Novel Porous Nitrogen Doped Graphene/Carbon Black Composites as Efficient Oxygen Reduction Reaction Electrocatalyst for Power Generation in Microbial Fuel Cell
Abstract
:1. Introduction
2. Materials and Methods
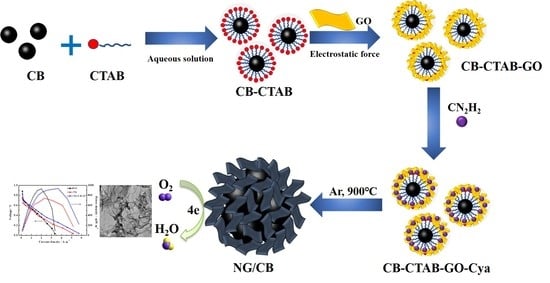
2.1. Synthesis of NG/CB Composites
2.2. Electrochemical Analysis
2.3. MFC Set-Up and Inoculation
2.4. Characterization of NG/CB-x
3. Results and Discussion
3.1. Electrocatalytic Activity for ORR in Neutral-pH Medium
3.2. Characterization of NG/CB-x
3.3. MFC Performance Using Different Electrocatalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, F.; Harnisch, F.; Schrorder, U.; Scholz, F.; Bogdanoff, P.; Herrmann, I. Challenges and constraints of using oxygen cathodes in microbial fuel cells. Environ. Sci. Technol. 2006, 40, 5193–5199. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Li, S.F.Y. Cathode reactions and applications in microbial fuel cells: A review. Crit. Rev. Environ. Sci. Technol. 2012, 42, 2504–2525. [Google Scholar] [CrossRef]
- Zhao, Y.; Watanabe, K.; Hashimoto, K. Self-supporting oxygen reduction electrocatalysts made from a nitrogen-rich network polymer. J. Am. Chem. Soc. 2012, 134, 19528–19531. [Google Scholar] [CrossRef] [PubMed]
- You, S.J.; Gong, X.B.; Wang, W.; Qi, D.P.; Wang, X.H.; Chen, X.D.; Ren, N.Q. Enhanced cathodic oxygen reduction and power production of microbial fuel cell based on noble–metal–free electrocatalyst derived from metal-organic frameworks. Adv. Energy Mater. 2016, 6. [Google Scholar] [CrossRef]
- Qu, L.T.; Liu, Y.; Baek, J.B.; Dai, L.M. Nitrogen-doped graphene as efficient metal-free electrocatalyst for oxygen reduction in fuel cells. ACS Nano 2010, 4, 1321–1326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.P.; Xia, Z.H. Mechanisms of oxygen reduction reaction on nitrogen-doped graphene for fuel cells. J. Phys. Chem. C 2011, 115, 11170–11176. [Google Scholar] [CrossRef]
- Dai, L.M.; Xue, Y.H.; Qu, L.T.; Choi, H.J.; Baek, J.B. Metal-free catalysts for oxygen reduction reaction. Chem. Rev. 2015, 115, 4823–4892. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Li, L.; Wei, Z.D. Recent advancements in Pt and Pt-free catalysts for oxygen reduction reaction. Chem. Soc. Rev. 2015, 44, 2168–2201. [Google Scholar] [CrossRef]
- Feng, L.Y.; Chen, Y.G.; Chen, L. Easy-to-operate and low-temperature synthesis of gram-scale nitrogen-doped graphene and its application as cathode catalyst in microbial fuel cells. ACS Nano 2011, 5, 9611–9618. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Wang, C.; Hou, S.X.; Yang, N. Sustainable energy recovery in wastewater treatment by microbial fuel cells: Stable power generation with nitrogen-doped graphene cathode. Environ. Sci. Technol. 2013, 47, 13889–13895. [Google Scholar] [CrossRef]
- Liu, Y.; Jin, X.J.; Dionysiou, D.D.; Liu, H.; Huang, Y.M. Homogeneous deposition-assisted synthesis of iron nitrogen composites on graphene as highly efficient non-precious metal electrocatalysts for microbial fuel cell power generation. J. Power Sources 2015, 278, 773–781. [Google Scholar] [CrossRef]
- Wu, J.J.; Ma, L.L.; Yadav, R.M.; Yang, Y.C.; Zhang, X.; Vajtai, R.; Lou, J.; Ajayan, P.M. Nitrogen-doped graphene with pyridinic dominance as a highly active and stable electrocatalyst for oxygen reduction. ACS Appl. Mater. Interfaces 2015, 7, 14763–14769. [Google Scholar] [CrossRef]
- Liu, M.K.; Song, Y.F.; He, S.X.; Tjiu, W.W.; Pan, J.S.; Xia, Y.Y.; Liu, T.X. Nitrogen-doped graphene nanoribbons as efficient metal-free electrocatalysts for oxygen reduction. ACS Appl. Mater. Interfaces 2014, 6, 4214–4222. [Google Scholar] [CrossRef]
- Shao, Y.Y.; Zhang, S.; Engelhard, M.H.; Li, G.S.; Shao, G.C.; Wang, Y.; Liu, J.; Aksay, I.A.; Lin, Y.H. Nitrogen-doped graphene and its electrochemical applications. J. Mater. Chem. 2010, 20, 7491–7496. [Google Scholar] [CrossRef]
- Ratso, S.; Kruusenberg, I.; Vikkisk, M.; Joost, U.; Shulga, E.; Kink, I.; Kallio, T.; Tammeveski, K. Highly active nitrogen-doped few-layer graphene/carbon nanotube composite electrocatalyst for oxygen reduction reaction in alkaline media. Carbon 2014, 73, 361–370. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, W.J.; Zhang, X.; Guo, L.; Hu, J.S.; Wei, Z.D.; Wan, L.J. Engineering self-assembled N-doped graphene-carbon nanotube composites towards efficient oxygen reduction electrocatalysts. Phys. Chem. Chem. Phys. 2014, 16, 13605–13609. [Google Scholar] [CrossRef] [PubMed]
- Xing, T.; Zheng, Y.; Li, L.H.; Cowie, B.C.C.; Gunzelmann, D.; Qiao, S.Z.; Huang, S.M.; Chen, Y. Observation of active sites for oxygen reduction reaction on nitrogen-doped multilayer graphene. ACS Nano 2014, 8, 6856–6862. [Google Scholar] [CrossRef] [PubMed]
- Tung, V.C.; Chen, L.M.; Allen, M.J.; Wassei, J.K.; Nelson, K.; Kaner, R.B.; Yang, Y. Low-temperature solution processing of graphene-carbon nanotube hybrid materials for high-performance transparent conductors. Nano Lett. 2009, 9, 1949–1955. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wei, T.; Shao, B.; Ma, F.Q.; Fan, Z.J.; Zhang, M.L.; Zheng, C.; Shang, Y.C.; Qian, W.Z.; Wei, F. Electrochemical properties of graphene nanosheet/carbon black composites as electrodes for supercapacitors. Carbon 2010, 48, 1731–1737. [Google Scholar] [CrossRef]
- Fan, W.; Miao, Y.E.; Huang, Y.P.; Tjiu, W.W.; Liu, T.X. Flexible free-standing 3D porous N-doped graphene-carbon nanotube hybrid paper for high-performance supercapacitors. RSC Adv. 2015, 5, 9228–9236. [Google Scholar] [CrossRef]
- Si, Y.C.; Samulski, E.T. Exfoliated graphene separated by platinum nanoparticles. Chem. Mater. 2008, 20, 6792–6797. [Google Scholar] [CrossRef]
- Chen, P.; Xiao, T.Y.; Qian, Y.H.; Li, S.S.; Yu, S.H. A Nitrogen-doped graphene/carbon nanotube nanocomposite with synergistically enhanced electrochemical activity. Adv. Mater. 2013, 25, 3192–3196. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jin, X.J.; Tuo, A.X.; Liu, H. Improved oxygen reduction reaction activity of three-dimensional porous N-doped graphene from a soft-template synthesis strategy in microbial fuel cells. RSC Adv. 2016, 6, 105211–105221. [Google Scholar] [CrossRef]
- Wang, L.; Sofer, Z.; Ambrosi, A.; Simek, P.; Pumera, M. 3D-graphene for electrocatalysis of oxygen reduction reaction: Increasing number of layers increases the catalytic effect. Electrochem. Commun. 2014, 46, 148–151. [Google Scholar] [CrossRef]
- Zhou, X.J.; Bai, Z.Y.; Wu, M.J.; Qiao, J.L.; Chen, Z.W. 3-Dimensional porous N-doped graphene foam as a non–precious catalyst for the oxygen reduction reaction. J. Mater. Chem. A 2015, 3, 3343–3350. [Google Scholar] [CrossRef]
- Wang, Z.J.; Cao, X.H.; Ping, J.F.; Wang, Y.X.; Lin, T.T.; Huang, X.; Ma, Q.L.; Wang, F.K.; He, C.B.; Zhang, H. Electrochemical doping of three-dimensional graphene networks used as efficient electrocatalysts for oxygen reduction reaction. Nanoscale 2015, 7, 9394–9398. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.D.; Qian, K.; Yang, J.; Zhang, J.; Li, L.; Yu, C.Z.; Zhao, D.Y. Functional nanoporous graphene foams with controlled pore sizes. Adv. Mater. 2012, 24, 4419–4423. [Google Scholar] [CrossRef]
- Xia, Y.D.; Yang, Z.X.; Mokaya, R. Templated nanoscale porous carbons. Nanoscale 2010, 2, 639–659. [Google Scholar] [CrossRef]
- Huang, X.D.; Sun, B.; Su, D.W.; Zhao, D.Y.; Wang, G.X. Sof-template synthesis of 3D porous graphene foams with tunable architectures for lithium-O2 batteries and oil adsorption applications. J. Mater. Chem. A 2014, 2, 7973–7979. [Google Scholar] [CrossRef]
- Huang, X.D.; Zhao, Y.F.; Ao, Z.M.; Wang, G.X. Micelle–template synthesis of nitrogen-doped mesoporous graphene as an efficient metal-free electrocatalyst for hydrogen production. Sci. Rep. 2014, 4, 7557. [Google Scholar] [CrossRef]
- Wang, L.; Sun, L.; Tian, C.G.; Tan, T.X.; Mu, G.; Zhang, H.X.; Fu, H.G. A novel soft template strategy to fabricate mesoporous carbon/graphene composites as high-performance supercapacitor electrodes. RSC Adv. 2012, 2, 8359–8367. [Google Scholar] [CrossRef]
- Fan, Z.J.; Yan, J.; Zhi, L.J.; Zhang, Q.; Wei, T.; Feng, J.; Zhang, M.L.; Qian, W.Z.; Wei, F. A three-dimensional carbon nanotube/graphene sandwich and its application as electrode in supercapacitors. Adv. Mater. 2010, 22, 3723–3728. [Google Scholar] [CrossRef] [PubMed]
- Jha, N.; Ramesh, P.; Bekyarova, E.; Itkis, M.E.; Haddon, R.C. High energy density supercapacitor based on a hybrid carbon nanotube-reduced graphite oxide architecture. Adv. Energy Mater. 2012, 2, 438–444. [Google Scholar] [CrossRef]
- Dong, X.C.; Ma, Y.W.; Zhu, G.Y.; Huang, Y.X.; Wang, J.; Chan-Park, M.B.; Wang, L.H.; Huang, W.; Chen, P. Synthesis of graphene-carbon nanotube hybrid foam and its use as a novel three–dimensional electrode for electrochemical sensing. J. Mater. Chem. 2012, 22, 17044–17048. [Google Scholar] [CrossRef]
- Han, P.X.; Yue, Y.H.; Liu, Z.H.; Xu, W.; Zhang, L.X.; Xu, H.X.; Dong, S.M.; Cui, G.L. Graphene oxide nanosheets/multi-walled carbon nanotubes hybrid as an excellent electrocatalytic material towards VO2+/VO2+ redox couples for vanadium redox flow batteries. Energy Environ. Sci. 2011, 4, 4710–4717. [Google Scholar] [CrossRef]
- Su, D.W.; Cortie, M.; Wang, G.X. Fabrication of N–doped graphene–carbon nanotube hybrids from prussian blue for lithium-sulfur batteries. Adv. Energy Mater. 2017, 7. [Google Scholar] [CrossRef]
- Shu, C.Z.; Li, B.; Zhang, B.S.; Su, D.S. Hierarchical Nitrogen–doped graphene/carbon nanotube composite cathode for lithium-oxygen batteries. Chemsuschem 2015, 8, 3973–3976. [Google Scholar] [CrossRef]
- Su, Q.; Liang, Y.Y.; Feng, X.L.; Mullen, K. Towards free-standing graphene/carbon nanotube composite films via acetylene-assisted thermolysis of organocobalt functionalized graphene sheets. Chem. Commun. 2010, 46, 8279–8281. [Google Scholar] [CrossRef]
- Liang, X.; Hart, C.; Pang, Q.; Garsuch, A.; Weiss, T.; Nazar, L.F. A highly efficient polysulfide mediator for lithium–sulfur batteries. Nat. Commun. 2015, 6, 5682. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Jiao, Y.; Chen, J.; Liu, J.; Liang, J.; Du, A.; Zhang, W.M.; Zhu, Z.H.; Smith, S.C.; Jaroniec, M.; et al. Nanoporous graphitic–C3N4@carbon metal–free electrocatalysts for highly efficient oxygen reduction. J. Am. Chem. Soc. 2011, 133, 20116–20119. [Google Scholar] [CrossRef]
- Dong, H.; Yu, H.; Wang, X.; Zhou, Q.; Feng, J. A novel structure of scalable air-cathode without Nafion and Pt by rolling activated carbon and PTFE as catalyst layer in microbial fuel cells. Water Res. 2012, 46, 5777–5787. [Google Scholar] [CrossRef]
- Li, Y.G.; Zhou, W.; Wang, H.L.; Xie, L.M.; Liang, Y.Y.; Wei, F.; Idrobo, J.C.; Pennycook, S.J.; Dai, H.J. An oxygen reduction electrocatalyst based on carbon nanotube-graphene complexes. Nat. Nanotechnol. 2012, 7, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Saito, T.; Cheng, S.A.; Hickner, M.A.; Logan, B.E. Microbial fuel cell cathodes with poly(dimethylsiloxane) diffusion layers constructed around stainless steel mesh current collectors. Environ. Sci. Technol. 2010, 44, 1490–1495. [Google Scholar] [CrossRef]
- Lovley, D.R.; Phillips, E.J.P. Novel mode of microbial energy metabolism: Organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl. Environ. Microbiol. 1988, 54, 1472–1480. [Google Scholar] [PubMed]
- Li, D.; Qu, Y.P.; Liu, J.; He, W.H.; Wang, H.M.; Feng, Y.J. Using ammonium bicarbonate as pore former in activated carbon catalyst layer to enhance performance of air cathode microbial fuel cell. J. Power Sources 2014, 272, 909–914. [Google Scholar] [CrossRef]
- Wang, H.M.; Li, D.; Liu, J.; Liu, L.C.; Zhou, X.T.; Qu, Y.P.; Zhang, J.; Feng, Y.J. Microwave-assisted synthesis of nitrogen–doped activated carbon as an oxygen reduction catalyst in microbial fuel cells. RSC Adv. 2016, 6, 90410–90416. [Google Scholar] [CrossRef]
- McAllister, M.J.; Li, J.L.; Adamson, D.H.; Schniepp, H.C.; Abdala, A.A.; Liu, J.; Herrera–Alonso, M.; Milius, D.L.; Car, R.; Prud’homme, R.K.; et al. Single sheet functionalized graphene by oxidation and thermal expansion of graphite. Chem. Mater. 2007, 19, 4396–4404. [Google Scholar] [CrossRef]
- Santoro, C.; Kodali, M.; Kabir, S.; Soavi, F.; Serov, A.; Atanassov, P. Three-dimensional graphene nanosheets as cathode catalysts in standard and supercapacitive microbial fuel cell. J. Power Sources 2017, 356, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Jaouen, F.; Proietti, E.; Lefèvre, M.; Chenitz, R.; Dodelet, J.P.; Wu, G.; Chung, H.T.; Johnston, C.M.; Zelenay, P. Recent advances in non-precious metal catalysis for oxygen–reduction reaction in polymer electrolyte fuel cells. Energy Environ. Sci. 2011, 4, 114–130. [Google Scholar] [CrossRef]
- Videla, A.H.A.M.; Zhang, L.; Kim, J.; Zeng, J.; Francia, C.; Zhang, J.; Specchia, S. Mesoporous carbons supported non–noble metal Fe-N(X) electrocatalysts for PEM fuel cell oxygen reduction reaction. J. Appl. Electrochem. 2013, 43, 159–169. [Google Scholar] [CrossRef]
- Mo, Z.; Zheng, R.; Peng, H.; Liang, H.; Liao, S. Nitrogen–doped graphene prepared by a transfer doping approach for the oxygen reduction reaction application. J. Power Sources 2014, 245, 801–807. [Google Scholar] [CrossRef]
- Su, Y.H.; Jiang, H.L.; Zhu, Y.H.; Zou, W.J.; Yang, X.L.; Chen, J.D.; Li, C.Z. Hierarchical porous iron and nitrogen co-doped carbons as efficient oxygen reduction electrocatalysts in neutral media. J. Power Sources 2014, 265, 246–253. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Li, K.X.; Liu, D.; Yang, T.T.; Wang, J.J.; Lu, J.F. The influence and mechanism of different acid treatment to activated carbon used as air-breathing cathode catalyst of microbial fuel cell. Electrochim. Acta 2017, 246, 830–840. [Google Scholar] [CrossRef]
- Parvez, K.; Yang, S.B.; Hernandez, Y.; Winter, A.; Turchanin, A.; Feng, X.L.; Mullen, K. Nitrogen-doped graphene and its iron-based composite as efficient electrocatalysts for oxygen reduction reaction. ACS Nano 2012, 6, 9541–9550. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.S.; Zhang, Q.; Dai, L.M. Highly efficient metal-free growth of nitrogen-doped single-walled carbon nanotubes on plasma-etched substrates for oxygen reduction. J. Am. Chem. Soc. 2010, 132, 15127–15129. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.Y.; Zhang, X.Y.; Lv, R.T.; Chen, X.; Xue, B.R.; Liang, P.; Huang, X. Binder-free nitrogen-doped graphene catalyst air-cathodes for microbial fuel cells. J. Mater. Chem. A 2016, 4, 12387–12391. [Google Scholar] [CrossRef]
- Guo, D.H.; Shibuya, R.; Akiba, C.; Saji, S.; Kondo, T.; Nakamura, J. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science 2016, 351, 361–365. [Google Scholar] [CrossRef] [PubMed]
- ElMekawy, A.; Hegab, H.M.; Losic, D.; Saint, C.P.; Pant, D. Applications of graphene in microbial fuel cells: The gap between promise and reality. Renew. Sustain. Energy Rev. 2017, 72, 1389–1403. [Google Scholar] [CrossRef]







| Samples | Onset Potential/V vs. RHE | n | H2O2 Yield/% | 10−2 j0/mA·cm−2 | Rs/Ω·cm2 | Rct/Ω·cm2 |
|---|---|---|---|---|---|---|
| NG | 0.798 | 3.58 ± 0.06 | 20.73 ± 3.15 | 1.06 | 5.85 | 226.7 |
| NG/CB-1 | 0.805 | 3.86 ± 0.04 | 7.35 ± 3.00 | 1.30 | 5.56 | 177.4 |
| NG/CB-2 | 0.832 | 30.87 ± 0.03 | 6.23 ± 1.50 | 1.83 | 5.75 | 138.2 |
| NG/CB-5 | 0.836 | 3.88 ± 0.02 | 5.81 ± 1.16 | 1.52 | 5.83 | 144.8 |
| NG/CB-10 | 0.864 | 3.92 ± 0.02 | 3.85 ± 0.93 | 2.85 | 5.38 | 108.7 |
| NG/CB-20 | 0.798 | 3.71 ± 0.05 | 14.70 ± 2.52 | 1.20 | 5.79 | 200.8 |
| Parameters | NG | NG/CB-1 | NG/CB-2 | NG/CB-5 | NG/CB-10 | NG/CB-20 |
|---|---|---|---|---|---|---|
| SBET/m2·g−1 | 336.62 | 252.58 | 331.14 | 371.52 | 376.94 | 382.91 |
| Smicropore/m2·g−1 | 43.73 | 14.47 | 71.8 | 83.14 | 52.54 | 59.49 |
| Smesopore/m2·g−1 | 292.89 | 238.11 | 259.34 | 288.38 | 324.40 | 323.42 |
| Total Vpore/cm3·g−1 | 0.76 | 0.58 | 0.67 | 0.69 | 0.77 | 0.77 |
| Vmicropore/cm3·g−1 | 0.07 | 0.03 | 0.05 | 0.06 | 0.07 | 0.08 |
| Vmesopore/cm3·g−1 | 0.69 | 0.55 | 0.62 | 0.63 | 0.70 | 0.69 |
| Mean pore diameter/nm | 9.02 | 6.73 | 8.09 | 7.35 | 8.05 | 8.34 |
| ID/IG | 1.17 | 1.04 | 1.05 | 1.08 | 1.09 | 1.14 |
| C1s/at.% | 88.80 | 93.80 | 90.27 | 89.64 | 87.80 | 88.55 |
| N1s/at.% | 6.57 | 3.14 | 5.95 | 6.34 | 7.79 | 7.19 |
| Pyridinic N | 2.63 (40.02%) a | 1.11 (35.39%) | 2.56 (43.05%) | 2.13 (33.64%) | 3.37 (43.26%) | 3.15 (43.78%) |
| Pyrrolic N | 1.95 (29.67%) | 1.00 (31.73%) | 1.70 (28.51%) | 2.04 (32.17%) | 2.26 (28.99%) | 2.03 (28.18%) |
| Graphitic N | 1.99 (30.31%) | 1.03 (32.88%) | 1.69 (28.44%) | 2.17 (34.19%) | 2.16 (27.75%) | 2.01 (28.03%) |
| O1s/at.% | 4.63 | 3.06 | 3.78 | 4.02 | 4.41 | 4.26 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Liu, Z.; Liu, H.; Liao, M. Novel Porous Nitrogen Doped Graphene/Carbon Black Composites as Efficient Oxygen Reduction Reaction Electrocatalyst for Power Generation in Microbial Fuel Cell. Nanomaterials 2019, 9, 836. https://doi.org/10.3390/nano9060836
Liu Y, Liu Z, Liu H, Liao M. Novel Porous Nitrogen Doped Graphene/Carbon Black Composites as Efficient Oxygen Reduction Reaction Electrocatalyst for Power Generation in Microbial Fuel Cell. Nanomaterials. 2019; 9(6):836. https://doi.org/10.3390/nano9060836
Chicago/Turabian StyleLiu, Yuan, Zhimei Liu, Hong Liu, and Meiling Liao. 2019. "Novel Porous Nitrogen Doped Graphene/Carbon Black Composites as Efficient Oxygen Reduction Reaction Electrocatalyst for Power Generation in Microbial Fuel Cell" Nanomaterials 9, no. 6: 836. https://doi.org/10.3390/nano9060836
APA StyleLiu, Y., Liu, Z., Liu, H., & Liao, M. (2019). Novel Porous Nitrogen Doped Graphene/Carbon Black Composites as Efficient Oxygen Reduction Reaction Electrocatalyst for Power Generation in Microbial Fuel Cell. Nanomaterials, 9(6), 836. https://doi.org/10.3390/nano9060836