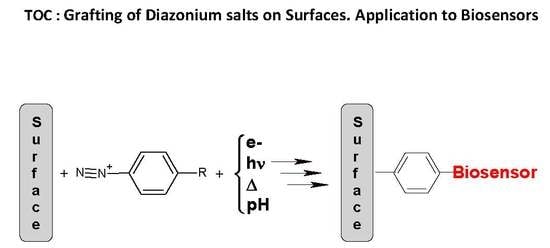
Grafting of Diazonium Salts on Surfaces: Application to Biosensors
Abstract
:1. An Overview of the Reactions of Diazonium Salts with Surfaces
1.1. The Principle of the Reaction
- * Diazonium salts are easily synthetized (isolated or not) from aromatic amines, many of which are commercially available.
- * All surfaces can be modified by this reaction, conductive or not.
- * The reaction can be performed by electrochemistry, spontaneously, by photochemistry, and by other methods.
- * The resulting modification is very stable due to the formation of a covalent bond between the surface and the aryl group.
- * The key species of this reaction is an aryl radical, and this reaction presents the typical behavior of radical reactions.
- * The reaction provides most often disordered oligomers (“multilayers”).
1.2. Synthesis and Stability of Diazonium Salts
1.3. Different Grafting Methods of Diazonium Salts
1.4. The Different Surfaces That Can be Grafted
1.5. The Surface Aryl Bond
1.6. The Structure of the Grafted Film
2. Applications to Biosensors
2.1. Detection of Small Molecules of Biological Interest
2.2. Detection of Polypeptides and Proteins
2.3. Detection of DNA
2.4. Cells
3. Concluding Remarks
Funding
Conflicts of Interest
References
- Allongue, P.; Delamar, M.; Desbat, B.; Fagebaume, O.; Hitmi, R.; Pinson, J.; Savéant, J.-M. Covalent Modification of Carbon Surfaces by Aryl Radicals Generated from the Electrochemical Reduction of Diazonium Salts. J. Am. Chem. Soc. 1997, 119, 201–207. [Google Scholar] [CrossRef]
- Berisha, A.; Chehimi, M.M.; Pinson, J.; Podvorica, F.I. Electrode surface modification using diazonium salts. Electroanal. Chem. 2016, 26, 115–225. [Google Scholar]
- Aryl Diazonium Salts. New Coupling Agents in Polymer and Surface Science; Chehimi, M.M., Ed.; Wiley-VCH: Weinheim, Germany, 2012. [Google Scholar]
- Jiang, C.; Moraes Silva, S.; Fan, S.; Wu, Y.; Alam, M.T.; Liu, G.; Gooding, J.J. Aryldiazonium salt derived mixed organic layers: From surface chemistry to their applications. J. Electroanal. Chem. 2017, 785, 265–278. [Google Scholar] [CrossRef]
- Assresahegn, B.D.; Brousse, T.; Belanger, D. Advances on the use of diazonium chemistry for functionalization of materials used in energy storage systems. Carbon 2015, 92, 362–381. [Google Scholar] [CrossRef]
- Delaporte, N.; Belanger, R.L.; Lajoie, G.; Trudeau, M.; Zaghib, K. Multi-carbonyl molecules immobilized on high surface area carbon by diazonium chemistry for energy storage applications. Electrochim. Acta 2019, 308, 99–114. [Google Scholar] [CrossRef]
- Bondarev, A.A.; Naumov, E.V.; Kassanova, A.Z.; Krasnokutskaya, E.A.; Stankevich, K.S.; Filimonov, V.D. First Study of the Thermal and Storage Stability of Arenediazonium Triflates Comparing to 4-Nitrobenzenediazonium Tosylate and Tetrafluoroborate by Calorimetric Methods. Org. Process Res. Dev. 2019, 23, 2405–2415. [Google Scholar] [CrossRef]
- Pazo-Llorente, R.; Bravo-Diaz, C.; Gonzalez-Romero, E. pH Effects on Ethanolysis of Some Arenediazonium Ions: Evidence for Homolytic Dediazoniation Proceeding through Formation of Transient Diazo Ethers. Eur. J. Org. Chem. 2004, 221–3226. [Google Scholar] [CrossRef]
- Pazo-Llorente, R.; Maskill, H.; Bravo-Diaz, C.; Gonzalez-Romero, E. Dediazoniation of 4-Nitrobenzenediazonium Ions in Acidic MeOH/H2O Mixtures: Role of Acidity and MeOH Concentration on the Formation of Transient Diazo Ethers that Initiate Homolytic Dediazoniation. Eur. J. Org. Chem. 2006, 201–2209. [Google Scholar] [CrossRef]
- Berisha, A.; Combellas, C.; Kanoufi, F.; Decorse, P.; Oturan, N.; Médard, J.; Seydou, M.; Maurel, M.; Pinson, J. Some Theoretical and Experimental Insights on the Mechanistic Routes Leading to the Spontaneous Grafting of Gold Surfaces by Diazonium Salts. Langmuir 2017, 33, 8730–8738. [Google Scholar] [CrossRef]
- Bui-Thi-Tuyet, V.; Cannizzo, C.; Legros, C.; Andrieux, M.; Chaussé, A. Modification of fluorine-doped tin oxide surface: Optimization of the electrochemical grafting of diazonium salt. Surf. Interfaces 2019, 15, 110–116. [Google Scholar] [CrossRef]
- Griffete, N.; Ahmad, R.; Benmehdi, H.; Lamouri, A.; Decorse, P.; Mangeney, C. Elaboration of hybrid silica particles using a diazonium salt chemistry approach. Colloids Surf. A 2013, 439, 145–150. [Google Scholar] [CrossRef]
- Schuurman, J.C.; McNeill, A.R.; Martinez-Gazoni, R.F.; Scott, J.I.; Reeves, R.J.; Allen, M.W.; Downard, A.J. The effect of covalently bonded aryl layers on the band bending and electron density of SnO2 surfaces probed by synchrotron X-ray photoelectron spectroscopy. Phys. Chem. Chem. Phys. 2019, 21, 17913–17922. [Google Scholar] [CrossRef] [PubMed]
- Gam-Derouich, S.; Pinson, J.; Decorse, P.; Luo, Y.; Herbaut, R.; Royon, L.; Mangeney, C. Diazonium salt chemistry for the design of nano-textured anti-icing surfaces. Chem. Commun. 2018, 54, 8983–8986. [Google Scholar] [CrossRef] [PubMed]
- Gam-Derouich, S.; Pinson, J.; Lamouri, A.; Decorse, P.; Bellynck, S.; Herbaut, R.; Royon, L.; Mangeney, C. Micro-patterned anti-icing coatings with dual hydrophobic/hydrophilic properties. J. Mater. Chem. A 2018, 6, 19353–19357. [Google Scholar] [CrossRef]
- Joselevich, M.; Williams, F.J. Synthesis and Characterization of Diazonium Functionalized Nanoparticles for Deposition on Metal Surfaces. Langmuir 2008, 24, 11711–11717. [Google Scholar] [CrossRef]
- Gorska, B.; Ratajczak, P.; Béguin, F. Faradaic processes on quinone-grafted carbons in protic ionic liquid electrolyte. Electrochim. Acta 2019, 328, 135090. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Shimizu, N. In Situ Functionalization of Graphene with Reactive End Group through Amine Diazotization. J. Phys. Chem. C 2017, 121, 25223–25228. [Google Scholar] [CrossRef]
- Thaçi, B.S.; Gashi, S.T.; Podvorica, F.I. Preparation of heterogeneous reverse osmosis membranes undergoing modification process. Desalin. Water Treat. 2018, 118, 96–102. [Google Scholar] [CrossRef]
- Zeb, G.; Viel, P.; Palacin, S.; Le, X.T. On the chemical grafting of titanium nitride by diazonium chemistry. RSC Adv. 2015, 5, 50298–50305. [Google Scholar] [CrossRef] [Green Version]
- Bouriga, M.; Chehimi, M.M.; Combellas, C.; Decorse, P.; Kanoufi, F.; Deronzier, A.; Pinson, J. Sensitized Photografting of Diazonium Salts by Visible Light. Chem. Mater. 2013, 25, 90–97. [Google Scholar] [CrossRef]
- Busson, M.; Berisha, A.; Combellas, C.; Kanoufi, F.; Pinson, J. Photochemical grafting of diazonium salts on metals. Chem. Commun. 2011, 47, 12631–12633. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.Q.; Ai, Y.; Martin, P.; Lacroix, J.-C. Plasmon-Induced Nanolocalized Reduction of Diazonium Salts. ACS Omega 2017, 2, 1947–1955. [Google Scholar] [CrossRef] [PubMed]
- Tijunelyte, I.; Kherbouche, I.; Gam-Derouich, S.; Nguyen, M.; Lidgi-Guigui, N.; Lamy de la Chapelle, M.; Lamouri, A.; Lévi, G.; Aubard, J.; Chevillot-Biraud, A.; et al. Multi-functionalization of lithographically designed gold nanodisks by plasmon-mediated reduction of aryl diazonium salts. Nanoscale Horiz. 2018, 3, 53–57. [Google Scholar] [CrossRef]
- Nguyen, M.; Kherbouche, I.; Gam-Derouich, S.; Ragheb, I.; Lau-Truong, S.; Lamouri, A.; Lévi, G.; Aubard, J.; Decorse, P.; Felidj, N.; et al. Regioselective surface functionalization of lithographically designed gold nanorods by plasmon-mediated reduction of aryl diazonium salts. Chem. Commun. 2017, 53, 11364–11367. [Google Scholar] [CrossRef]
- Wu, F.; Thomas, P.A.; Kravets, V.G.; Arola, H.O.; Soikkeli, M.; Iljin, K.; Kim, G.; Kim, M.; Shin, H.S.; Andreeva, D.V.; et al. Layered material platform for surface plasmon resonance biosensing. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Chehimi, M.M.; Pinson, J. (Eds.) Applied Surface Chemistry of Nanomaterials; Nova Publishers: New York, NY, USA, 2013. [Google Scholar]
- Unwin, P.R.; Guell, A.G.; Zhang, G. Nanoscale Electrochemistry of sp2 Carbon Materials: From Graphite and Graphene to Carbon Nanotubes. Acc. Chem. Res. 2016, 49, 2041–2048. [Google Scholar] [CrossRef] [Green Version]
- James, D.K.; Tour, J.M. Graphene: Powder, Flakes, Ribbons, and Sheets. Acc. Chem. Res. 2013, 46, 2307–2318. [Google Scholar] [CrossRef]
- Schirowski, M.; Hauke, F.; Hirsch, A. Controlling the Degree of Functionalization: In-Depth Quantification and Side-Product Analysis of Diazonium Chemistry on SWCNTs. Chem. Eur. J. 2019, 25, 12761–12768. [Google Scholar] [CrossRef] [Green Version]
- Romero-Ben, E.; Cid, J.J.; Assali, M.; Fernández-García, E.; Wellinger, R.E.; Khiar, N. Surface modulation of single-walled carbon nanotubes for selective bacterial cell agglutination. Int. J. Nanomed. 2019, 14, 3245–3263. [Google Scholar] [CrossRef] [Green Version]
- Clément, P.; Trinchera, P.; Cervantes-Salguero, K.; Ye, Q.; Jones, C.R.; Palma, M. A One-Step Chemical Strategy for the Formation of Carbon Nanotube Junctions in Aqueous Solution: Reaction of DNA Wrapped Carbon Nanotubes with Diazonium Salts. ChemPlusChem 2019, 84, 1235–1238. [Google Scholar] [CrossRef]
- Ejigu, A.; Kinloch, I.A.; Dryfe, R.A.W. Single Stage Simultaneous Electrochemical Exfoliation and Functionalization of Graphene. ACS Appl. Mater. Interfaces 2017, 9, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Ossonon, B.D.; Bélanger, D. Synthesis and characterization of sulfophenyl-functionalized reduced graphene oxide sheets. RSC Adv. 2017, 7, 27224–27234. [Google Scholar] [CrossRef] [Green Version]
- Kirkman, P.M.; Güell, A.G.; Cuharuc, A.S.; Unwin, P.R. Spatial and Temporal Control of the Diazonium Modification of sp2 Carbon Surfaces. J. Am. Chem. Soc. 2014, 136, 36–39. [Google Scholar] [CrossRef]
- Bellunato, A.; Schneider, G.F. Electrophilic radical coupling at the edge of Graphene. Nanoscale 2018, 10, 12011–12017. [Google Scholar] [CrossRef] [PubMed]
- Bernal, M.M.; Di Pierro, A.; Novara, C.; Giorgis, F.; Mortazavi, B.; Saracco, G.; Fina, A. Edge-Grafted Molecular Junctions between Graphene Nanoplatelets: Applied Chemistry to Enhance Heat Transfer in Nanomaterials. Adv. Funct. Mater. 2018, 28, 1706954. [Google Scholar] [CrossRef] [Green Version]
- van Nguyen, Q.; Martin, P.; Frath, D.; Della Rocca, M.L.; Lafolet, F.; Barraud, C.; Lafarge, P.; Mukundan, V.; James, D.; McCreery, R.L.; et al. Control of rectification in molecular junctions: Contact effects and molecular signature. J. Am. Chem. Soc. 2017, 139, 11913–11922. [Google Scholar] [CrossRef] [Green Version]
- McCreery, R.L.; Bergren, A.J. Progress with Molecular Electronic Junctions: Meeting Experimental Challenges in Design and Fabrication. Adv. Mater. 2009, 21, 4303–4322. [Google Scholar] [CrossRef]
- Supur, M.; Van Dyck, C.; Bergren, A.J.; McCreery, R.L. Bottom-up, Robust Graphene Ribbon Electronics in All-Carbon Molecular Junctions. ACS Appl. Mater. Interfaces 2018, 10, 6090–6095. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Gan, F.; Dong, N.; Zhang, B.; Wang, J.; Chen, Y. Fabrication and nonlinear optical characterization of fluorinated zinc phthalocyanine covalently modified black phosphorus/PMMA films using the nanosecond Z-scan technique. J. Mater. Chem. C 2019, 7, 10789–10794. [Google Scholar] [CrossRef]
- Li, D.O.; Gilliam, M.S.; Chu, X.S.; Yousaf, A.; Guo, Y.; Green, A.A.; Wang, Q.H. Covalent chemical functionalization of semiconducting layered chalcogenide nanosheets. Mol. Syst. Des. Eng. 2019, 4, 962–973. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, J.; Wu, Y.; Huang, H.; Jiang, Q. Chemically functionalized two-dimensional titanium carbide MXene by in situ grafting-intercalating with diazonium ions to enhance supercapacitive performance. J. Phys. Chem. Solids 2018, 115, 172–179. [Google Scholar] [CrossRef]
- Kainz, Q.M.; Reiser, M. Polymer- and Dendrimer-Coated Magnetic Nanoparticles as Versatile Supports for Catalysts, Scavengers, and Reagents. Acc. Chem. Res. 2014, 47, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Griffete, N.; Herbst, F.; Pinson, J.; Ammar, S.; Mangeney, C. Preparation of Water-Soluble Magnetic Nanocrystals Using Aryl Diazonium Salt Chemistry. J. Am. Chem. Soc. 2011, 133, 1646–1649. [Google Scholar] [CrossRef]
- Charlotte Boitard, C.; Lamouri, M.C.; Griffete, N. Whole Protein Imprinting over Magnetic Nanoparticles Using Photo-Polymerization. ACS Appl. Polym. Mater. 2019, 1, 928–932. [Google Scholar] [CrossRef]
- Sanders, S.; Golden, T.D. Functionalization of Cerium Oxide Nanoparticles to Influence Hydrophobic Properties. Langmuir 2019, 35, 5841–5847. [Google Scholar] [CrossRef]
- Mousli, F.; Chaouchi, A.; Hocine, S.; Lamouri, A.; Rei Vilar, M.; Kadri, A.; Chehimi, M.M. Diazonium-modified TiO2/polyaniline core/shell nanoparticles. Structural characterization, interfacial aspects and photocatalytic performances. Appl. Surf. Sci. 2019, 465, 1078–1095. [Google Scholar] [CrossRef]
- Jasmin, J.-P.; Cannizzo, C.; Dumas, E.; Chaussé, A. Fabrication and characterization of all-covalent nanocomposite functionalized screen-printed voltammetric sensors. Electrochim. Acta 2014, 133, 467–474. [Google Scholar] [CrossRef]
- Mooste, M.; Kibena-Põldsepp, E.; Diby Ossonon, B.; Bélanger, D.; Tammeveski, K. Oxygen reduction on graphene sheets functionalised by anthraquinone diazonium compound during electrochemical exfoliation of graphite. Electrochim. Acta 2018, 267, 246–254. [Google Scholar] [CrossRef]
- Laurentius, L.; Stoyanov, S.R.; Gusarov, S.; Kovalenko, A.; Du, R.; Lopinski, G.P.; McDermott, M.T. Diazonium-derived aryl films on gold nanoparticles: Evidence for a carbon gold covalent bond. ACS Nano 2011, 5, 4219–4227. [Google Scholar] [CrossRef]
- Sampathkumar, K.; Diez-Cabanes, V.; Kovaricek, P.; del Corro, E.; Bouša, M.; Hošek, J.; Kalbac, M.; Frank, O. On the Suitability of Raman Spectroscopy to Monitor the Degree of Graphene Functionalization by Diazonium Salts. J. Phys. Chem. C 2019, 123, 22397–22402. [Google Scholar] [CrossRef] [Green Version]
- Berger, F.J.; Luttgens, J.; Nowack, T.; Kutsch, T.; Lindenthal, S.; Kistner, L.; Muller, C.C.; Bongartz, L.M.; Lumsargis, V.A.; Zakharko, Y.; et al. Brightening of Long, Polymer—Wrapped Carbon Nanotubes by sp3 Functionalization in Organic Solvents. ACS Nano 2019, 13, 9259–9269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wepasnick, K.A.; Smith, B.A.; Bitter, J.L.; Fairbrother, D.H. Chemical and structural characterization of carbon nanotube surfaces. Anal. Bioanal. Chem. 2010, 396, 1003–1014. [Google Scholar] [CrossRef] [PubMed]
- Van Dyck, C.; Bergren, A.J.; Mukundan, V.; Fereiro, J.A.; DiLabio, G.A. Extent of conjugation in diazonium-derived layers in molecular junction devices determined by experiment and modelling. Phys. Chem. Chem. Phys. 2019, 21, 16762–16770. [Google Scholar] [CrossRef]
- Descroix, S.; Hallais, G.; Corinne Lagrost, C.; Pinson, J. Regular poly (para-phenylene) films bound to gold surfaces through the electrochemical reduction of diazonium salts followed by electropolymerization in an ionic liquid. Electrochim. Acta 2013, 106, 172–180. [Google Scholar] [CrossRef] [Green Version]
- Greenwood, J.; Phan, T.H.; Fujita, Y.; Li, Z.; Ivasenko, O.; Vanderlinden, W.; Van Gorp, H.; Frederickx, W.; Lu, G.; Tahara, K.; et al. Covalent Modification of Graphene and Graphite Using Diazonium Chemistry: Tunable Grafting and Nanomanipulation. ACS Nano 2015, 9, 5520–5535. [Google Scholar] [CrossRef] [Green Version]
- Anariba, F.; DuVall, S.H.; McCreery, R.L. Mono- and Multilayer Formation by Diazonium Reduction on Carbon Surfaces Monitored with Atomic Force Microscopy “Scratching”. Anal. Chem. 2003, 75, 3837–3844. [Google Scholar] [CrossRef]
- Ceccato, M.; Bousquet, A.; Hinge, M.; Pedersen, S.U.; Daasbjerg, K. Using a Mediating Effect in the Electroreduction of Aryldiazonium Salts To Prepare Conducting Organic Films of High Thickness. Chem. Mater. 2011, 23, 1551–1557. [Google Scholar] [CrossRef]
- Combellas, C.; Kanoufi, F.; Pinson, J.; Podvorica, F.I. Sterically Hindered Diazonium Salts for the Grafting of a Monolayer on Metals. J. Am. Chem. Soc. 2008, 130, 8576–8577. [Google Scholar] [CrossRef]
- Leroux, Y.R.; Fei, H.; Noël, J.-M.; Roux, C.; Hapiot, P. Efficient covalent modification of a carbon surface: Use of a silyl protecting group to form an active monolayer. J. Am. Chem. Soc. 2010, 132, 14039–14041. [Google Scholar] [CrossRef]
- Pichereau, L.; López, I.; Cesbron, M.; Dabos-Seignon, S.; Gautier, C.; Breton, T. Controlled diazonium electrografting driven by overpotential reduction: A general strategy to prepare ultrathin layers. Chem. Commun. 2019, 55, 455–457. [Google Scholar] [CrossRef]
- Xia, Z.; Leonardi, F.; Gobbi, M.; Liu, Y.; Bellani, V.; Liscio, A.; Kovtun, A.; Li, R.; Feng, X.; Orgiu, E.; et al. Electrochemical Functionalization of Graphene at the Nanoscale with Self-Assembling Diazonium Salts. ACS Nano 2016, 10, 7125–7134. [Google Scholar] [CrossRef] [PubMed]
- Dincer, C.; Bruch, R.; Costa-Rama, E.; Fernández-Abedul, M.T.; Merkoçi, A.; Manz, A.; Urban, G.A.; Güder, F. Disposable Sensors in Diagnostics, Food, and Environmental Monitoring. Adv. Mater. 2019, 31, 1806739. [Google Scholar] [CrossRef] [PubMed]
- Gooding, J.J. Advances in interfacial design sensors: Aryl diazonium salts for electrochemical biosensors and for modifying carbon and metal electrodes. Electroanalysis 2008, 2, 573–582. [Google Scholar] [CrossRef]
- Mahouche-Chergui, S.; Gam-Derouich, S.; Mangeney, C.; Chehimi, M.M. Aryl diazonium salts: A new class of coupling agents for bonding polymers, biomacromolecules and nanoparticles to surfaces. Chem. Soc. Rev. 2011, 40, 4143–4166. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chiu, C.-W.; Liang, H. Interfacial Structures and Properties of Organic Materials for Biosensors: An Overview. Sensors 2012, 12, 15036–15062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdellaoui, S.; Corgier, B.C.; Mandon, C.A.; Doumèche, B.; Marquette, C.A.; Blum, L.J. Biomolecules Immobilization Using the Aryl Diazonium Electrografting. Electroanalysis 2013, 25, 671–684. [Google Scholar] [CrossRef]
- Cao, C.; Zhang, Y.; Jiang, C.; Qi, M.; Liu, G. Advances on Aryldiazonium Salt Chemistry Based Interfacial Fabrication for Sensing Applications. ACS Appl. Mater. Interfaces 2017, 9, 5031–5049. [Google Scholar] [CrossRef]
- Yáñez-Sedeño, P.; Campuzano, S.; Pingarrón, J.M. Integrated Affinity Biosensing Platforms on Screen-Printed Electrodes Electrografted with Diazonium Salts. Sensors 2018, 18, 675. [Google Scholar] [CrossRef] [Green Version]
- Yates, N.D.J.; Fascione, M.A.; Parkin, A. Methodologies for “Wiring” Redox Proteins/Enzymes to Electrode Surfaces. Chem. A Eur. J. 2018, 24, 12164–12182. [Google Scholar] [CrossRef] [Green Version]
- Yáñez-Sedeño, P.; González-Cortés, A.; Campuzano, S.; Pingarrón, J.M. Copper(I)-Catalyzed Click Chemistry as a Tool for the Functionalization of Nanomaterials and the Preparation of Electrochemical (Bio)Sensors. Sensors 2019, 19, 2379. [Google Scholar] [CrossRef] [Green Version]
- Bourdillon, C.; Delamar, M.; Demaille, C.; Hitmi, R.; Moiroux, J.; Pinson, J. Immobilization of glucose oxidase on a carbon surface derivatized by electrochemical reduction of diazonium salts. J. Electroanal. Chem. 1992, 336, 113–123. [Google Scholar] [CrossRef]
- Yang, X.; Hall, S.B.; Tan, S.N. Electrochemical Reduction of a Conjugated Cinnamic Acid Diazonium Salt as an Immobilization Matrix for Glucose Biosensor. Electroanalysis 2003, 15, 885–891. [Google Scholar] [CrossRef]
- Liu, G.; Paddon-Row, M.N.; Gooding, J.J. A molecular wire modified glassy carbon electrode for achieving direct electron transfer to native glucose oxidase. Electrochem. Commun. 2007, 9, 92218–92223. [Google Scholar] [CrossRef]
- Qi, M.; Zhang, Y.; Cao, C.; Luc, Y.; Liu, G. Increased sensitivity of extracellular glucose monitoring based on AuNP decorated GO nanocomposites. RSC Adv. 2016, 6, 39180–39186. [Google Scholar] [CrossRef]
- Ferreyra, N.; Coche-Guérente, L.; Labbé, P. Construction of layer-by-layer self-assemblies of glucose oxidase and cationic polyelectrolyte onto glassy carbon electrodes and electrochemical study of the redox-mediated enzymatic activity. Electrochim. Acta 2004, 49, 477–484. [Google Scholar] [CrossRef]
- Zhao, K.; Zhuang, S.; Chang, Z.; Songm, H.; Dai, L.; He, P.; Fanga, Y. Amperometric Glucose Biosensor Based on Platinum Nanoparticles Combined Aligned Carbon Nanotubes Electrode. Electroanalysis 2007, 19, 1069–1074. [Google Scholar] [CrossRef]
- Le Goff, A.; Moggia, F.; Debou, N.; Jegou, P.; Artero, V.; Fontecave, M.; Jousselme, B.; Palacin, S. Facile and tunable functionalization of carbon nanotube electrodes with ferrocene by covalent coupling and p-stacking interactions and their relevance to glucose bio-sensing. J. Electroanal. Chem. 2010, 641, 57–63. [Google Scholar] [CrossRef]
- Nasri, A.; Shams, E.; Ahmadi, M. A glucose biosensor based on direct attachment of in situ generated Nile blue diazonium cations to the electrode surface. J. Electroanal. Chem. 2013, 703, 146–152. [Google Scholar] [CrossRef]
- Sharma, D.; Lim, Y.; Lee, Y.; Shin, H. Glucose sensor based on redox-cycling between selectively modified and unmodified combs of carbon interdigitated array nanoelectrodes. Anal. Chim. Acta 2015, 889, 194–202. [Google Scholar] [CrossRef]
- Raicopol, M.D.; Andronescu, C.; Atasiei, R.; Hanganu, A.; Vasile, E.; Brezoiu, A.M.; Pilan, L. Organic layers via aryl diazonium electrochemistry: Towards modifying platinum electrodes for interference free glucose biosensors. Electrochim. Acta 2016, 206, 226–237. [Google Scholar] [CrossRef]
- Li, M.; Cui, L.; Niu, F.; Ji, X.; Xu, Y.; Liu, J. Efficient and Facile Fabrication of Glucose Biosensor Based on Electrochemically Etched Porous HOPG platform. Electroanalysis 2017, 29, 944–949. [Google Scholar] [CrossRef]
- Morita, K.; Hirayama, N.; Imura, H.; Yamaguchi, A.; Teramae, N. Grafting of phenylboronic acid on a glassy carbon electrode and its application as a reagentless glucose sensor. J. Electroanal. Chem. 2011, 656, 192–197. [Google Scholar] [CrossRef] [Green Version]
- Shervedani, R.K.; Amini, A.; Sadeghi, N. Electrografting of thionine diazonium cationon to the graphene edges and decorating with Au nano-dendrites or glucoseoxidase: Characterization and electrocatalytic applications. Biosens. Bioelectron. 2016, 77, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Ott, C.; Raicopol, M.D.; Andronescu, C.; Vasile, E.; Hanganue, A.; Prunaf, A.; Pilan, L. Functionalized polypyrrole/sulfonated graphene nanocomposites: Improved biosensing platforms through aryl diazonium electrochemistry. Synth. Met. 2018, 235, 20–28. [Google Scholar] [CrossRef]
- Harper, J.C.; Polsky, R.; Dirk, S.M.; Wheeler, D.R.; Brozik, S.M. Electroaddressable Selective Functionalization of Electrode Arrays: Catalytic NADH Detection Using Aryl Diazonium Modified Gold Electrodes. Electroanalysis 2007, 19, 1268–1274. [Google Scholar] [CrossRef]
- Nasri, Z.; Shams, E.; Ahmadi, M. Direct Modification of a Glassy Carbon Electrode with ToluidineBlue Diazonium Salt: Application to NADH Determination and Biosensing of Ethanol. Electroanalysis 2007, 19, 1268–1274. [Google Scholar]
- Venarussoa, L.B.; Tammeveski, K.; Maia, G. Versatile charge transfer through anthraquinone films for electrochemical sensing applications. Electrochim. Acta 2011, 56, 8926–8933. [Google Scholar] [CrossRef]
- Revenga-Parra, M.; Gomez-Anquela, C.; Garcia-Mendiola, T.; Gonzalez, E.; Parientea, F.; Lorenzo, E. Grafted Azure A modified electrodes as disposable β-nicotinamide adenine dinucleotide sensors. Anal. Chim. Acta 2012, 747, 84–91. [Google Scholar] [CrossRef]
- Kesavan, S.; Revin, S.B.; John, S.A. Potentiodynamic formation of gold nanoparticles film on glassy carbon electrode using aminophenyl diazonium cations grafted gold nanoparticles: Determination of histamine H2 receptor antagonist. Electrochim. Acta 2014, 119, 214–224. [Google Scholar] [CrossRef]
- Alarfaj, N.A.; El-Tohamy, M.F. A label-free electrochemical immunosensor based on gold nanoparticles and graphene oxide for the detection of tumor marker calcitonin. New J. Chem. 2017, 41, 11029–11035. [Google Scholar] [CrossRef]
- William Richard, W.; Evrard, D.; Gros, P. A Novel Electrochemical Sensor Based on a Mixed Diazonium/PEDOT Surface Functionalization for the Simultaneous Assay of Ascorbic and Uric Acids. Towards an Improvement in Amperometric Response Stability. Electroanalysis 2014, 26, 1390–1399. [Google Scholar] [CrossRef]
- Assaud, L.; Massonnet, N.; Evrard, D.; Vergnes, H.; Salvagnac, L.; Conédéra, V.; Noé, L.; Monthioux, M.; Gros, P.; Temple-Boyer, P.; et al. A new route for the integration of a graphene/diazonium/PEDOT electrode towards antioxidant biomarker detection. J. Electroanal. Chem. 2016, 771, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Himori, S.; Nishitani, S.; Sakata, T. Control of Potential Response to Small Biomolecules with Electrochemically Grafted Aryl-Based Monolayer in Field-Effect Transistor-Based Sensors. Langmuir 2019, 35, 3701–3709. [Google Scholar] [CrossRef] [PubMed]
- Rather, J.A.; Khudaish, E.A.; Kannan, P. Graphene-amplified femtosensitive aptasensing of estradiol, an endocrine disruptor. Analyst 2018, 143, 1835–1845. [Google Scholar] [CrossRef]
- Istamboulié, G.; Paniel, N.; Zara, L.; Reguillo Granados, L.; Barthelmebs, L.; Noguer, T. Development of an impedimetric aptasensor for the determination of aflatoxin M1 in milk. Talanta 2016, 146, 464–469. [Google Scholar] [CrossRef]
- Hayat, A.; Barthelmebs, L.; Marty, J.-L. Electrochemical impedimetric immunosensor for the detection of okadaic acid in mussel sample. Sens. Actuators B 2012, 171, 810–815. [Google Scholar] [CrossRef]
- Eissa, S.; Zourob, M. A graphene-based electrochemical competitive immunosensor for the sensitive detection of okadaic acid in shellfish. Nanoscale 2012, 4, 7593–7599. [Google Scholar] [CrossRef]
- Sánchez-Tirado, E.; Arellano, L.M.; González-Cortés, A.; Yáñez-Sedeño, P.; Langa, F.; Pingarrón, J.M. Viologen-functionalized single-walled carbon nanotubes as carrier nanotags for electrochemical immunosensing. Application to TGF-β1 cytokine. Biosens. Bioelectron. 2017, 98, 240–247. [Google Scholar] [CrossRef]
- Chrouda, A.; Sbartai, A.; Bessueille, F.; Renaud, L.; Maaref, A.; Jaffrezic-Renault, N. Electrically addressable deposition of diazonium functionalized antibodies on boron-doped diamond microcells for the detection of ochratoxin A. Anal. Methods 2015, 7, 2444–2451. [Google Scholar] [CrossRef]
- Alonso-Lomillo, M.A.; Domínguez-Renedo, O.; Matos, P.; Arcos-Martín, M.J. Disposable biosensors for determination of biogenic amines. Anal. Chim. Acta 2010, 665, 26–31. [Google Scholar] [CrossRef]
- Corgier, B.P.; Marquette, C.A.; Blum, L.J. Diazonium-Protein Adducts for Graphite Electrode Microarrays Modification: Direct and Addressed Electrochemical Immobilization. J. Am. Chem. Soc. 2005, 127, 18328–18332. [Google Scholar] [CrossRef] [PubMed]
- Corgier, B.P.; Laurent, A.; Perriat, P.; Blum, L.J.; Marquette, C.A. A Versatile Method for Direct and Covalent Immobilization of DNA and Proteins on Biochips. Angew. Chem. Int. Ed. 2007, 46, 4108–4110. [Google Scholar] [CrossRef]
- Corgier, B.P.; Li, F.; Blum, L.J.; Marquette, C.A. Direct electrochemical addressing of immunoglobulins: Immuno-chip on screen-printed microarray. Biosens. Bioelectron. 2007, 22, 1522–1526. [Google Scholar] [CrossRef] [PubMed]
- Marquette, C.A.; Bouteille, F.; Corgier, B.P.; Degiuli, A.; Blum, L.J. Disposable screen-printed chemiluminescent biochips for the simultaneous determination of four point-of-care relevant proteins. Anal. Bioanal. Chem. 2009, 393, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Corgier, B.P.; Li, F.; Blum, L.J.; Marquette, C.A. On-Chip Chemiluminescent Signal Enhancement Using Nanostructured Gold-Modified Carbon Microarrays. Langmuir 2007, 23, 8619–8623. [Google Scholar] [CrossRef] [PubMed]
- Corgier, B.P.; Bellon, S.; Anger-Leroy, M.; Blum, L.J.; Marquette, C.A. Protein-Diazonium Adduct Direct Electrografting onto SPRi-Biochip. Langmuir 2009, 25, 9619–9623. [Google Scholar] [CrossRef]
- Polsky, R.; Harper, J.C.; Wheeler, D.R.; Dirk, S.M.; Arango, D.C.; Brozik, S.M. Electrically addressable diazonium-functionalized antibodies for multianalyte electrochemical sensor applications. Biosens. Bioelectron. 2008, 23, 757–764. [Google Scholar] [CrossRef]
- Bertok, T.; Lorencova, L.; Hroncekova, S.; Gajdosova, V.; Jane, E.; Hires, M.; Kasak, P.; Kaman, O.; Sokol, R.; Bella, V.; et al. Advanced impedimetric biosensor configuration and assay protocol for glycoprofiling of a prostate oncomarker using Au nanoshells with a magnetic core. Biosens. Bioelectron. 2019, 131, 24–29. [Google Scholar] [CrossRef]
- Mandon, C.A.; Blum, L.J.; Marquette, C.A. Aryl Diazonium for Biomolecules Immobilization onto SPRi Chips. ChemPhysChem 2009, 10, 3273–3277. [Google Scholar] [CrossRef]
- Goldsmith, B.R.; Mitala, J.J., Jr.; Josue, J.; Castro, A.; Lerner, M.B.; Bayburt, T.H.; Khamis, S.M.; Jones, R.A.; Brand, J.G.; Sligar, S.G.; et al. Biomimetic Chemical Sensors Using Nanoelectronic Readout of Olfactory Receptor Proteins. ACS Nano 2011, 5, 5408–5416. [Google Scholar] [CrossRef]
- Wang, R.; Xue, C. A sensitive electrochemical immunosensor for alpha-fetoprotein based on covalently incorporating a biorecognition element onto a graphene modified electrode via diazonium chemistry. Anal. Methods 2013, 5, 5195–5200. [Google Scholar] [CrossRef]
- Ocaña, C.; Hayat, A.; Mishra, R.; Vasilescu, A.; del Vallea, M.; Marty, J.-L. A novel electrochemical aptamer–antibody sandwich assay for lysozyme detection. Analyst 2015, 140, 4148–4153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adabo, A.H.; Zeggari, R.; Saïd, N.M.; Bazzi, R.; Elie-Caille, C.; Marquette, C.; Martini, M.; Tillement, O.; Perriat, P.; Chaix, C.; et al. Enhanced chemiluminescence-based detection on gold substrate after electrografting of diazonium precursor-coated gold nanoparticles. J. Coll. Interface Sci. 2016, 467, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Zhang, Y.; Cao, C.; Zhang, M.; Liu, S.; Liu, G. Decoration of Reduced Graphene Oxide Nanosheets with Aryldiazonium Salts and Gold Nanoparticles toward a Label-Free Amperometric Immunosensor for Detecting Cytokine Tumor Necrosis Factor-α in Live Cells. Anal. Chem. 2016, 88, 9614–9621. [Google Scholar] [CrossRef]
- Jiang, C.; Alam, M.T.; Moraes Silva, S.; Taufik, S.; Fan, S.; Gooding, J.J. Unique Sensing Interface That Allows the Development of an Electrochemical Immunosensor for the Detection of Tumor Necrosis Factor α in Whole Blood. ACS Sens. 2016, 1, 1432–1438. [Google Scholar] [CrossRef]
- Fioresi, F.; Rouleau, A.; Maximova, K.; Vieillard, J.; Boireau, W.; Elie Caille, C.; Soulignac, C.; Zeggarib, R.; Clamens, T.; Lesouhaitier, O.; et al. Electrografting of diazonium salt for SPR application. Mater. Today Proc. 2019, 6, 340–344. [Google Scholar] [CrossRef]
- Li, N.; Chow, A.M.; Ganesh, H.V.S.; Ratnam, M.; Brown, I.R.; Kerman, K. Diazonium-Modified Screen-Printed Electrodes for Immunosensing Growth Hormone in Blood Samples. Biosensors 2019, 9, 88. [Google Scholar] [CrossRef] [Green Version]
- van den Hurk, R.; Baghelani, M.; Chen, J.; Daneshmand, M.; Evoy, S. Al-Mo nanocomposite functionalization for membrane-based resonance detection of bovine Herpesvirus-1. Sens. Actuators A 2019, 296, 186–191. [Google Scholar] [CrossRef]
- Anjum, S.; Qi, W.; Gao, W.; Zhao, J.; Hanif, S.; Rehman, A.-U.; Xu, G. Fabrication of biomembrane-like films on carbon electrodes using alkanethiol and diazonium salt and their application for direct electrochemistry of myoglobin. Biosens. Bioelectron. 2015, 65, 159–165. [Google Scholar] [CrossRef]
- Chung, D.-J.; Oh, S.-H.; Komathi, S.; Gopalan, A.I.; Lee, K.-P.; Choi, S.-H. One-step modification of various electrode surfaces using diazonium salt compounds and the application of this technology to electrochemical DNA (E-DNA) sensors. Electrochim. Acta 2012, 76, 394–403. [Google Scholar] [CrossRef]
- Baker, S.E.; Tse, K.-Y.; Hindin, E.; Nichols, B.M.; Clare, T.L.; Robert, J.; Hamers, R.J. Covalent Functionalization for Biomolecular Recognition on Vertically Aligned Carbon Nanofibers. Chem. Mater. 2005, 17, 4971–4978. [Google Scholar] [CrossRef]
- Nebel, C.E.; Yang, N.; Uetsuka, H.; Osawa, E.; Tokuda, N.; Williams, O. Diamond nano-wires, a new approach towards next generation electrochemical gene sensor platforms. Diam. Relat. Mater. 2009, 18, 910–917. [Google Scholar] [CrossRef] [Green Version]
- Hai, L.V.; Reisberg, S.; Chevillot-Biraud, A.; Noël, V.; Pham, M.C.; Piro, B. Simultaneous Electroreduction of Different Diazonium Salts for Direct Electrochemical DNA Biosensor Development. Electrochim. Acta 2014, 140, 49–58. [Google Scholar] [CrossRef]
- Bartolome, J.P.; Echegoyen, L.; Fragoso, A. Reactive Carbon Nano-Onion Modified Glassy Carbon Surfaces as DNA Sensors for Human Papillomavirus Oncogene Detection with Enhanced Sensitivity. Anal. Chem. 2015, 87, 6744–6751. [Google Scholar] [CrossRef]
- Civit, L.; Fragoso, A.; O’Sullivan, C.K. Thermal stability of diazonium derived and thiol-derived layers on gold for application in genosensors. Electrochem. Commun. 2010, 12, 1045–1048. [Google Scholar] [CrossRef]
- Yang, L.; Xu, Y.; Wang, X.; Zhu, J.; Zhang, R.; He, P.; Fang, Y. The application of cyclodextrin derivative functionalized aligned carbon nanotubes for electrochemically DNA sensing via host–guest recognition. Anal. Chim. Acta 2011, 689, 39–46. [Google Scholar] [CrossRef]
- Revenga-Parra, M.; Garcia-Mendiola, T.; Gonzalez-Costas, J.; Gonzalez-Romero, E.; Garcia Marin, A.; Pau, J.L.; Pariente, F.; Lorenzo, E. Simple diazonium chemistry to develop specific gene sensing platforms. Anal. Chim. Acta 2014, 813, 41–47. [Google Scholar] [CrossRef]
- Kuo, T.M.; Shen, M.Y.; Huang, S.Y.; Li, Y.K.; Chuang, M.C. Facile Fabrication of a Sensor with a Bifunctional Interface for Logic Analysis of the New Delhi Metallo-β-Lactamase (NDM)-Coding Gene. ACS Sens. 2016, 1, 124–130. [Google Scholar] [CrossRef]
- Ge, L.; Wang, W.; Li, F. Electro-Grafted Electrode with Graphene-Oxide-Like DNA Affinity for Ratiometric Homogeneous Electrochemical Biosensing of MicroRNA. Anal. Chem. 2017, 89, 11560–11567. [Google Scholar] [CrossRef]
- Mousavisania, S.Z.; Raoof, J.-B.; Turner, A.P.F.; Ojani, R.; Mak, W.C. Label-free DNA sensor based on diazonium immobilisation for detection of DNA damage in breast cancer 1 gene. Sens. Actuators B 2018, 264, 59–66. [Google Scholar] [CrossRef]
- Guselnikova, O.; Dvorankova, B.; Kakisheva, K.; Kalachyova, Y.; Postnikov, P.; Svorcik, V.; Lyutakov, O. Rapid SERS-based recognition of cell secretome on the folic acid-functionalized gold gratings. Anal. Bioanal. Chem. 2019, 411, 3309–3319. [Google Scholar] [CrossRef] [PubMed]























| [1] | Some of advantages and disadvantages of alkanethiol and diazonium salts for the modification of electrode surfaces, and selected examples |
| [66] | The use of diazonium salts as surface modifiers and coupling agents, as well as some applications in biosensing |
| [67] | A general overview of biosensors including different methods that permit attaching sensing groups to a surface |
| [68] | A review of the author’s work concerning biosensing of antibodies, oligonucleotides, and enzymes onto conductive supports |
| [69] | Advances in the use of aryldiazonium salts for modifying interfaces in sensors and biosensors |
| [70] | A review that outlines the potential of diazonium chemistry to prepare single or multianalyte electrochemical affinity biosensors on screen-printed electrodes (SPEs) |
| [71] | A review that evaluates the methods through which redox proteins can be attached to electrode surfaces in a “wired” configuration that facilitates direct electron transfer |
| [72] | Copper(I)-catalyzed click chemistry as a tool for the preparation of electrochemical (bio)sensors |
| Surface (a) | Attached Aryl Group | Characteristics c = Concentration Range Γ (GOx) mol∙cm−2, kET T = Turnover S= Sensitivity | Reference | |
|---|---|---|---|---|
| Mediator | ||||
| GC | 4-phenylacetic | Fc-CH2OH (b) | Γ ~1.8 × 10−13 | [73] |
| GC | 4-phenylcinnamic | Fc-CH2OH (b) | - | [74] |
| GC | 4-phenylacetic + layer by-layer (c) | Fc-CH2OH b) | Γ ~1.1 × 10−12 | [77] |
| Aligned CNT | 4-aminophenyl (d) | Pt nanoparticles | c = 1 × 10−2–7 mM Stability 20 days | [78] |
| CNT | 4-(2-aminoethyl) phenyl | Fc-CH2-CH2-COOH (e) | c = 5–50 mM s = 0.83 µA mM−1 | [79] |
| GC | Nile blue | O2 f) | c = up to 2.5 mM | [80] |
| GC (g) | 4-nitrophenyl | Fe(CN)63−/4− | c= up to10 mM | [81] |
| Pt (h) | 4-fluoro 4-methyl 4-methoxy | Fc-CH2OH | c = 0.2–10 mM In the presence of ascorbic acid and uric acid | [82] |
| Porous HOPG (i) | To create the porosity | Fc-CH2OH | 5 µM–100 mM | [83] |
| Direct Electron Transfer | ||||
| GC-Ar-GO-nP | 4-carboxyphenyl | Direct electron transfer to GOx | C = 0.3–20 mM kET = 8.3 s−1 T = 112 s−1. | [76] |
| GC | 4-carboxyphenyl + oligo(phenylethynyl) (j) | Direct electron transfer to GOx | 0–25 mM T = 1.1 s−1 | [75] |
| GC | 3-phenylboronic | Direct complexation of GOx | −50 mM | [84] |
| GC + GO (k) | Thionine | Electrostatic adsorption of negatively charged GOx on positively charged functions of thionine | c = 0.5–6.0 mM s = 43.2 mA mM−1 cm−2 | [85] |
| Pt/GO-SO3−/PPy (l) | 4-carboxyphenyl | c = 0.2 × 12 mM s = 0.56 μA mM−1 cm−2 In the presence of ascorbic acid (AA) and uric acid (UA) | [86] |
| Surface | Diazonium Salt + Attached Recognizing Group | Analyte | Detectable Label and Detection Limit | Reference |
|---|---|---|---|---|
| Attachment of toxins to surfaces modified by diazonium chemistry | ||||
| SPE (a) | 4-carboxybenzenediazonium + hexaethyleneglycol-modified 21-mer oligonucleotide | Aflatoxin M1 in milk  | Direct detection by EIS (b) and CV (c)c = 20–1000 ng/kg. | [97] |
| SPE | 4-carboxybenzenediazonium+ anti-okadaic acid monoclonal antibody (anti-OA-MAb) | Okadaic acid | Direct detection by EIS | [98] |
| Graphene-modified SPE | Direct detection by SWV (d) | [99] | ||
| SPE | 4-carboxybenzenediazonium+ streptavidine + biotin-anti-TGFβ1 | TGFβ1 protein | CV of viologen-modified SWCNT | [100] |
| SPR chip with Cu/graphene layer | 3,5-bis-fluoro 4-carboxybenzenediazonium | HT-2 toxin | Label-free, surface plasmon resonance | [26] |
| Toxins modified with an aryl diazonium group | ||||
| Boron-doped diamond electrochemical microcell | Anti-ochratoxin polyclonal antibodies modified by a diazonium salt after coupling with 4-carboxymethylaniline | Ochratoxin A | [101] | |
| Surface | Diazonium Salt + Attached Recognizing Group | Analyte | Detectable Label and Detection Limit | Reference |
| Proteins modified with an aryl diazonium group | ||||
| SPE array | Immunoglobulin modified by a diazonium salt after coupling with 4-carboxymethylaniline | Anti-rabbit IgG antibodies | Peroxidase-labeled anti-rabbit or anti-human IgG antibodies. LOD: 50 fmol (a) | [103] |
| SPE array | Anti-human IgG | Human IgG | Horseradish peroxidase (HRP)-modified secondary antibody. Detection limit: 60 nm human IgG | [104,105,106] (b) |
| SPE array | Rabbit IgG modified by a diazonium salt after coupling with 4-carboxymethylaniline | Rheumatoid factor (RF) | Horseradish peroxidase (HRP)-modified secondary antibody. Detection range: 5.3–485 IU∙mL−1 | |
| SPE array | HRP modified by a diazonium salt after coupling with 4-carboxymethylaniline | HRP | ||
| Gold NPs (c) on SPE | Human prostate-specific antigen (PSA) modified by a diazonium salt after coupling with 4-carboxymethylaniline | Prostate-specific antigen (PSA) monoclonal antibody labeled with biotin | Horseradish peroxidase-labeled streptavidin Detection range of 5–80 ng/mL | [107] |
| (SPRi) (e) gold chip | Anti-ovalbumin IgG modified by a diazonium salt after coupling with 4-carboxymethylaniline | Ovalbumin | Direct reflectivity change. LOD: 100 ng/mL (2 nM). | [108] (d) |
| GC, Au | Biotinylated anti-TNF-antibody (Tumor Necrosis Factor) modified by a diazonium salt after coupling with 4-carboxyaniline | Anti-TNF-antibody | Avidin modified gold NPs or avidin-HRP | [109] (e) |
| Au | Carboxybetaine aryldiazonium derivative + lectin | Prostate-specific antigen (PSA) | EIS detection of anti-PSA antibody-modified Au nanoshells with a magnetic core | [110] (f) |
| Attachment of proteins to surfaces modified by diazonium chemistry | ||||
| Modified Surface | Analyte | Detectable Label and Detection Limit | ||
| SPRi gold chip | Gold surface modified | Protein A | Direct reflectivity change upon coupling the protein to the attached carboxylic group | [111] (g) |
| CNT | CNT modified by reaction of 4-carboxymethylbenzenediazonium + attachment of mouse olfactory receptor proteins | Various odoriferant organic compounds in vapor phase | Detection of odors: e-nose For example, 7 ppb of toluene | [112] (h) |
| Graphene-modified GC | Graphene surface modified by reaction of 4-aminobenzenediazonium + coupling with A-Fetoprotein antibody | A-Fetoprein | HRP-labeled anti-AFP antibody LOD: 0.03 ng mL−1 | [113] (i) |
| SPE | Gold surface modified by reaction of 4-carboxybenzenediazonium | Lysozyme | Biotinylated antibody avidin–alkaline phosphatase LOD: 4.3 fM | [114] (j) |
| Gold | Gold NPs modified with 4-mercaptoaniline, attached to gold surface by diazonium coupling | Five-amino-acid polypeptide with a biotin group | Peroxidase-labeled streptavidin | [115] |
| Nanocomposite: gold NPs loaded on reduced graphene oxide | Gold NPs modified with 4-carbxyphenyl and 4-aminophenylphosphorylcholine + coupling with anti-TNF-α capture antibody | Cytokine tumor necrosis factor-alpha (TNF-α) | Coupling anti-TNF-α detection antibody (Ab2) attached to graphene oxides modified with ferrocenyl groups. Electrochemical detection of ferrocene. LOD: 0.1 pg∙mL−1 | [116] (k) |
| Indium tin oxide (ITO) | Gold surface modified by reaction of the diazonium salt of 4-aminobutyric acid + peptidic coupling of antibody (Ab1) | Tumor necrosis factor | HRP-conjugated detection antibody (Ab2) LOD: 10 pg/mL | [117] (l) |
| SPRi biochip | 4-Carboxybenzenediazonium + anti-ovalbumin antibody | Ovalbumine | Direct detection by SPR | [118] |
| SPE | SPE surface modified by reaction of 4-methoxybenzenediazonium + oxidation of the grafted methoxy group+ attachment of anti-growth hormone antibodies | Growth hormone | EIS detection of 100 pg/mL growth hormone in undiluted whole blood LOD: 5 pg∙mL−1 | [119] (m) |
| Al–Mo nanoparticle membrane on Si | 4-formylbenzenediazonium | Monoclonal antibodies specific for bovine herpes virus 1 (BHV-1) | Bovine herpes virus 1 Measurement of the resonance frequency shift of the membrane | [120] |
| Biomembrane-like films | ||||
| GC | Gold NPs capped with myoglobin on 4-carboxaldehyde diazonium salt + docecyl thiol | Myoglobin | Electrochemical detection of H2O2 0.3 μM | [121] |
| Surface | Diazonium Salt + Attached Recognizing Group | Analyte | Detectable Label and Detection Limit | Reference |
|---|---|---|---|---|
| Modification of the Surface | ||||
| Carbon nanofibers | 4-Nitrobenzenediazonium and reduction of 4-nitrophenyl to 4-aminophenyl groups after electrografting + reaction of a maleimide linker (a) | Thiol-terminated DNA attaches to the linker | Fluorescently labeled, perfect complement to the grafted oligonucleotides | [123] |
| Vertically aligned diamond nano-wires | 4-Nitrobenzenediazonium and reduction of 4-nitrophenyl to 4-aminophenyl groups after electrografting + maleimide linker (a) + thiol-terminated DNA, 23-mer cancer marker cytokeratin | The complementary DNA sequence | The complementary sequence detected by Differential Pulsed Voltammetry (DPV) LOD ~2 pM | [124] (b) |
| Array of gold electrodes | 4-Carboxybenzenediazonium and a bis-diazonium salt with a COOH terminal group + amino-terminated DNA | Human papillomavirus sequences terminated by tetramethylbenzidine | Electrochemical detection of tetramethylbenzidine | [127] |
| Vertically aligned carbon nanotubes | Diazonium salt of an aminophenyl group substituted + β-cyclodextrin (β-CD) | DNA probe substituted on one end by a dabcyl group and a CdS nanoparticle at the other end | In the presence of the complementary sequence, the probe could be captured by the β-CD-modified CNT electrode LOD by DPV: 5.0 × 10−13 M | [128] (c) |
| ITO, gold, GC | 4-Carboxybenzenediazonium + attachment of avidin | Biotinylated DNA from influenza virus (type A) | Avidin–biotin recognition. Detection through the CV of ferro/ferricyanide LOD: 8.51 × 10−14 M | [122] |
| SPE | 4-Nitrobenzenediazonium and reduction of 4-nitrophenyl to 4-aminophenyl groups after electrografting | Amine-modified (polyA)25 DNA probe. | Reaction between the diazonium group and NH2-DNA and recognition of the hybridization by EIS and DPV LOD: 4.65 nm | [129] (d) |
| GC | 4-Carboxybenzenediazonium and a naphthoquinone (e) to give a mixed layer + DNA probe attached to the surface | Fluorescent complementary DNA strand | Hybridization was detected by fluorescence and Alternative Current (AC) voltammetry Detection limit ca. 10 pM | [125] |
| Au | Mixed layer obtained from the diazonium salts bearing (i) a sulfobetaine group, (ii) a phenylmaleimido group + DNA probe attached to the surface | New Delhi metallo-β-lactamase (NDM)-Coding Gene | CV and chronoamperometric detection of the charges on the phosphate groups of DNA Detection limit: 54 pM | [130] (f) |
| The diazonium salts of 4-aminophenylacetic acid + covalent immobilization of streptavidin and incubation of a biotinylated DNA capture probe | Biotinylated DNA target sequence associated with the human papillomavirus | HRP-DNA probe + electrochemical detection of tetramethyl benzidine Detection limit: 0.50 nM | [126] (g) | |
| ITO | 1-Naphthalenesulfonate diazonium salt | MicroRNA | Discrimination ability over single-mismatch, high sensitivity in the aM range thanks to the use of isothermal amplification strategy Detection limit: 25 aM | [131] (h) |
| SPE | 4-Carboxybenzenediazonium + peptidic attachment of a DNA probe | Determination of DNA damage by various reagents using EIS | [132] (i) | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hetemi, D.; Noël, V.; Pinson, J. Grafting of Diazonium Salts on Surfaces: Application to Biosensors. Biosensors 2020, 10, 4. https://doi.org/10.3390/bios10010004
Hetemi D, Noël V, Pinson J. Grafting of Diazonium Salts on Surfaces: Application to Biosensors. Biosensors. 2020; 10(1):4. https://doi.org/10.3390/bios10010004
Chicago/Turabian StyleHetemi, Dardan, Vincent Noël, and Jean Pinson. 2020. "Grafting of Diazonium Salts on Surfaces: Application to Biosensors" Biosensors 10, no. 1: 4. https://doi.org/10.3390/bios10010004
APA StyleHetemi, D., Noël, V., & Pinson, J. (2020). Grafting of Diazonium Salts on Surfaces: Application to Biosensors. Biosensors, 10(1), 4. https://doi.org/10.3390/bios10010004