Solid Lipid Nanoparticles Surface Modification Modulates Cell Internalization and Improves Chemotoxic Treatment in an Oral Carcinoma Cell Line
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Solid Lipid Nanoparticles
2.3. Photon Correlation Spectroscopy
2.4. Transmission Electron Microscopy
2.5. Incorporation of All-Trans Retinoic Acid in SLN
2.6. Cell Culturing
2.7. In Vitro Cell Cytotoxicity Assay
2.8. Cell Incorporation Assay by Flow Cytometry
2.9. Cell Incorporation Assay by Confocal Microscopy
2.10. Statistical Analysis
3. Results
3.1. Development and Characterization of PE–PEG Coated SLN
3.2. Incorporation of PEG–SLN in Cell Culture
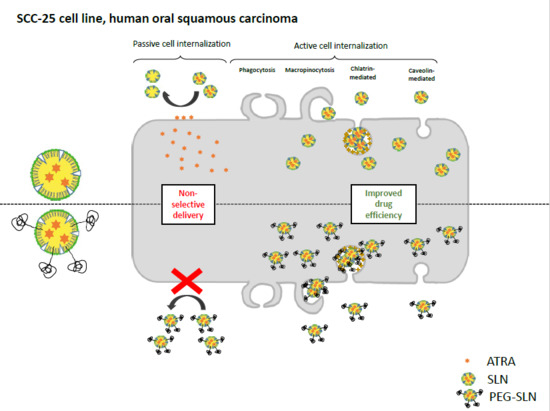
3.2.1. PE–PEG Coating Improves Active fSLN Internalization Mechanisms in SCC-25 Cells but not in THP-1 Cells
3.2.2. PE–PEG-Coating Does Not Alter Intracellular Distribution of SLN in SCC-25 Cells
3.2.3. PE–PEG-Coating Affects Cell Internalization Pathway of fSLN in SCC-25 Cells
3.3. PEG Coating Improves the Efficiency of ATRA-Loaded SLN Treatment
3.3.1. ATRA Can Be Easily Loaded into SLN Using an Organic Solvent-Free Method
3.3.2. PEG–SLN Are More Efficient Drug Carriers than SLN without Surface Modification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kalaydina, R.V.; Bajwa, K.; Qorri, B.; Decarlo, A.; Szewczuk, M.R. Recent Advances in “Smart” Delivery Systems for Extended Drug Release in Cancer Therapy. Int. J. Nanomed. 2018, 13, 4727–4745. [Google Scholar] [CrossRef] [PubMed]
- Geszke-Moritz, M.; Moritz, M. Solid Lipid Nanoparticles as Attractive Drug Vehicles: Composition, Properties and Therapeutic Strategies. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 68, 982–994. [Google Scholar] [CrossRef] [PubMed]
- Tapeinos, C.; Battaglini, M.; Ciofani, G. Advances in the Design of Solid Lipid Nanoparticles and Nanostructured Lipid Carriers for Targeting Brain Diseases. J. Control. Release 2017, 264, 306–332. [Google Scholar] [CrossRef] [PubMed]
- Mehnert, W.; Mader, K. Solid Lipid Nanoparticles: Production, Characterization and Applications. Adv. Drug Deliv. Rev. 2001, 47, 165–196. [Google Scholar] [CrossRef]
- Marengo, E.; Cavalli, R.; Caputo, O.; Rodriguez, L.; Gasco, M.R. Scale-up of the Preparation Process of Solid Lipid Nanospheres. Part I. Int. J. Pharm. 2000, 205, 3–13. [Google Scholar] [CrossRef]
- Wissing, S.A.; Kayser, O.; Muller, R.H. Solid Lipid Nanoparticles for Parenteral Drug Delivery. Adv. Drug Deliv. Rev. 2004, 56, 1257–1272. [Google Scholar] [CrossRef] [PubMed]
- Blasi, P.; Giovagnoli, S.; Schoubben, A.; Ricci, M.; Rossi, C. Solid Lipid Nanoparticles for Targeted Brain Drug Delivery. Adv. Drug Deliv. Rev. 2007, 59, 454–477. [Google Scholar] [CrossRef]
- Sarin, H. Overcoming the Challenges in the Effective Delivery of Chemotherapies to Cns Solid Tumors. Ther. Deliv. 2010, 1, 289–305. [Google Scholar] [CrossRef]
- Chen, Y.; Dalwadi, G.; Benson, H.A. Drug Delivery across the Blood-Brain Barrier. Curr. Drug Deliv. 2004, 1, 361–376. [Google Scholar] [CrossRef]
- Maeda, H.; Nakamura, H.; Fang, J. The Epr Effect for Macromolecular Drug Delivery to Solid Tumors: Improvement of Tumor Uptake, Lowering of Systemic Toxicity, and Distinct Tumor Imaging in Vivo. Adv. Drug Deliv. Rev. 2012, 65, 71–79. [Google Scholar] [CrossRef]
- Leonessa, F.; Clarke, R. Atp Binding Cassette Transporters and Drug Resistance in Breast Cancer. Endocr. Relat. Cancer 2003, 10, 43–73. [Google Scholar] [CrossRef]
- De Juan, B.S.; Von Briesen, H.; Gelperina, S.E.; Kreuter, J. Cytotoxicity of Doxorubicin Bound to Poly(Butyl Cyanoacrylate) Nanoparticles in Rat Glioma Cell Lines Using Different Assays. J. Drug Target. 2006, 14, 614–622. [Google Scholar] [CrossRef]
- Miller, D.S.; Bauer, B.; Hartz, A.M. Modulation of P-Glycoprotein at the Blood-Brain Barrier: Opportunities to Improve Central Nervous System Pharmacotherapy. Pharm. Rev. 2008, 60, 196–209. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zheng, Y.; Shi, Y.; Cui, Z. Overcoming Tumor Cell Chemoresistance Using Nanoparticles: Lysosomes Are Beneficial for (Stearoyl) Gemcitabine-Incorporated Solid Lipid Nanoparticles. Int. J. Nanomed. 2018, 13, 319–336. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.; Costa-Lima, S.; Carneiro, T.; Cordeiro-da-Silva, A.; Souto, E.B.; Ferreira, D.C. Solid Lipid Nanoparticles as Intracellular Drug Transporters: An Investigation of the Uptake Mechanism and Pathway. Int. J. Pharm. 2012, 430, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Jindal, A.B. The Effect of Particle Shape on Cellular Interaction and Drug Delivery Applications of Micro- and Nanoparticles. Int. J. Pharm. 2017, 532, 450–465. [Google Scholar] [CrossRef]
- Klibanov, A.L.; Maruyama, K.; Torchilin, V.P.; Huang, L. Amphipathic Polyethyleneglycols Effectively Prolong the Circulation Time of Liposomes. FEBS Lett. 1990, 268, 235–237. [Google Scholar] [CrossRef]
- Allen, T.M.; Hansen, C. Pharmacokinetics of Stealth Versus Conventional Liposomes: Effect of Dose. Biochim. Biophys. Acta 1991, 1068, 133–141. [Google Scholar] [CrossRef]
- Kang, X.; Chen, H.; Li, S.; Jie, L.; Hu, J.; Wang, X.; Qi, J.; Ying, X.; Du, Y. Magnesium Lithospermate B Loaded Pegylated Solid Lipid Nanoparticles for Improved Oral Bioavailability. Colloids Surf. B Biointerfaces 2018, 161, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Madan, J.; Pandey, R.S.; Jain, V.; Katare, O.P.; Chandra, R.; Katyal, A. Poly (Ethylene)-Glycol Conjugated Solid Lipid Nanoparticles of Noscapine Improve Biological Half-Life, Brain Delivery and Efficacy in Glioblastoma Cells. Nanomedicine 2012, 9, 492–503. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. Pegylation as a Strategy for Improving Nanoparticle-Based Drug and Gene Delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Jones, S.W.; Parker, C.L.; Zamboni, W.C.; Bear, J.E.; Lai, S.K. Evading Immune Cell Uptake and Clearance Requires Peg Grafting at Densities Substantially Exceeding the Minimum for Brush Conformation. Mol. Pharm. 2014, 11, 1250–1258. [Google Scholar] [CrossRef] [PubMed]
- Alferiev, I.S.; Iyer, R.; Croucher, J.L.; Adamo, R.F.; Zhang, K.; Mangino, J.L.; Kolla, V.; Fishbein, I.; Brodeur, G.M.; Levy, R.J.; et al. Nanoparticle-Mediated Delivery of a Rapidly Activatable Prodrug of Sn-38 for Neuroblastoma Therapy. Biomaterials 2015, 51, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Pietzonka, P.; Rothen-Rutishauser, B.; Langguth, P.; Wunderli-Allenspach, H.; Walter, E.; Merkle, H.P. Transfer of Lipophilic Markers from Plga and Polystyrene Nanoparticles to Caco-2 Monolayers Mimics Particle Uptake. Pharm. Res. 2002, 19, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, D.; Messerschmidt, C.; Bannwarth, M.B.; Landfester, K.; Mailander, V. Drug Delivery without Nanoparticle Uptake: Delivery by a Kiss-and-Run Mechanism on the Cell Membrane. Chem. Commun. 2014, 50, 1369–1371. [Google Scholar] [CrossRef]
- Arana, L.; Salado, C.; Vega, S.; Aizpurua-Olaizola, O.; de la Arada, I.; Suarez, T.; Usobiaga, A.; Arrondo, J.L.; Alonso, A.; Goni, F.M.; et al. Solid Lipid Nanoparticles for Delivery of Calendula Officinalis Extract. Colloids Surf. B Biointerfaces 2015, 135, 18–26. [Google Scholar] [CrossRef]
- Chen, M.C.; Hsu, S.L.; Lin, H.; Yang, T.Y. Retinoic Acid and Cancer Treatment. Biomedicine 2014, 4, 22. [Google Scholar] [CrossRef]
- Xie, X.; Liao, J.; Shao, X.; Li, Q.; Lin, Y. The Effect of Shape on Cellular Uptake of Gold Nanoparticles in the Forms of Stars, Rods, and Triangles. Sci. Rep. 2017, 7, 3827. [Google Scholar] [CrossRef]
- Chiu, H.Y.; Deng, W.; Engelke, H.; Helma, J.; Leonhardt, H.; Bein, T. Intracellular Chromobody Delivery by Mesoporous Silica Nanoparticles for Antigen Targeting and Visualization in Real Time. Sci. Rep. 2016, 6, 25019. [Google Scholar] [CrossRef]
- Smith, P.J.; Giroud, M.; Wiggins, H.L.; Gower, F.; Thorley, J.A.; Stolpe, B.; Mazzolini, J.; Dyson, R.J.; Rappoport, J.Z. Cellular Entry of Nanoparticles Via Serum Sensitive Clathrin-Mediated Endocytosis, and Plasma Membrane Permeabilization. Int. J. Nanomed. 2012, 7, 2045–2055. [Google Scholar]
- Vidarsson, G.; Stemerding, A.M.; Stapleton, N.M.; Spliethoff, S.E.; Janssen, H.; Rebers, F.E.; de Haas, M.; van de Winkel, J.G. Fcrn: An Igg Receptor on Phagocytes with a Novel Role in Phagocytosis. Blood 2006, 108, 3573–3579. [Google Scholar] [CrossRef]
- Kontogiannopoulos, K.N.; Tsermentseli, S.K.; Assimopoulou, A.N.; Papageorgiou, V.P. Sterically Stabilized Liposomes as a Potent Carrier for Shikonin. J. Liposome Res. 2014, 24, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J. Surface Modification of Monodisperse Magnetite Nanoparticles for Improved Intracellular Uptake to Breast Cancer Cells. J. Colloid Interface Sci. 2005, 283, 352–357. [Google Scholar] [CrossRef]
- Yu, M.; Huang, S.; Yu, K.J.; Clyne, A.M. Dextran and Polymer Polyethylene Glycol (Peg) Coating Reduce Both 5 and 30 Nm Iron Oxide Nanoparticle Cytotoxicity in 2d and 3d Cell Culture. Int. J. Mol. Sci. 2012, 13, 5554–5570. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Kucharski, C.; Doschak, M.R.; Sebald, W.; Uludag, H. Polyethylenimine-Peg Coated Albumin Nanoparticles for Bmp-2 Delivery. Biomaterials 2010, 31, 952–963. [Google Scholar] [CrossRef]
- Li, H.H.; Li, J.; Wasserloos, K.J.; Wallace, C.; Sullivan, M.G.; Bauer, P.M.; Stolz, D.B.; Lee, J.S.; Watkins, S.C.; St Croix, C.M.; et al. Caveolae-Dependent and -Independent Uptake of Albumin in Cultured Rodent Pulmonary Endothelial Cells. PLoS ONE 2013, 8, e81903. [Google Scholar] [CrossRef] [PubMed]
- Rezgui, R.; Blumer, K.; Yeoh-Tan, G.; Trexler, A.J.; Magzoub, M. Precise Quantification of Cellular Uptake of Cell-Penetrating Peptides Using Fluorescence-Activated Cell Sorting and Fluorescence Correlation Spectroscopy. Biochim. Biophys. Acta 2016, 1858, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- Schenk, T.; Stengel, S.; Zelent, A. Unlocking the Potential of Retinoic Acid in Anticancer Therapy. Br. J. Cancer 2014, 111, 2039–2045. [Google Scholar] [CrossRef] [PubMed]
- Le, Q.; Dawson, M.I.; Soprano, D.R.; Soprano, K.J. Modulation of Retinoic Acid Receptor Function Alters the Growth Inhibitory Response of Oral Scc Cells to Retinoids. Oncogene 2000, 19, 1457–1465. [Google Scholar] [CrossRef]
- Lim, S.J.; Kim, C.K. Formulation Parameters Determining the Physicochemical Characteristics of Solid Lipid Nanoparticles Loaded with All-Trans Retinoic Acid. Int. J. Pharm. 2002, 243, 135–146. [Google Scholar] [CrossRef]
- Choi, S.W.; Kim, W.S.; Kim, J.H. Surface Modification of Functional Nanoparticles for Controlled Drug Delivery. J. Dispers. Sci. Technol. 2003, 24, 475–487. [Google Scholar] [CrossRef]
- Jeong, K.J.; Kohane, D.S. Surface Modification and Drug Delivery for Biointegration. Ther. Deliv. 2011, 2, 737–752. [Google Scholar] [CrossRef] [PubMed]
- Ugarte-Uribe, B.; Perez-Rentero, S.; Lucas, R.; Avino, A.; Reina, J.J.; Alkorta, I.; Eritja, R.; Morales, J.C. Synthesis, Cell-Surface Binding, and Cellular Uptake of Fluorescently Labeled Glucose-DNA Conjugates with Different Carbohydrate Presentation. Bioconjug. Chem. 2010, 21, 1280–1287. [Google Scholar] [CrossRef]
- Cardoso, A.M.; Morais, C.M.; Cruz, A.R.; Cardoso, A.L.; Silva, S.G.; do Vale, M.L.; Marques, E.F.; Pedroso de Lima, M.C.; Jurado, A.S. Gemini surfactants mediate efficient mitochondrial gene delivery and expression. Mol. Pharm. 2015, 12, 716–730. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Guo, J.; Xing, X.; Zhang, X.; Du, Y.; Lu, Q. Rgd Modified and Pegylated Lipid Nanoparticles Loaded with Puerarin: Formulation, Characterization and Protective Effects on Acute Myocardial Ischemia Model. Biomed. Pharm. 2017, 89, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Wan, F.; You, J.; Sun, Y.; Zhang, X.G.; Cui, F.D.; Du, Y.Z.; Yuan, H.; Hu, F.Q. Studies on Peg-Modified Slns Loading Vinorelbine Bitartrate (I): Preparation and Evaluation in Vitro. Int. J. Pharm. 2008, 359, 104–110. [Google Scholar] [CrossRef]
- Parr, M.J.; Ansell, S.M.; Choi, L.S.; Cullis, P.R. Factors Influencing the Retention and Chemical Stability of Poly(Ethylene Glycol)-Lipid Conjugates Incorporated into Large Unilamellar Vesicles. Biochim. Biophys. Acta 1994, 1195, 21–30. [Google Scholar] [CrossRef]
- Uner, M.; Yener, G. Importance of Solid Lipid Nanoparticles (Sln) in Various Administration Routes and Future Perspectives. Int. J. Nanomed. 2007, 2, 289–300. [Google Scholar]
- Wang, L.H.; Rothberg, K.G.; Anderson, R.G. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell Biol. 1993, 123, 1107–1117. [Google Scholar] [CrossRef] [Green Version]
- Murata, T.; Maruoka, N.; Omata, N.; Takashima, Y.; Fujibayashi, Y.; Yonekura, Y.; Wada, Y. A comparative study of the plasma membrane permeabilization and fluidization induced by antipsychotic drugs in the rat brain. Int. J. Neuropsychopharmacol. 2007, 10, 683–689. [Google Scholar] [CrossRef]
- Orlandi, P.A.; Fishman, P.H. Filipin-dependent inhibition of cholera toxin: Evidence for toxin internalization and activation through caveolae-like domains. J. Cell Biol. 1998, 141, 905–915. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, A.; Harashima, H. Endocytosis of gene delivery vectors: From clathrin-dependent to lipid raft-mediated endocytosis. Mol. Ther. 2013, 21, 1118–1130. [Google Scholar] [CrossRef]
- Casella, J.F.; Flanagan, M.D.; Lin, S. Cytochalasin D inhibits actin polymerization and induces depolymerization of actin filaments formed during platelet shape change. Nature 1981, 293, 302. [Google Scholar] [CrossRef]
- Arranja, A.; Gouveia, L.F.; Gener, P.; Rafael, D.F.; Pereira, C.; Schwartz, S., Jr.; Videira, M.A. Self-Assembly Pegylation Assists Sln-Paclitaxel Delivery Inducing Cancer Cell Apoptosis Upon Internalization. Int. J. Pharm. 2016, 501, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.J.; Lee, M.K.; Kim, C.K. Altered Chemical and Biological Activities of All-Trans Retinoic Acid Incorporated in Solid Lipid Nanoparticle Powders. J. Control. Release 2004, 100, 53–61. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arana, L.; Bayón-Cordero, L.; Sarasola, L.I.; Berasategi, M.; Ruiz, S.; Alkorta, I. Solid Lipid Nanoparticles Surface Modification Modulates Cell Internalization and Improves Chemotoxic Treatment in an Oral Carcinoma Cell Line. Nanomaterials 2019, 9, 464. https://doi.org/10.3390/nano9030464
Arana L, Bayón-Cordero L, Sarasola LI, Berasategi M, Ruiz S, Alkorta I. Solid Lipid Nanoparticles Surface Modification Modulates Cell Internalization and Improves Chemotoxic Treatment in an Oral Carcinoma Cell Line. Nanomaterials. 2019; 9(3):464. https://doi.org/10.3390/nano9030464
Chicago/Turabian StyleArana, Lide, Laura Bayón-Cordero, Laura Isabel Sarasola, Miren Berasategi, Sandra Ruiz, and Itziar Alkorta. 2019. "Solid Lipid Nanoparticles Surface Modification Modulates Cell Internalization and Improves Chemotoxic Treatment in an Oral Carcinoma Cell Line" Nanomaterials 9, no. 3: 464. https://doi.org/10.3390/nano9030464
APA StyleArana, L., Bayón-Cordero, L., Sarasola, L. I., Berasategi, M., Ruiz, S., & Alkorta, I. (2019). Solid Lipid Nanoparticles Surface Modification Modulates Cell Internalization and Improves Chemotoxic Treatment in an Oral Carcinoma Cell Line. Nanomaterials, 9(3), 464. https://doi.org/10.3390/nano9030464