A Model for Non-Arrhenius Ionic Conductivity
Abstract
:1. Introduction
2. Model of Non-Arrhenius Ionic Conductivity
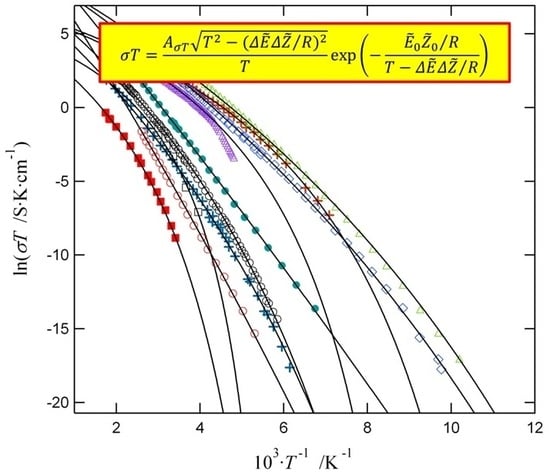
2.1. Ionic Conductivity in Solids
2.2. Ionic Conductivity in Liquids
3. Possible Size Effect
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kincs, J.; Martin, S.W. Non-Arrhenius conductivity in glass: Mobility and conductivity saturation effects. Phys. Rev. Lett. 1996, 76, 70–73. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, S.; Shiotsu, T.; Hayashi, S. Non-Arrhenius temperature dependence of conductivity in lanthanum lithium tantalate. J. Ceram. Soc. Jpn. 2005, 113, 317–319. [Google Scholar] [CrossRef]
- Šalkus, T.; Kazakevičius, E.; Kežionis, A.; Orliukas, A.F.; Badot, J.C.; Bohnke, O. Determination of the non-Arrhenius behavior of the bulk conductivity of fast ionic conductors LLTO at high temperature. Solid State Ion. 2011, 188, 69–72. [Google Scholar] [CrossRef]
- Murugavel, S. Origin of non-Arrhenius conductivity in fast ion conducting glasses. Phys. Rev. B 2005, 72, 134204. [Google Scholar] [CrossRef]
- Kuwata, N.; Saito, T.; Tatsumisago, M.; Minami, T.; Kawamura, J. Non-Arrhenius ionic conductivity in α-AgI-stabilized composites and rapid quenched glasses. Solid State Ion. 2004, 175, 679–682. [Google Scholar] [CrossRef]
- Ribes, M.; Taillades, G.; Pradel, A. Non-Arrhenius conductivity in glassy and crystallized fast ion conductors. A manifestation of cationic disorder. Solid State Ion. 1998, 105, 159–165. [Google Scholar] [CrossRef]
- León, C.; Santamaría, J.; París, M.A.; Sanz, J.; Ibarra, J.; Várez, A. Non-Debye conductivity relaxation in the non-Arrhenius Li0.5La0.5TiO3 fast ionic conductor. A nuclear magnetic resonance and complex impedance study. J. Non-Cryst. Solids 1998, 235–237, 753–760. [Google Scholar] [CrossRef]
- Mykolaychuk, O.G.; Moroz, N.V.; Demchenko, P.Yu.; Akselrud, L.G.; Gladyshevskii, R.E. Electrical conductivity of Ag8SnS6-Ag2SnS3-AgBr alloys. Inorg. Mater. 2010, 46, 707–710. [Google Scholar] [CrossRef]
- Balapanov, M.K.; Zinnurov, I.B.; Mukhamed’yanov, U.K.; Musalimov, R.S. Ionic conduction and chemical diffusion in Cu2-xS codoped with magnesium and aluminum. Inorg. Mater. 2007, 43, 449–451. [Google Scholar] [CrossRef]
- París, M.A.; Sanz, J.; León, C.; Santamaría, J.; Ibarra, J.; Várez, A. Li mobility in the orthorhombic Li0.18La0.61TiO3 perovskite studied by NMR and impedance spectroscopies. Chem. Mater. 2000, 12, 1694–1701. [Google Scholar] [CrossRef]
- Jimenez, R.; Rivera, A.; Varez, A.; Sanz, J. Li mobility in Li0.5-xNaxLa0.5TiO3 perovskites (0≤x≤0.5) Influence of structural and compositional parameters. Solid State Ion. 2009, 180, 1362–1371. [Google Scholar] [CrossRef]
- Tong, X.; Thangadurai, V.; Wachsman, E.D. Highly conductive Li garnets by a multielement doping strategy. Inorg. Chem. 2015, 54, 3600–3607. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, H.S.; Cho, M.S.; Kalubarme, R.S.; Lee, J.S.; Jung, K.N.; Shin, K.H.; Park, C.J. Influence of B2O3 addition on the ionic conductivity of Li1.5Al0.5Ge1.5(PO4)3 glass ceramics. J. Power Sour. 2013, 241, 502–508. [Google Scholar] [CrossRef]
- Sigaryov, S. Vogel-Fulcher-Tammann behavior of ionic conductivity in KTiOPO4. J. Phys. D: Appl. Phys. 1993, 26, 1326–1327. [Google Scholar] [CrossRef]
- Kharton, V.V.; Marques, F.M.B.; Atkinson, A. Transport properties of solid oxide electrolyte ceramics: A brief review. Solid State Ion. 2004, 174, 135–149. [Google Scholar] [CrossRef]
- Badwal, S.P.S.; Ciacchi, F.T.; Milosevic, D. Scandia-zirconia electrolytes for intermediate temperature solid oxide fuel cell operation. Solid State Ion. 2000, 136–137, 91–99. [Google Scholar] [CrossRef]
- Arikawa, H.; Nishiguchi, H.; Ishihara, T.; Takita, Y. Oxide ion conductivity in Sr-doped La10Ge6O27 apatite oxide. Solid State Ion. 2000, 136–137, 31–37. [Google Scholar] [CrossRef]
- Zerouale, A.; Cros, B.; Deroide, B.; Ribes, M. Electrical properties of Ag7GeSe5I. Solid State Ion. 1988, 28–30, 1317–1319. [Google Scholar] [CrossRef]
- Itoh, M.; Inaguma, Y.; Jung, W.H.; Chen, L.; Nakamura, T. High lithium ion conductivity in the perovskite-type compounds Ln1/2Li1/2TiO3 (Ln=La,Pr,Nd,Sm). Solid State Ion. 1994, 70–71, 203–207. [Google Scholar] [CrossRef]
- Fehr, T.; Schmidbauer, E. Electrical conductivity of Li2TiO3 ceramics. Solid State Ion. 2007, 178, 35–41. [Google Scholar] [CrossRef]
- Daugėla, S.; Kežionis, A.; Šalkus, T.; Orliukas, A.F.; Belous, A.G.; V’yunov, O.I.; Kobylianska, S.D.; Vasylechko, L.O. Peculiarities of ionic conduction in Li0.5-yNayLi0.5Nb2O6 system at high temperatures. Solid State Ion. 2017, 300, 86–90. [Google Scholar] [CrossRef]
- Mei, Q.; Meyer, B.; Martin, D.; Martin, S.W. Ion trapping model and the non-Arrhenius ionic conductivity in fast ion conducting glasses. Solid State Ion. 2004, 168, 75–85. [Google Scholar] [CrossRef]
- Ngai, K.L.; Rizos, A.K. Parameterless explanation of the non-Arrhenius conductivity in glassy fast ionic conductors. Phys. Rev. Lett. 1996, 76, 1296–1299. [Google Scholar] [CrossRef] [PubMed]
- Maass, P.; Meyer, M.; Bunde, A.; Dieterich, W. Microscopic explanation of the non-Arrhenius conductivity in glassy fast ionic conductors. Phys. Rev. Lett. 1996, 77, 1528–1531. [Google Scholar] [CrossRef] [PubMed]
- Indoh, T.; Aniya, M. A simple model for the non-Arrhenius ionic conductivity in superionic glasses. J. Phys. Soc. Jpn. 2010, 79 (Suppl. A), 113–114. [Google Scholar] [CrossRef]
- Okada, Y.; Ikeda, M.; Aniya, M. Non-Arrhenius ionic conductivity in solid electrolytes: A theoretical model and its relation with the bonding nature. Solid State Ion. 2015, 281, 43–48. [Google Scholar] [CrossRef]
- Palchucan, C.A.; Peña Lara, D.; Correa, H. A unified equation for modeling the dependency of conductivity with temperature in ionic systems. Physica A 2019, 525, 635–641. [Google Scholar] [CrossRef]
- Aniya, M. A model for the fragility of the melt. J. Therm. Anal. Calorim. 2002, 69, 971–978. [Google Scholar] [CrossRef]
- Ikeda, M.; Aniya, M. Understanding the Vogel-Fulcher-Tammann law in terms of the bond strength-coordination number fluctuation model. J. Non-Cryst. Solids 2013, 371–372, 53–57. [Google Scholar] [CrossRef]
- Ikeda, M.; Aniya, M. Elastic properties and activation energy profiles of viscosity in Ge-Se system across the glass-transition range. J. Non-Cryst. Solids 2016, 431, 52–56. [Google Scholar] [CrossRef]
- Sun, C.Q. Size dependence of nanostructures: Impact of bond order deficiency. Prog. Solid State Chem. 2007, 35, 1–159. [Google Scholar] [CrossRef]
- Maier, J. Nanoionics: Size effects and storage in small systems. J. Electroceram. 2015, 34, 69–73. [Google Scholar] [CrossRef]
- Qi, W. Nanoscopic thermodynamics. Acc. Chem. Res. 2016, 49, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Zwanzig, R. On the relation between self-diffusion and viscosity of liquids. J. Chem. Phys. 1983, 79, 4507–4508. [Google Scholar] [CrossRef]
- Moynihan, C.T.; Gavin, D.L.; Syed, R. Pre-exponential term in the Arrhenius equation for electrical conductivity of glass. J. Phys. Colloq. 1982, 43, 395–398. [Google Scholar] [CrossRef]
- Bendler, J.T.; Fontanella, J.J.; Shlesinger, M.F.; Wintersgill, M.C. The need to reconsider traditional free volume theory for polymer electrolytes. Electrochim. Acta 2003, 48, 2267–2272. [Google Scholar] [CrossRef]
- Rao, K.J. Structural Chemistry of Glasses; Elsevier: Oxford, UK, 2002; p. 206. ISBN 0-08-043958-6. [Google Scholar]
- Souquet, J.L.; Ferreira Nascimento, M.C.; Martins Rodrigues, A.C. Charge carrier concentration and mobility in alkali silicates. J. Chem. Phys. 2010, 132, 034704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeda, M.; Aniya, M. A relationship between diffusivity and cooperativity of supercooled liquids in the proximity of glass transition. J. Non-Cryst. Solids 2014, 383, 44–48. [Google Scholar] [CrossRef]
- Ikeda, M.; Aniya, M. Analysis of the ionic conductivity in lithium salt-containing ionic liquids based on the bond strength-coordination number fluctuation model. Solid State Ion. 2014, 262, 476–481. [Google Scholar] [CrossRef]
- Ikeda, M.; Aniya, M. Ionization condition scaled with molecular cooperativity in ionic liquids-lithium salt mixtures. In Proceedings of the 14th Asian Conference on Solid State Ionics (ACSSI 2014), Singapore, 24–27 June 2014; pp. 207–216. [Google Scholar] [CrossRef]
- Saito, Y.; Umecky, T.; Niwa, J.; Sakai, T.; Maeda, S. Existing condition and migration property of ions in lithium electrolytes with ionic liquid solvent. J. Phys. Chem. B 2007, 111, 11794–11802. [Google Scholar] [CrossRef]
- Tokuda, H.; Hayamizu, K.; Ishii, K.; Susan, M.A.B.H.; Watanabe, M. Physicochemical properties and structures of room temperature ionic liquids. 2. Variation of alkyl chain length in imidazolium cation. J. Phys. Chem. B 2005, 109, 6103–6110. [Google Scholar] [CrossRef] [PubMed]
- Fukao, K.; Miyamoto, Y. Slow Dynamics near glass transitions in thin polymer films. Phys. Rev. E 2001, 64, 011803. [Google Scholar] [CrossRef]
- Taniguchi, N.; Fukao, K.; Sotta, P.; Long, D.R. Dielectric relaxation of thin films of polyamide random copolymers. Phys. Rev. E 2015, 91, 052605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madkour, S.; Szymoniak, P.; Hertwig, A.; Heidari, M.; von Klitzing, R.; Napolitano, S.; Sferrazza, M.; Schönhals, A. Decoupling of dynamic and thermal glass transition in thin films of PVME/PS blend. ACS Macro Lett. 2017, 6, 1156–1161. [Google Scholar] [CrossRef]
- Vyas, M.K.; Chandra, A. Role of organic/inorganic salts and nanofillers in polymer nanocomposites: Enhanced conduction, rheological, and thermal properties. J. Mater. Sci. 2018, 53, 4987–5003. [Google Scholar] [CrossRef]




| Materials | Ref. | |||
|---|---|---|---|---|
| Ag7GeS5I | 910 | 74 | 6.28 | [6] |
| Ag6SnS4Br2 | 950 | 95 | 6.19 | [8] |
| 60AgI+13.3Ag2O+26.7MoO3 | 1850 | 33 | 9.29 | [5] |
| 74AgI+8.7Ag2O+17.3MoO3 | 1600 | 37 | 9.20 | [5] |
| Li0.5La0.5TiO3 | 2200 | 70 | 7.31 | [7] |
| Li0.18La0.61TiO3 | 2500 | 58 | 6.79 | [10] |
| Li0.5-xNaxLa0.5TiO3 (x = 0.0) | 3400 | 10 | 10.81 | [11] |
| Li1.47Al0.49Ge1.49(PO4)2.97 | 1120 | 156 | 4.60 | [13] |
| 0.8Na2S+0.2B2S3 | 4340 | 20 | 11.39 | [1] |
| KTiOPO4 | 1990 | 139 | 4.26 | [14] |
| Materials | (nm) | (K) | (K) |
|---|---|---|---|
| a-Polystyrene | 18 | 1887 | 313 |
| 247 | 1733 | 324 | |
| Polyamide copolymer PA66/6I | 40 | 1730 | 292 |
| 56 | 1569 | 298 | |
| 99 | 1458 | 295 | |
| 114 | 1280 | 302 | |
| 556 | 1076 | 307 | |
| PVME/PS blend | 9 | 537 | 354 |
| 28 | 1166 | 344 | |
| 50 | 1153 | 327 | |
| 84 | 1212 | 330 | |
| 148 | 1491 | 304 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aniya, M.; Ikeda, M. A Model for Non-Arrhenius Ionic Conductivity. Nanomaterials 2019, 9, 911. https://doi.org/10.3390/nano9060911
Aniya M, Ikeda M. A Model for Non-Arrhenius Ionic Conductivity. Nanomaterials. 2019; 9(6):911. https://doi.org/10.3390/nano9060911
Chicago/Turabian StyleAniya, Masaru, and Masahiro Ikeda. 2019. "A Model for Non-Arrhenius Ionic Conductivity" Nanomaterials 9, no. 6: 911. https://doi.org/10.3390/nano9060911
APA StyleAniya, M., & Ikeda, M. (2019). A Model for Non-Arrhenius Ionic Conductivity. Nanomaterials, 9(6), 911. https://doi.org/10.3390/nano9060911