g-C3N4 | Conventional structure | UV light | [124] |
| Au/TiO2, Ag/TiO2 | Conventional structure | Visible light | [125] |
| Cu/TiO2, Ni/TiO2, CuNi/TiO2 | Conventional structure | UV light | [126] |
| Pt3Co/CdS, Pt3Co/ TiO2 | Conventional structure | UV light | [127] |
| Au/ZnO | Conventional structure | Simulated sunlight | [128] |
| Au/MoS2 | Conventional structure | Visible light | [129] |
| Au/ZnO | Conventional structure | Visible light | [130] |
| Au/CdS | Conventional structure | Simulated sunlight | [131] |
| Ni/CdS | Conventional structure | UV light | [132] |
| Pt/TiO2, Au/TiO2 | Conventional structure | Simulated sunlight | [133] |
| SnRux/TiO2 | Conventional structure | UV light | [134] |
| Au/Cu2O | Conventional, core-shell structure | Visible light | [135] |
| Au@CdS | Core-shell structure | Visible light | [136] |
| Au@TiO2 | Yolk-shell structure | UV light | [137] |
| Au@TiO2 | Yolk-shell structure | UV light | [138] |
| Au/TiO2 | Janus structure | Visible light | [63] |
| Au/CdS | Array structure | Visible light | [67] |
| CdS/Au/ZnO | Multi-junction structure | UV light | [139] |
| CdS/Au/MoS2 | Multi-junction structure | Visible light | [140] |
| CdS/Pt/ZnO | Multi-junction structure | Simulated sunlight | [141] |
| CdS/M/TiO2 (M = Au, Ag, Pt, Pd) | Multi-junction structure | Simulated sunlight | [142] |
| CoOx/Ir/Ta3N5 | Multi-junction structure | Simulated sunlight | [143] |
| Cr2O3/Rh/ (Ga1-xZnx)(N1-xOx) | Multi-junction structure | Visible light | [144] |
| Photocatalyst | Structure | Bacteria | Light Source | Reference |
|---|---|---|---|---|
| Ag/g-C3N4 | Conventional structure | Escherichia coli | UV and visible light | [151] |
| Ag/TiO2 | Conventional structure | Escherichia coli | UV light | [152] |
| Ag/TiO2 | Conventional structure | Escherichia coli | Sunlight | [153] |
| Ag/TiO2 | Conventional structure | Escherichia coli | Simulated sunlight | [154] |
| Cu/TiO2 | Conventional structure | Escherichia coli | UV and visible light | [155] |
| Ag/BiOI | Conventional structure | Escherichia coli | Visible light | [156] |
| Ag/AgX (X = Cl, Br, I) | Conventional structure | Escherichia coli | Visible light | [157] |
| Ag/ZnO | Conventional structure | Escherichia coli | Visible light | [158] |
| Ag@ZnO | Core–shell structure | Vibrio cholerae | Sunlight | [159] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.-s.; Li, J.; Li, J. Metal/Semiconductor Nanocomposites for Photocatalysis: Fundamentals, Structures, Applications and Properties. Nanomaterials 2019, 9, 359. https://doi.org/10.3390/nano9030359
Fu Y-s, Li J, Li J. Metal/Semiconductor Nanocomposites for Photocatalysis: Fundamentals, Structures, Applications and Properties. Nanomaterials. 2019; 9(3):359. https://doi.org/10.3390/nano9030359
Chicago/Turabian StyleFu, Yong-sheng, Jun Li, and Jianguo Li. 2019. "Metal/Semiconductor Nanocomposites for Photocatalysis: Fundamentals, Structures, Applications and Properties" Nanomaterials 9, no. 3: 359. https://doi.org/10.3390/nano9030359
APA StyleFu, Y. -s., Li, J., & Li, J. (2019). Metal/Semiconductor Nanocomposites for Photocatalysis: Fundamentals, Structures, Applications and Properties. Nanomaterials, 9(3), 359. https://doi.org/10.3390/nano9030359