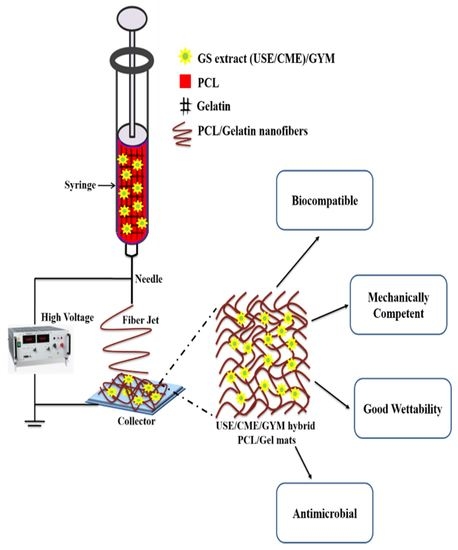
Poly-ε-Caprolactone/Gelatin Hybrid Electrospun Composite Nanofibrous Mats Containing Ultrasound Assisted Herbal Extract: Antimicrobial and Cell Proliferation Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Microbial Strains Used
2.1.2. Cell Lines Used
2.1.3. Bioactive Compound and Leaf Extracts
2.2. Processing of Gymnema sylvestre Leaves
2.3. Electrospinning of PCL/Gelatin Nanofibers
2.4. Field Emission Scanning Electron Microscopy (FESEM) Analysis
2.5. Fourier Transform Infra-Red Spectroscopy
2.6. Mechanical Properties of Hybrid Mats
2.7. Wettability Studies
2.8. Release Kinetics and Scaffold Degradation Studies
2.9. Biocompatibility Studies of USE/CME/GYM Nanofibers
2.10. Antimicrobial Studies
2.10.1. Radial Disc Diffusion Assay
2.10.2. Bacterial Cell Viability Assay
2.11. Statistical Analysis
3. Results and Discussion
3.1. FE-SEM Analysis to Visualise the Surface Morphology and Determining the Fiber Diameter Distribution
3.2. FTIR Analysis of Composite Mats
3.3. Mechanical Properties of Electrospun Hybrid Mats
3.4. Wettability of Electrospun Mats
3.5. Release Kinetics and Scaffold Degradation Studies
3.6. Cell Proliferation Assessment on the Electrospun Nanofibers
3.6.1. MTS Assay
3.6.2. F-Actin Staining
3.6.3. Collagen Expression
3.7. Antibacterial Activity of Electrospun Gymnema Sylvestre Mats
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wang, L.; Yang, J.; Ran, B.; Yang, X.; Zheng, W.; Long, Y.; Jiang, X. Small Molecular TGF- β 1 Inhibitor Loaded Electrospun Fibrous Scaffolds for Preventing Hypertrophic Scars. ACS Appl. Mater. Interfaces 2017, 9, 32545–32553. [Google Scholar] [CrossRef] [PubMed]
- Rameshbabu, A.P.; Bankoti, K.; Datta, S.; Subramani, E.; Apoorva, A.; Ghosh, P.; Maity, P.P.; Manchikanti, P.; Chaudhury, K.; Dhara, S. Silk Sponges Ornamented with Placenta-Derived Extracellular Matrix Augments Full-thickness Cutaneous Wound Healing by Stimulating Neovascularization and Cellular Migration. ACS Appl. Mater. Interfaces 2018, 10, 16977–16991. [Google Scholar] [CrossRef] [PubMed]
- Raja, I.S.; Fathima, N.N. Gelatin-Cerium Oxide Nanocomposite for Enhanced Excisional Wound Healing. ACS Appl. Biomater. 2018, 1, 487–495. [Google Scholar] [CrossRef]
- Baranowska-Korczyc, A.; Warowicka, A.; Jasiurkowska-Delaporte, M.; Grześkowiak, B.; Jarek, M.; Maciejewska, B.M.; Jurga-Stopa, J.; Jurga, S. Antimicrobial electrospun poly(ε-caprolactone) scaffolds for gingival fibroblast growth. RSC Adv. 2016, 6, 19647–19656. [Google Scholar] [CrossRef]
- Cegelski, L.; Marshall, G.R.; Eldridge, G.R.; Hultgren, S.J. The biology and future prospects of antivirulence therapies. Nat. Rev. Microbiol. 2008, 6, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Rumbo, C.; Tamayo-ramos, J.A.; Caso, M.F.; Romero-santacreu, L.; Quesada, R.; Cuesta, S. Colonization of electrospun polycaprolactone fibers by relevant pathogenic bacterial strains. ACS Appl. Mater. Interfaces 2018, 10, 11467–11473. [Google Scholar] [CrossRef] [PubMed]
- Adeli, H.; Khorasani, M.T.; Parvazinia, M. Wound dressing based on electrospun PVA/chitosan/starch nanofibrous mats: Fabrication, antibacterial and cytocompatibility evaluation and in vitro healing assay. Int. J. Biol. Macromol. 2019, 122, 238–254. [Google Scholar] [CrossRef]
- Worley, B.V.; Soto, R.J.; Kinsley, P.C.; Schoenfisch, M.H. Active Release of Nitric Oxide-Releasing Dendrimers from Electrospun Polyurethane Fibers. ACS Biomater. Sci. Eng. 2016, 2, 426–437. [Google Scholar] [CrossRef]
- Luo, D.; Zhang, X.; Shahid, S.; Cattell, M.J.; Gould, D.J.; Sukhorukov, G.B. Electrospun poly(lactic acid) fibers containing novel chlorhexidine particles with sustained antibacterial activity. Biomater. Sci. 2017, 5, 111–119. [Google Scholar] [CrossRef]
- Vigani, B.; Rossi, S.; Milanesi, G.; Bonferoni, M.; Sandri, G.; Bruni, G.; Ferrari, F. Electrospun Alginate Fibers: Mixing of Two Different Poly(ethylene oxide) Grades to Improve Fiber Functional Properties. Nanomaterials 2018, 8, 971. [Google Scholar] [CrossRef]
- Lopez de Dicastillo, C.; Patino, C.; Galotto, M.; Palma, J.; Alburquenque, D.; Escrig, J. Novel Antimicrobial Titanium Dioxide Nanotubes Obtained through a Combination of Atomic Layer Deposition and Electrospinning Technologies. Nanomaterials 2018, 8, 128. [Google Scholar] [CrossRef]
- Serio, F.; Miola, M.; Verne, E.; Pisignano, D.; Boccaccini, A.; Liverani, L. Electrospun Filaments Embedding Bioactive Glass Particles with Ion Release and Enhanced Mineralization. Nanomaterials 2019, 9, 182. [Google Scholar] [CrossRef]
- Aldana, A.; Malatto, L.; Rehman, M.; Boccaccini, A.; Abraham, G. Fabrication of Gelatin Methacrylate (GelMA) Scaffolds with Nano- and Micro-Topographical and Morphological Features. Nanomaterials 2019, 9, 120. [Google Scholar] [CrossRef]
- Deng, A.; Yang, Y.; Du, S.; Yang, S. Electrospinning of In situ crosslinked recombinant human collagen peptide/chitosan nanofibers for wound healing. Biomater. Sci. 2018, 6, 2197–2208. [Google Scholar] [CrossRef]
- Jiang, S.; Ma, B.C.; Reinholz, J.; Li, Q.; Wang, J.; Zhang, K.A.I.; Landfester, K.; Crespy, D. Efficient Nanofibrous Membranes for Antibacterial Wound Dressing and UV Protection. Appl. Mater. Interfaces 2016, 8, 29915–29922. [Google Scholar] [CrossRef]
- Bai, R.; Kang, J.; Simalou, O.; Liu, W.; Ren, H.; Gao, T.; Gao, Y.; Chen, W.; Dong, A.; Jia, R. Novel N-Br Bond-Containing N-Halamine Nanofibers with Antibacterial Activities. ACS Biomater. Sci. Eng. 2018, 4, 2193–2202. [Google Scholar] [CrossRef]
- Arslan, A.; Simsek, M.; Aldemir, S.D.; Kazaroglu, N.M.; Gumusderelioglu, M. Honey-based PET or PET/chitosan fibrous wound dressings: Effect of honey on electrospinning process. J. Biomater. Sci. Polym. Ed. 2014, 25, 999–1012. [Google Scholar] [CrossRef]
- Maleki, H.; Gharehaghaji, A.A.; Dijkstra, P.J. A novel honey-based nanofibrous scaffold for wound dressing application. J. Appl. Polym. Sci. 2013, 127, 4086–4092. [Google Scholar] [CrossRef]
- Zhang, W.; Ronca, S.; Mele, E. Electrospun Nanofibres Containing Antimicrobial Plant Extracts. Nanomaterials 2017, 7, 42. [Google Scholar] [CrossRef]
- Chuysinuan, P.; Techasakul, S.; Suksamrarn, S.; Wetprasit, N.; Hongmanee, P.; Supaphol, P. Preparation and characterization of electrospun polyacrylonitrile fiber mats containing Garcinia mangostana. Polym. Bull. 2018, 75, 1311–1327. [Google Scholar] [CrossRef]
- Jin, G.; Prabhakaran, M.P.; Kai, D.; Kumar, S.; Arunachalam, K.D.; Ramakrishna, S. Tissue engineered plant extracts as nanofibrous wound dressing. Biomaterials 2013, 34, 724–734. [Google Scholar] [CrossRef]
- Selvaraj, S.; Fathima, N.N. Fenugreek Incorporated Silk Fibroin Nanofibers—A Potential Antioxidant Scaffold for Enhanced Wound Healing. ACS Appl. Mater. Interfaces 2017, 9, 5916–5926. [Google Scholar] [CrossRef] [PubMed]
- Heo, M.; Lee, S.J.; Heo, D.N.; Lee, D.; Lim, H.N.; Moon, J.H.; Kwon, I.K. Multilayered co-electrospun scaffold containing silver sulfadiazine as a prophylactic against osteomyelitis: Characterization and biological in vitro evaluations. Appl. Surf. Sci. 2018, 432, 308–316. [Google Scholar] [CrossRef]
- Tallosy, S.P.; Janovak, L.; Nagy, E.; Deak, Á.; Juhasz, Á.; Csapo, E.; Buzas, N.; Dekany, I. Adhesion and inactivation of Gram-negative and Gram-positive bacteria on photoreactive TiO2/polymer and Ag-TiO2/polymer nanohybrid films. Appl. Surf. Sci. 2016, 371, 139–150. [Google Scholar] [CrossRef]
- Beyth, N.; Houri-Haddad, Y.; Domb, A.; Khan, W.; Hazan, R. Alternative antimicrobial approach: Nano-antimicrobial materials. Evid.-Based Complement. Altern. Med. 2015, 2015, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Lopez, K.J.; Vicente, A.A.; Reis, M.A.M.; Torres-Giner, S.; Lagaron, J.M. Antimicrobial and Antioxidant Performance of Various Essential Oils and Natural Extracts and Their Incorporation into Biowaste Derived Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Layers Made from Electrospun Ultrathin Fibers. Nanomaterials 2019, 9, 144. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, S.; Zhang, R.; Lan, W.; Qin, W. Development of Poly(lactic acid)/Chitosan Fibers Loaded with Essential Oil for Antimicrobial Applications. Nanomaterials 2017, 7, 194. [Google Scholar] [CrossRef]
- Liakos, I.L.; Holban, A.M.; Carzino, R.; Lauciello, S.; Grumezescu, A.M. Electrospun Fiber Pads of Cellulose Acetate and Essential Oils with Antimicrobial Activity. Nanomaterials 2017, 7, 84. [Google Scholar] [CrossRef]
- Dhand, C.; Harini, S.; Venkatesh, M.; Dwivedi, N.; Ng, A.; Liu, S.; Verma, N.K.; Ramakrishna, S.; Beuerman, R.W.; Loh, X.J.; et al. Multifunctional Polyphenols- and Catecholamines-Based Self-Defensive Films for Health Care Applications. ACS Appl. Mater. Interfaces 2016, 8, 1220–1232. [Google Scholar] [CrossRef]
- Chen, X.; Hu, B.; Xing, X.; Liu, Z.; Zuo, Y.; Xiang, Q. Preparation of grafted cationic polymer/silver chloride modified cellulose fibers and their antibacterial properties. J. Appl. Polym. Sci. 2015, 132, 1–7. [Google Scholar] [CrossRef]
- Fallah, M.; Bahrami, S.H.; Ranjbar-Mohammadi, M. Fabrication and characterization of PCL/gelatin/curcumin nanofibers and their antibacterial properties. J. Ind. Text. 2016, 46, 562–577. [Google Scholar] [CrossRef]
- Jain, S.; Meka, S.R.K.; Chatterjee, K. Engineering a Piperine Eluting Nanofibrous Patch for Cancer Treatment. ACS Biomater. Sci. Eng. 2016, 2, 1376–1385. [Google Scholar] [CrossRef]
- Ahmed, A.B.A.; Rao, A.S.; Rao, M.V. In vitro callus and in vivo leaf extract of Gymnema sylvestre stimulate β-cells regeneration and anti-diabetic activity in Wistar rats. Phytomedicine 2010, 17, 1033–1039. [Google Scholar] [CrossRef]
- Di Fabio, G.; Romanucci, V.; Zarrelli, M.; Giordano, M.; Zarrelli, A. C-4 gem-dimethylated oleanes of Gymnema sylvestre and their pharmacological activities. Molecules 2013, 18, 14892–14919. [Google Scholar] [CrossRef] [PubMed]
- Chodisetti, B.; Rao, K.; Giri, A. Phytochemical analysis of Gymnema sylvestre and evaluation of its antimicrobial activity. Nat. Prod. Res. 2013, 27, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.; Mishra, B.N.; Sangwan, N.S. Phytochemical and Pharmacological Properties of: An Important Medicinal Plant. Biomed. Res. Int. 2014, 2014, 830285. [Google Scholar] [CrossRef]
- David, B.C.; Sudarsanam, G. Antimicrobial activity of Gymnema sylvestre (Asclepiadaceae). J. Acute Dis. 2013, 2, 222–225. [Google Scholar] [CrossRef]
- Satdive, R.K.; Abhilash, P.; Fulzele, D.P. Antimicrobial activity of Gymnema sylvestre leaf extract. Fitoterapia 2003, 74, 699–701. [Google Scholar] [CrossRef]
- Xu, T.; Jin, W.; Wang, Z.; Cheng, H.; Huang, X.; Guo, X.; Ying, Y. Electrospun CuO-Nanoparticles-Modified Polycaprolactone @Polypyrrole Fibers: An Application to Sensing Glucose in Saliva. Nanomaterials 2018, 8, 133. [Google Scholar]
- Liverani, L.; Boccaccini, A. Versatile Production of Poly(Epsilon-Caprolactone) Fibers by Electrospinning Using Benign Solvents. Nanomaterials 2016, 6, 75. [Google Scholar] [CrossRef]
- Pal, P.; Srivas, P.K.; Dadhich, P.; Das, B.; Maulik, D.; Dhara, S. Nano-/Microfibrous Cotton-Wool-Like 3D Scaffold with Core–Shell Architecture by Emulsion Electrospinning for Skin Tissue Regeneration. ACS Biomater. Sci. Eng. 2017, 3, 3563–3575. [Google Scholar] [CrossRef]
- Blackstone, B.N.; Hahn, J.M.; Mcfarland, K.L.; Danielle, M.; Supp, D.M.; Powell, H.M. Inflammatory Response and Biomechanical Properties of Coaxial Scaffolds for Engineered Skin In Vitro and Post-Grafting. Acta Biomater. 2018, 80, 247–257. [Google Scholar] [CrossRef]
- Ramalingam, R.; Dhand, C.; Leung, C.M.; Ong, S.T.; Annamalai, S.K.; Kamruddin, M.; Verma, N.K.; Ramakrishna, S.; Lakshminarayanan, R.; Arunachalam, K.D. Antimicrobial properties and biocompatibility of electrospun poly-ε-caprolactone fibrous mats containing Gymnema sylvestre leaf extract. Mater. Sci. Eng. C 2019, 98, 503–514. [Google Scholar] [CrossRef]
- Dai, X.; Liu, J.; Zheng, H.; Wichmann, J.; Hopfner, U.; Sudhop, S.; Prein, C.; Shen, Y.; Machens, H.G.; Schilling, A.F. Nano-formulated curcumin accelerates acute wound healing through Dkk-1-mediated fibroblast mobilization and MCP-1-mediated anti-inflammation. Npg Asia Mater. 2017, 9, e368. [Google Scholar] [CrossRef]
- Verma, N.K.; Conroy, J.; Lyons, P.E.; Coleman, J.; O’Sullivan, M.P.; Kornfeld, H.; Kelleher, D.; Volkov, Y. Autophagy induction by silver nanowires: A new aspect in the biocompatibility assessment of nanocomposite thin films. Toxicol. Appl. Pharm. 2012, 264, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Dhand, C.; Balakrishnan, Y.; Ong, S.T.; Dwivedi, N.; Venugopal, J.R.; Harini, S.; Leung, C.M.; Low, K.Z.W.; Loh, X.J.; Beuerman, R.W.; et al. Antimicrobial quaternary ammonium organosilane cross-linked nanofibrous collagen scaffolds for tissue engineering. Int. J. Nanomed. 2018, 13, 4473–4492. [Google Scholar] [CrossRef] [PubMed]
- Dhand, C.; Venkatesh, M.; Barathi, V.A.; Harini, S.; Bairagi, S.; Goh Tze Leng, E.; Muruganandham, N.; Low, K.Z.W.; Fazil, M.H.U.T.; Loh, X.J.; et al. Bio-inspired crosslinking and matrix-drug interactions for advanced wound dressings with long-term antimicrobial activity. Biomaterials 2017, 138, 153–168. [Google Scholar] [CrossRef] [PubMed]
- Hadisi, Z.; Nourmohammadi, J.; Nassiri, S.M. The antibacterial and anti-inflammatory investigation of Lawsonia Inermis-gelatin-starch nano-fibrous dressing in burn wound. Int. J. Biol. Macromol. 2018, 107, 2008–2019. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.K.; Tiwari, A.P.; Pant, H.R.; Shrestha, B.K.; Kim, H.J.; Park, C.-H.; Kim, C.S. In situ generation of cellulose nanocrystals in polycaprolactone nanofibers: Effects on crystallinity, mechanical strength, biocompatibility, and biomimetic mineralization. ACS Appl. Mater. Interfaces 2015, 7, 19672–19683. [Google Scholar] [CrossRef]
- Wong, S.C.; Baji, A.; Leng, S. Effect of fiber diameter on tensile properties of electrospun poly(ε-caprolactone). Polymer 2008, 49, 4713–4722. [Google Scholar] [CrossRef]
- Kim, H.H.; Kim, M.J.; Ryu, S.J.; Ki, C.S.; Park, Y.H. Effect of fiber diameter on surface morphology, mechanical property, and cell behavior of electrospun poly(ε-caprolactone) mat. Fibers Polym. 2016, 17, 1033–1042. [Google Scholar] [CrossRef]
- Goonoo, N.; Bhaw-Luximon, A.; Rodriguez, I.A.; Wesner, D.; Schönherr, H.; Bowlin, G.L.; Jhurry, D. Poly(ester-ether)s: III. assessment of cell behaviour on nanofibrous scaffolds of PCL, PLLA and PDX blended with amorphous PMeDX. J. Mater. Chem. B 2015, 3, 673–687. [Google Scholar] [CrossRef] [Green Version]
- Munj, H.R.; Lannutti, J.J.; Tomasko, D.L. Understanding drug release from PCL/gelatin electrospun blends. J. Biomater. Appl. 2016, 31, 933–949. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; He, M.; Liu, H.; Niu, Y.; Crawford, A.; Coates, P.D.; Chen, D.; Shi, R.; Zhang, L. Drug loaded homogeneous electrospun PCL/gelatin hybrid nano fiber structures for anti-infective tissue regeneration membranes. Biomaterials 2014, 35, 9395–9405. [Google Scholar] [CrossRef] [PubMed]
- Wojtowicz, A.M.; Oliveira, S.; Carlson, M.W.; Zawadzka, A.; Rousseau, C.F.; Baksh, D. The importance of both fibroblasts and keratinocytes in a bilayered living cellular construct used in wound healing. Wound Repair Regen. 2014, 22, 246–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werner, S.; Krieg, T.; Smola, H. Keratinocyte – Fibroblast Interactions in Wound Healing. J. Invest. Derm. 2007, 127, 998–1008. [Google Scholar] [CrossRef]
- Pastar, I.; Stojadinovic, O.; Yin, N.C.; Ramirez, H.; Nusbaum, A.G.; Sawaya, A.; Patel, S.B.; Khalid, L.; Isseroff, R.R.; Tomic-canic, M. Epithelialization in Wound Healing: A Comprehensive Review. Adv. Wound Care 2014, 3, 445–464. [Google Scholar] [CrossRef] [Green Version]
- Bashur, C.A.; Dahlgren, L.A.; Goldstein, A.S. Effect of fiber diameter and orientation on fibroblast morphology and proliferation on electrospun poly(d,l-lactic-co-glycolic acid) meshes. Biomaterials 2006, 27, 5681–5688. [Google Scholar] [CrossRef]
- Sheoran, S.; Panda, B.P.; Admane, P.S.; Panda, A.K.; Wajid, S. Ultrasound-assisted extraction of gymnemic acids from Gymnema sylvestre leaves and its effect on insulin-producing RINm-5F β cell lines. Phytochem. Anal. 2015, 26, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Ahamad, J.; Amin, S.; Mir, S.R. Simultaneous quantification of gymnemic acid as gymnemagenin and charantin as b-sitosterol using validated HPTLC densitometric method. J. Chromatogr. Sci. 2015, 53, 1203–1209. [Google Scholar] [CrossRef] [PubMed]
- Arun, L.B.; Arunachalam, A.M.; Arunachalam, K.D.; Annamalai, S.K. functional properties of Gymnemic Acid Isolated from Gymnema sylvestre R Br. BMC Complement. Altern. Med. 2014, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ye, W.; Yu, B.; Zhao, S.; Wu, H.; Che, C. Two new flavonol glycosides from Gymnema sylvestre and Euphorbia ebracteolata. Carbohydr. Res. 2004, 339, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Sahu, N.P.; Mahato, S.B.; Sarkar, S.K.; Poddar, G. Triterpenoid saponins from Gymnema sylvestre. Phytochemistry 1996, 41, 1181–1185. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, T.-H.; Zhang, M.-Q.; Li, X.; Xu, Y.-J.; Jiang, H.-Y.; Liu, T.-H.; Xu, D.-M. Chemical constituents from the stems of Gymnema sylvestre. Chin. J. Nat. Med. 2014, 12, 300–304. [Google Scholar] [CrossRef]








| Sample | Tensile Modulus (MPa) | Ultimate Tensile Stress (MPa) | Ultimate Tensile Strain (%) | Toughness (MJ·m−3) |
|---|---|---|---|---|
| PCL | 5.58 ± 0.86 | 1.5 ± 0.21 | 100.5 ± 12.5 | 10.81 ± 3.25 |
| PCL/Gel | 12.52 ± 2.84 ns | 4.9 ± 0.58 ns | 75.67 ± 9.06 ns | 2.33 ± 0.92 ns |
| PCL/Gel+USE 25 | 76.32 ± 12.3 **** | 10.44 ± 1.56 *** | 69.78 ± 7.6 ns | 6.01 ± 0.5 ns |
| PCL/Gel+CME 25 | 41.73 ± 9.57 *** | 8.93 ± 1.21 *** | 58.36 ± 12.16 ns | 5.22 ± 1.19 ns |
| PCL/Gel+GYM | 11.6 ± 0.47 ns | 5.88 ± 2.4 * | 64.34 ± 5.52 ns | 3.65 ± 1.21 ns |
| Microorganism | Zone of Inhibition (mm) Excluding Fiber Diameter | |
|---|---|---|
| PCL/Gel+USE | PCL/Gel+CME | |
| Staphylococcus aureus 29213 | 15.2 ± 3.6 | 7.6 ± 2.2 |
| Methicillin-Resistant Staphylococcus aureus 700699 | 10.4 ± 1 | 5.2 ± 1.7 |
| Staphylococcus epidermidis 12228 | 18.5 ± 2.2 | 10.6 ± 1.5 |
| Pseudomonas aeruginosa 9027 | 6.3 ± 1.1 | 3.1 ± 0.3 |
| Escherichia coli 8739 | 4.2 ± 0.8 | 1.9 ± 0.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramalingam, R.; Dhand, C.; Leung, C.M.; Ezhilarasu, H.; Prasannan, P.; Ong, S.T.; Subramanian, S.; Kamruddin, M.; Lakshminarayanan, R.; Ramakrishna, S.; et al. Poly-ε-Caprolactone/Gelatin Hybrid Electrospun Composite Nanofibrous Mats Containing Ultrasound Assisted Herbal Extract: Antimicrobial and Cell Proliferation Study. Nanomaterials 2019, 9, 462. https://doi.org/10.3390/nano9030462
Ramalingam R, Dhand C, Leung CM, Ezhilarasu H, Prasannan P, Ong ST, Subramanian S, Kamruddin M, Lakshminarayanan R, Ramakrishna S, et al. Poly-ε-Caprolactone/Gelatin Hybrid Electrospun Composite Nanofibrous Mats Containing Ultrasound Assisted Herbal Extract: Antimicrobial and Cell Proliferation Study. Nanomaterials. 2019; 9(3):462. https://doi.org/10.3390/nano9030462
Chicago/Turabian StyleRamalingam, Raghavendra, Chetna Dhand, Chak Ming Leung, Hariharan Ezhilarasu, Praseetha Prasannan, Seow Theng Ong, Sundarapandian Subramanian, Mohammed Kamruddin, Rajamani Lakshminarayanan, Seeram Ramakrishna, and et al. 2019. "Poly-ε-Caprolactone/Gelatin Hybrid Electrospun Composite Nanofibrous Mats Containing Ultrasound Assisted Herbal Extract: Antimicrobial and Cell Proliferation Study" Nanomaterials 9, no. 3: 462. https://doi.org/10.3390/nano9030462
APA StyleRamalingam, R., Dhand, C., Leung, C. M., Ezhilarasu, H., Prasannan, P., Ong, S. T., Subramanian, S., Kamruddin, M., Lakshminarayanan, R., Ramakrishna, S., Verma, N. K., & Arunachalam, K. D. (2019). Poly-ε-Caprolactone/Gelatin Hybrid Electrospun Composite Nanofibrous Mats Containing Ultrasound Assisted Herbal Extract: Antimicrobial and Cell Proliferation Study. Nanomaterials, 9(3), 462. https://doi.org/10.3390/nano9030462