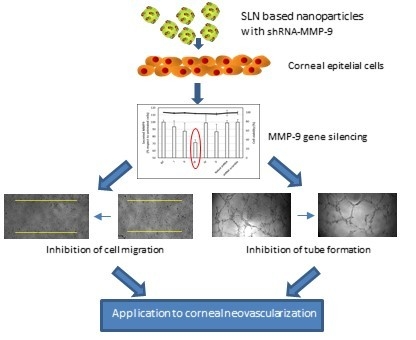
MMP-9 Downregulation with Lipid Nanoparticles for Inhibiting Corneal Neovascularization by Gene Silencing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Elaboration of the p-shRNA-MMP-9-Bearing Vectors
2.2.2. The Characterization of the Vectors
2.2.3. Cell Culture Conditions
2.2.4. In Vitro Transfection Assays
Percentage of Transfected Cells and GFP Production
Silencing of MMP-9
2.2.5. Cell Viability
2.2.6. Cellular Uptake of Non-Viral Vectors
2.2.7. Intracellular Disposition of the Vectors
2.2.8. Cell Migration Assay
2.2.9. HUVEC Tube Formation Assay
2.2.10. Statistical Analysis
3. Results
3.1. Characterization
3.2. In Vitro Transfection and Cell Viability
3.3. Cellular Uptake and Intracellular Trafficking
3.4. Migration Assay
3.5. HUVEC Tube Formation Assay
4. Discussion
5. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Torrecilla, J.; del Pozo-Rodríguez, A.; Vicente-Pascual, M.; Solinís, M.Á.; Rodríguez-Gascón, A. Targeting corneal inflammation by gene therapy: Emerging strategies for keratitis. Exp. Eye Res. 2018, 176, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.S.; Carnt, N.A.; Truong, N.R.; Pattamatta, U.; White, A.J.; Samarawickrama, C.; Cunningham, A.L. Dendritic cells in the cornea during Herpes simplex viral infection and inflammation. Surv. Ophthalmol. 2018, 63, 565–578. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, K.W.; Joo, K.; Kim, J.C. Angiogenin ameliorates corneal opacity and neovascularization via regulating immune response in corneal fibroblasts. BMC Ophthalmol. 2016, 16, 57. [Google Scholar] [CrossRef] [PubMed]
- Sivak, J.M.; Fini, M.E. MMPs in the eye: Emerging roles for matrix metalloproteinases in ocular physiology. Prog. Retin. Eye Res. 2002, 21, 1–14. [Google Scholar] [CrossRef]
- Mohan, R.; Chintala, S.K.; Jung, J.C.; Villar, W.V.; McCabe, F.; Russo, L.A.; Lee, Y.; McCarthy, B.E.; Wollenberg, K.R.; Jester, J.V.; et al. Matrix metalloproteinase gelatinase B (MMP-9) coordinates and effects epithelial regeneration. J. Biol. Chem. 2002, 277, 2065–2072. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.N.; Wang, F.; Zhou, W.; Wu, Z.Q.; Xing, Y.Q. TNF-α stimulates MMP-2 and MMP-9 activities in human corneal epithelial cells via the activation of FAK/ERK signaling. Ophthalmic Res. 2012, 48, 165–170. [Google Scholar] [CrossRef]
- Feizi, S.; Azari, A.A.; Safapour, S. Therapeutic approaches for corneal neovascularization. Eye Vis. 2017, 4, 28. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.X.; Zhao, S.Z. Gene-based therapeutic tools in the treatment of Cornea Disease. Curr. Gene Ther. 2018. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Romano, V.; Steger, B.; Kaye, S.B.; Hamill, K.J.; Willoughby, C.E. Gene-based antiangiogenic applications for corneal neovascularization. Surv. Ophthalmol. 2018, 63, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Kersey, J.P.; Broadway, D.C. Corticosteroid-induced glaucoma: A review of the literature. Eye 2006, 20, 407–416. [Google Scholar] [CrossRef]
- O’Doherty, M.; Murphy, C.C. Update on immunosuppressive therapy for corneal transplantation. Int. Ophthalmol. Clin. 2010, 5, 113–122. [Google Scholar] [CrossRef]
- Guzman-Aranguez, A.; Loma, P.; Pintor, J. Small-interfering RNAs (siRNAs) as a promising tool for ocular therapy. Br. J. Pharmacol. 2013, 170, 730–747. [Google Scholar] [CrossRef] [Green Version]
- Torrecilla, J.; Rodríguez-Gascón, A.; Solinís, M.Á.; Del Pozo-Rodríguez, A. Lipid nanoparticles as carriers for RNAi against viral infections: Current status and future perspectives. BioMed Res. Int. 2014, 2014, 161794. Available online: https://www.hindawi.com/journals/bmri/2014/161794/ (accessed on 25 March 2019). [CrossRef] [PubMed]
- Jin, X.; Sun, T.; Zhao, C.; Zheng, Y.; Zhang, Y.; Cai, W.; He, Q.; Taira, K.; Zhang, L.; Zhou, D. Strand antagonism in RNAi: An explanation of differences in potency between intracellularly expressed siRNA and shRNA. Nucleic Acids Res. 2012, 40, 1797–1806. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Gascón, A.; del Pozo-Rodríguez, A.; Isla, A. Gene Therapy in the Cornea; In: eLS; John Wiley Sons, Ltd.: Chichester, UK, 2016; Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/9780470015902.a0024274 (accessed on 25 March 2019).
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ (accessed on 8 March 2019).
- Gene Therapy Clinical Trials Worldwide. Available online: http://www.abedia.com/wiley/ (accessed on 8 March 2019).
- Mohan, R.R.; Sinha, S.; Tandon, A.; Gupta, R.; Tovey, J.C.; Sharma, A. Efficacious and safe tissue-selective controlled gene therapy approaches for the cornea. PLoS ONE 2011, 6, e18771. [Google Scholar] [CrossRef]
- Rodier, J.T.; Tripathi, R.; Fink, M.K.; Sharma, A.; Korampally, M.; Gangopadhyay, S.; Giuliano, E.A.; Sinha, P.R.; Mohan, R.R. Linear Polyethylenimine-DNA Nanoconstruct for Corneal Gene Delivery. J. Ocul. Pharmacol. Ther. 2019, 35, 23–31. [Google Scholar] [CrossRef]
- Vicente-Pascual, M.; Albano, A.; Solinís, M.Á.; Serpe, L.; Rodríguez-Gascón, A.; Foglietta, F.; Muntoni, E.; Torrecilla, J.; Pozo-Rodríguez, A.D.; Battaglia, L. Gene delivery in the cornea: In vitro & ex vivo evaluation of solid lipid nanoparticle-based vectors. Nanomedicine 2018, 13, 1847–1864. [Google Scholar]
- Delgado, D.; del Pozo-Rodríguez, A.; Solinís, M.Á.; Avilés-Triqueros, M.; Weber, B.H.; Fernández, E.; Gascón, A.R. Dextran and Protamine-Based Solid Lipid Nanoparticles as Potential Vectors for the Treatment of X-Linked Juvenile Retinoschisis. Hum. Gene Ther. 2012, 23, 345–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apaolaza, P.S.; Del Pozo-Rodríguez, A.; Solinís, M.A.; Rodríguez, J.M.; Friedrich, U.; Torrecilla, J.; Weber, B.H.; Rodríguez-Gascón, A. Structural recovery of the retina in a retinoschisin-deficient mouse after gene replacement therapy by solid lipid nanoparticles. Biomaterials 2016, 90, 40–49. [Google Scholar] [CrossRef]
- Tapeinos, C.; Marino, A.; Battaglini, M.; Migliorin, S.; Brescia, R.; Scarpellini, A.; De Julián Fernández, C.; Prato, M.; Drago, F.; Ciofani, G. Stimuli-responsive lipid-based magnetic nanovectors increase apoptosis in glioblastoma cells through synergic intracellular hyperthermia and chemotherapy. Nanoscale 2019, 11, 72–88. [Google Scholar] [CrossRef]
- Mukherjee, S.; Ray, S.; Thakur, R.S. Solid lipid nanoparticles: A modern formulation approach in drug delivery system. Indian J. Pharm. Sci. 2009, 71, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo-Rodríguez, A.; Solinís, M.Á.; Rodríguez-Gascón, A. Applications of lipid nanoparticles in gene therapy. Eur. J. Pharm. Biopharm. 2016, 109, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, L.; Serpe, L.; Foglietta, F.; Muntoni, E.; Gallarate, M.; Del Pozo Rodriguez, A.; Solinis, M.A. Application of lipid nanoparticles to ocular drug delivery. Expert Opin. Drug Deliv. 2016, 13, 1743–1757. [Google Scholar] [CrossRef] [PubMed]
- Torrecilla, J.; Del Pozo-Rodríguez, A.; Solinís, M.Á.; Apaolaza, P.S.; Berzal-Herranz, B.; Romero-López, C.; Berzal-Herranz, A.; Rodríguez-Gascón, A. Silencing of hepatitis C virus replication by a non-viral vector based on solid lipid nanoparticles containing a shRNA targeted to the internal ribosome entry site (IRES). Colloid Surf. B Biointerfaces 2016, 146, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Torrecilla, J.; Del Pozo-Rodríguez, A.; Apaolaza, P.S.; Solinís, M.Á.; Rodríguez-Gascón, A. Solid lipid nanoparticles as non-viral vector for the treatment of chronic hepatitis C by RNA interference. Int. J. Pharm. 2015, 479, 181–188. [Google Scholar] [CrossRef]
- Ke, Y.; Wu, Y.; Cui, X.; Liu, X.; Yu, M.; Yang, C.; Li, X. Polysaccharide hydrogel combined with mesenchymal stem cells promotes the healing of corneal alkali burn in rats. PLoS ONE 2015, 10, e0119725. [Google Scholar] [CrossRef]
- Carpentier, G. Angiogenesis Analyzer for Image J. Available online: http://rsb.info.nih.gov/ij/macros/toolsets/Analogenesis%20Analyzer.txt (accessed on 25 March 2019).
- Azkur, A.K.; Kim, B.; Suvas, S.; Lee, Y.; Kumaraguru, U.; Rouse, B.T. Blocking mouse MMP-9 production in tumor cells and mouse cornea by short hairpin (sh) RNA encoding plasmids. Oligonucleotides 2005, 15, 72–84. [Google Scholar] [CrossRef]
- Ruiz de Garibay, A.P.; Solinís, M.A.; del Pozo-Rodríguez, A.; Apaolaza, P.S.; Shen, J.S.; Rodríguez-Gascón, A. Solid lipid nanoparticles as non-viral vectors for gene transfection in a cell model of Fabry disease. J. Biomed. Nanotechnol. 2015, 11, 500–511. [Google Scholar] [CrossRef]
- Apaolaza, P.S.; Delgado, D.; Del Pozo-Rodríguez, A.; Rodríguez-Gascón, A.; Solinís, M.Á. A novel gene therapy vector based on hyaluronic acid and solid lipid nanoparticles for ocular diseases. Int. J. Pharm. 2014, 465, 413–426. [Google Scholar] [CrossRef]
- Delgado, D.; Del Pozo-Rodríguez, A.; Solinís, M.Á.; Rodríguez-Gascón, A. Understanding the mechanism of protamine in solid lipid nanoparticle-based lipofection: The importance of the entry pathway. Eur. J. Pharm. Biopharm. 2011, 79, 495–502. [Google Scholar] [CrossRef]
- Delgado, D.; Gascón, A.R.; Del Pozo-Rodríguez, A.; Echevarría, E.; Ruiz de Garibay, A.P.; Rodríguez, J.M.; Solinís, M.Á. Dextran-protamine-solid lipid nanoparticles as a non-viral vector for gene therapy: In vitro characterization and in vivo transfection after intravenous administration to mice. Int. J. Pharm. 2012, 425, 35–43. [Google Scholar] [CrossRef]
- Apaolaza, P.S.; Del Pozo-Rodríguez, A.; Torrecilla, J.; Rodríguez-Gascón, A.; Rodríguez, J.M.; Friedrich, U.; Weber, B.H.; Solinís, M.A. Solid lipid nanoparticle-based vectors intended for the treatment of X-linked juvenile retinoschisis by gene therapy: In vivo approaches in Rs1h-deficient mouse model. J. Control. Release 2015, 217, 273–283. [Google Scholar] [CrossRef] [PubMed]
- del Pozo-Rodríguez, A.; Delgado, D.; Solinís, M.A.; Gascón, A.R.; Pedraz, J.L. Solid lipid nanoparticles: Formulation factors affecting cell transfection capacity. Int. J. Pharm. 2007, 339, 261–268. [Google Scholar] [CrossRef]
- Ueta, M.; Kinoshita, S. Ocular surface inflammation is regulated by innate immunity. Prog. Retin. Eye Res. 2012, 31, 551–575. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K. Molecular mechanism of the disruption of barrier function in cultured human corneal epithelial cells induced by tumor necrosis factor-alpha, a proinflammatory cytokine. Nihon. Ganka Gakkai Zasshi 2010, 114, 935–943. [Google Scholar]
- Kimura, K.; Morita, Y.; Orita, T.; Haruta, J.; Takeji, Y.; Sonoda, K.H. Protection of human corneal epithelial cells from TNF-α- induced disruption of barrier function by rebamipide. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2752–2760. [Google Scholar] [CrossRef]
- Li, Q.; Jie, Y.; Wang, C.; Zhang, Y.; Guo, H.; Pan, Z. Tryptase compromises corneal epithelial barrier function. Cell Biochem. Funct. 2014, 32, 183–187. [Google Scholar] [CrossRef]
- Goktas, S.; Erdogan, E.; Sakarya, R.; Sakarya, Y.; Yılmaz, M.; Ozcimen, M.; Unlukal, N.; Alpfidan, I.; Tas, F.; Erdogan, E.; et al. Inhibition of corneal neovascularization by topical and subconjunctival tigecycline. J. Ophthalmol. 2014, 2014, 452685. Available online: https://www.hindawi.com/journals/joph/2014/452685/ (accessed on 25 March 2019). [CrossRef] [PubMed]
- Gordon, G.M.; Ledee, D.R.; Feuer, W.J.; Fini, M.E. Cytokines and signaling pathways regulating matrix metalloproteinase-9 (MMP-9) expression in corneal epithelial cellsy. J. Cell. Physiol. 2009, 221, 402–411. [Google Scholar] [CrossRef]
- Mohan, R.R.; Tovey, J.C.K.; Sharmaa, A.; Tandon, A. Gene Therapy in the Cornea: 2005-present. Prog. Retin. Eye Res. 2012, 31, 43–64. [Google Scholar] [CrossRef]
- Clements, J.L.; Dana, R. Inflammatory corneal neovascularization: Etiopathogenesis. Semin. Ophthalmol. 2011, 26, 235–245. [Google Scholar] [CrossRef]
- Haas, T.L. Endothelial cell regulation of matrix metalloproteinases. Can. J. Physiol. Pharmacol. 2005, 83, 1–7. [Google Scholar] [CrossRef]
- Ramaesh, T.; Ramaesh, K.; Riley, S.C.; West, J.D.; Dhillon, B. Effects of N-acetylcysteine on matrix metalloproteinase-9 secretion and cell migration of human corneal epithelial cells. Eye 2012, 26, 1138–1144. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Lin, X.; Liu, Z.; Wu, Y.; Yang, Y.; Ouyang, W.; Li, W.; Liu, Z. Topical application of mizoribine suppresses CD4+ T-cell-mediated pathogenesis in murine dry eye. Investig. Ophthalmol. Vis. Sci. 2017, 58, 6056–6064. [Google Scholar] [CrossRef]
- Webb, A.H.; Gao, B.T.; Goldsmith, Z.K.; Irvine, A.S.; Saleh, N.; Lee, R.P.; Lendermon, J.B.; Bheemreddy, R.; Zhang, Q.; Brennan, R.C.; et al. Inhibition of MMP-2 and MMP-9 decreases cellular migration, and angiogenesis in in vitro models of retinoblastoma. BMC Cancer 2017, 17, 434. [Google Scholar] [CrossRef]
- Lee, S.; Zheng, M.; Kim, B.; Rouse, B.T. Role of matrix metalloproteinase-9 in angiogenesis caused by ocular infection with herpes simplex virus. J. Clin. Investig. 2002, 110, 1105–1111. [Google Scholar] [CrossRef] [Green Version]
- Swamynathan, S.; Loughner, C.L.; Swamynathan, S.K. Inhibition of HUVEC tube formation via suppression of NFκB suggests an anti-angiogenic role for SLURP1 in the transparent cornea. Exp. Eye Res. 2017, 164, 118–128. [Google Scholar] [CrossRef] [PubMed]









| Vector | DX:P:p-shRNA-MMP-9:SLN Ratio | Size (nm) | ZP (mV) | PdI |
|---|---|---|---|---|
| I | 1:2:1:5 | 186 ± 5 | +40.7 ± 1.0 ** | 0.21 ± 0.01 |
| II | 1:1:1:5 | 216 ± 4 * | +36.1 ± 1.0 * | 0.28 ± 0.02 |
| III | 2:1:1:5 | 190 ± 3 | +45.1 ± 1.2 | 0.22 ± 0.01 |
| IV | 2:0.5:1:5 | 189 ± 4 | +42.8 ± 0.8 ** | 0.21 ± 0.01 |
| V | 3:1:1:5 | 182 ± 2 | +45.7 ± 1.4 | 0.23 ± 0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torrecilla, J.; Gómez-Aguado, I.; Vicente-Pascual, M.; del Pozo-Rodríguez, A.; Solinís, M.Á.; Rodríguez-Gascón, A. MMP-9 Downregulation with Lipid Nanoparticles for Inhibiting Corneal Neovascularization by Gene Silencing. Nanomaterials 2019, 9, 631. https://doi.org/10.3390/nano9040631
Torrecilla J, Gómez-Aguado I, Vicente-Pascual M, del Pozo-Rodríguez A, Solinís MÁ, Rodríguez-Gascón A. MMP-9 Downregulation with Lipid Nanoparticles for Inhibiting Corneal Neovascularization by Gene Silencing. Nanomaterials. 2019; 9(4):631. https://doi.org/10.3390/nano9040631
Chicago/Turabian StyleTorrecilla, Josune, Itziar Gómez-Aguado, Mónica Vicente-Pascual, Ana del Pozo-Rodríguez, María Ángeles Solinís, and Alicia Rodríguez-Gascón. 2019. "MMP-9 Downregulation with Lipid Nanoparticles for Inhibiting Corneal Neovascularization by Gene Silencing" Nanomaterials 9, no. 4: 631. https://doi.org/10.3390/nano9040631
APA StyleTorrecilla, J., Gómez-Aguado, I., Vicente-Pascual, M., del Pozo-Rodríguez, A., Solinís, M. Á., & Rodríguez-Gascón, A. (2019). MMP-9 Downregulation with Lipid Nanoparticles for Inhibiting Corneal Neovascularization by Gene Silencing. Nanomaterials, 9(4), 631. https://doi.org/10.3390/nano9040631