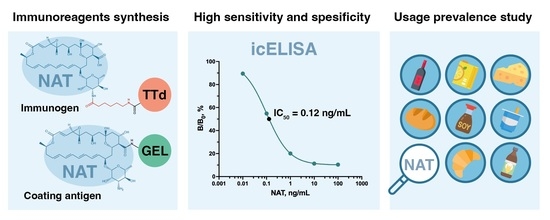
Immunoassay for Natamycin Trace Screening: Bread, Wine and Other Edibles Analysis
Abstract
:1. Introduction
2. Methods
2.1. Chemicals
2.2. Synthesis of Immunogens and Coating Antigens
2.3. Immunization Procedure and Antibody Preparation
2.4. ELISA Optimization
2.5. Sample Collection, Pretreatment and Analysis
3. Results and Discussion
3.1. Immunogen Synthesis
3.2. Selection of Immunoreagents and Examination of Assay Specificity
3.3. Evaluation of NAT Determination in Its Formulations with Cyclodextrins
3.4. Extraction Optimization and Recovery Experiments
3.5. Determination of NAT in Real Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Struyk, A.P.; Drost, G.; Haisvisz, J.M.; Van Eek, T.; Hoogerheide, J.C. Pimaricin, a new antifungal antibiotic. Antibiot. Annu. 1957, 5, 878–885. [Google Scholar] [PubMed]
- Guo, X.; Zhang, J.; Li, X.; Xiao, E.; Lange, J.D.; Rienstra, C.M.; Burke, M.D.; Mitchell, D.A. Sterol sponge mechanism is conserved for glycosylated polyene macrolides. ACS Cent. Sci. 2021, 7, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Bagga, B.; Singal, D.; Nagpal, R.; Kate, A.; Saluja, G.; Maharana, P.K. Fungal keratitis: A review of clinical presentations, treatment strategies and outcomes. Ocul. Surf. 2022, 24, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.; Lakhani, P.; Majumdar, S. Current perspectives on natamycin in ocular fungal infections. J. Drug Deliv. Sci. Technol. 2017, 41, 206–212. [Google Scholar] [CrossRef]
- Williams, C.; Whitehouse, J.; Lister, T.; Wrigley, P. Oral anticandidal prophylaxis in patients undergoing chemotherapy for acute leukemia. Med. Pediatr. Oncol. 1977, 3, 275–280. [Google Scholar] [CrossRef]
- Masterton, G.; Sengupta, S.; Schofield, C. Natamycin in genital candidosis in men. Sex. Transm. Infect. 1975, 51, 210–212. [Google Scholar] [CrossRef] [Green Version]
- Krotin, P.; Kirilenko, O. Combination of antifungal agent and prebiotic for acute vulvovaginal candidiasis. Russ. J. Woman Child Health 2019, 2, 120–125. [Google Scholar]
- Currie, D.; Lueck, C.; Milburn, H.; Harvey, C.; Longbottom, J.; Darbyshire, J.; Nunn, A.; Cole, P. Controlled trial of natamycin in the treatment of allergic bronchopulmonary aspergillosis. Thorax 1990, 45, 447–450. [Google Scholar] [CrossRef] [Green Version]
- Davidson, P.M.; Doan, C. Natamycin. In Antimicrobials in Food; CRC Press: Boca Raton, FL, USA, 2020; pp. 339–356. [Google Scholar]
- Meena, M.; Prajapati, P.; Ravichandran, C.; Sehrawat, R. Natamycin: A natural preservative for food applications—A review. Food Sci. Biotechnol. 2021, 30, 1481–1496. [Google Scholar] [CrossRef]
- Delves-Broughton, J.; Steenson, L.; Dorko, C.; Erdmann, J.; Mallory, S.; Norbury, F.; Thompson, B. Use of natamycin as a preservative on the surface of baked goods: A case study. In Case Studies in Novel Food Processing Technologies; Elsevier: Amsterdam, The Netherlands, 2010; pp. 303–320. [Google Scholar]
- Yang, Y.; Huan, C.; Liang, X.; Fang, S.; Wang, J.; Chen, J. Development of starch-based antifungal coatings by incorporation of natamycin/methyl-β-cyclodextrin inclusion complex for postharvest treatments on cherry tomato against Botrytis cinerea. Molecules 2019, 24, 3962. [Google Scholar] [CrossRef] [Green Version]
- Anari, H.N.B.; Majdinasab, M.; Shaghaghian, S.; Khalesi, M. Development of a natamycin-based non-migratory antimicrobial active packaging for extending shelf-life of yogurt drink (Doogh). Food Chem. 2022, 366, 130606. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EU). No 1129/2011 of 11 November 2011 amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council by establishing a Union list of food additives. Off. J. Eur. Union 2011, 295, 1–177. [Google Scholar]
- Dalhoff, A.A.; Levy, S.B. Does use of the polyene natamycin as a food preservative jeopardise the clinical efficacy of amphotericin B? A word of concern. Int. J. Antimicrob. Agents 2015, 45, 564–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, S.; Peng, X.; Liang, X.; Shen, J.; Wang, J.; Chen, J.; Meng, Y. Enhancing water solubility and stability of natamycin by molecular encapsulation in methyl-β-cyclodextrin and its mechanisms by molecular dynamics simulations. Food Biophys. 2020, 15, 188–195. [Google Scholar] [CrossRef]
- Burkin, M.A.; Galvidis, I.A. Simultaneous immunodetection of ionophore antibiotics, salinomycin and narasin, in poultry products and milk. Anal. Methods 2021, 13, 1550–1558. [Google Scholar] [CrossRef]
- Galvidis, I.A.; Burkin, K.M.; Eremin, S.A.; Burkin, M.A. Group-specific detection of 2-deoxystreptamine aminoglycosides in honey based on antibodies against ribostamycin. Anal. Methods 2019, 11, 4620–4628. [Google Scholar] [CrossRef]
- Nuriev, R.; Galvidis, I.; Burkin, M. Immunochemical characteristics of Streptococcus pneumoniae type 3 capsular polysaccharide glycoconjugate constructs correlate with its immunogenicity in mice model. Vaccine 2020, 38, 8292–8301. [Google Scholar] [CrossRef]
- Hu, S.; Huang, Z.; Wang, C.; Peng, J.; Lai, W.-H. Using hapten cross-reactivity to screen heterologous competitive antigens for improving the sensitivity of ELISA. Food Chem. 2020, 303, 125379. [Google Scholar] [CrossRef]
- Burkin, M.A.; Galvidis, I.A. Hapten modification approach for switching immunoassay specificity from selective to generic. J. Immunol. Methods 2013, 388, 60–67. [Google Scholar] [CrossRef]
- Chen, Y.; Kong, D.; Liu, L.; Song, S.; Kuang, H.; Xu, C. Development of an enzyme-linked immunosorbent assay (ELISA) for natamycin residues in foods based on a specific monoclonal antibody. Anal. Methods 2015, 7, 3559–3565. [Google Scholar] [CrossRef]
- Liu, L.; Chen, Y.; Song, S.; Zheng, Q.; Wu, X.; Kuang, H. Development of an antibody-based colloidal gold immunochromatographic lateral flow strip test for natamycin in milk and yoghurt samples. Food Agric. Immunol. 2017, 28, 1283–1292. [Google Scholar] [CrossRef] [Green Version]
- Burkin, M.A.; Surovoy, Y.A.; Arzumanian, V.G.; Galvidis, I.A. Development and application of amphotericin B immunoassay for pharmacokinetic studies and therapeutic drug monitoring in critically ill patients. J. Pharm. Biomed. Anal. 2022, 218, 114875. [Google Scholar] [CrossRef] [PubMed]
- Szente, L.; Szejtli, J. Cyclodextrins as food ingredients. Trends Food Sci. Technol. 2004, 15, 137–142. [Google Scholar] [CrossRef]
- Navarro-Orcajada, S.; Conesa, I.; Matencio, A.; Rodríguez-Bonilla, P.; García-Carmona, F.; López-Nicolás, J.M. The use of cyclodextrins as solubility enhancers in the ORAC method may cause interference in the measurement of antioxidant activity. Talanta 2022, 243, 123336. [Google Scholar] [CrossRef]
- Koontz, J.L.; Marcy, J.E.; Barbeau, W.E.; Duncan, S.E. Stability of natamycin and its cyclodextrin inclusion complexes in aqueous solution. J. Agric. Food Chem. 2003, 51, 7111–7114. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS). Scientific Opinion on the use of natamycin (E 235) as a food additive. EFSA J. 2009, 7, 1412. [Google Scholar] [CrossRef]
- Paseiro-Cerrato, R.; Otero-Pazos, P.; de Quirós, A.R.-B.; Sendón, R.; Angulo, I.; Paseiro-Losada, P. Rapid method to determine natamycin by HPLC-DAD in food samples for compliance with EU food legislation. Food Control 2013, 33, 262–267. [Google Scholar] [CrossRef]
- Mielech-Łukasiewicz, K.; Leoniuk, M. Voltammetric determination of natamycin using a cathodically pretreated boron-doped diamond electrode in the presence of sodium dodecyl sulfate. Microchem. J. 2020, 159, 105570. [Google Scholar] [CrossRef]





| AGs | ELISA Variants Based on “Antibody Coating Antigens” Reagents | |||
|---|---|---|---|---|
| Anti-TTd-adh-NAT–Gel(pi)-NAT | Anti-TTd-aca-NAT–Gel-NAT(ae) | |||
| IC50, ng/mL | CR, % | IC50, ng/mL | CR, % | |
| NAT | 0.63 | 100 | 0.12 | 100 |
| Amphotericin B | >1000 | <0.1 | >1000 | <0.01 |
| Nystatin | >1000 | <0.1 | >1000 | <0.01 |
| Immuno Assays | Antibody, Species | Immunogen, Coating Antigen | IC50, ng/mL | Working Range, ng/mL | LOD, ng/mL | Matrix | Reference |
|---|---|---|---|---|---|---|---|
| ELISA | McAb mouse | BSA-NAT(ga) OVA-NAT(ga) | 1.69 | 0.64–4.46 | 0.59 | Milk Juice Yoghurt Cheese | [21] |
| LFIA | McAb mouse | BSA-NAT(ga) OVA-NAT(ae) | nd | 5–20 | 5.0 10.0 | Milk Yoghurt | [22] |
| ELISA | PcAb rabbit | TTd-ATB(cuaac) GEL(pi)-NAT | 6.0 | 0.6–50 | 0.1 | Serum | [23] |
| ELISA | PcAb rabbit | TTd-aca-NAT Gel-NAT(ae) | 0.12 | 0.04–1.2 | 0.02 | Bakery products Wine and Beer Beverages Yoghurt Cheese | Present study |
| Matrix | Fortification Level, µg/kg or µg/L | Extract Dilution | RC, % | CV, % |
|---|---|---|---|---|
| White bread | 1000 | 200 | 102 | 10.2 |
| (wheat) | 100 | 200 | 98 | 10.9 |
| 10 | 20 | 75.4 | 6.5 | |
| 1 | 20 | 89.4 | 5.7 | |
| Black bread | 1000 | 200 | 81.6 | 10.3 |
| (rye) | 100 | 200 | 79.8 | 11.2 |
| 10 | 20 | 88.7 | 10.1 | |
| 1 | 20 | 86.8 | 7.3 | |
| Wine | 1000 | 200 | 96.0 | 8.3 |
| (red dry) | 100 | 200 | 84.4 | 3.1 |
| Wine | 1000 | 200 | 96.4 | 8.8 |
| (white semisweet) | 100 | 200 | 84.4 | 10.9 |
| Beer | 1000 100 | 200 200 | 101.2 95.3 | 4.5 3.6 |
| Juice | 1000 | 200 | 98.6 | 9.5 |
| (apple) | 100 | 200 | 91.7 | 9.2 |
| Juice | 1000 | 200 | 106 | 9.9 |
| (banana) | 100 | 200 | 94.0 | 10.0 |
| Juice | 1000 | 200 | 92.7 | 9.6 |
| (orange) | 100 | 200 | 89.6 | 9.1 |
| Juice | 1000 | 200 | 95.4 | 11.1 |
| (tomato) | 100 | 200 | 73.5 | 9.2 |
| Juice | 10 | 15 | 105 | 9.8 |
| (pear) | 1 | 15 | 94.4 | 12.0 |
| Sauce | 1000 | 200 | 85.8 | 4.3 |
| (soy) | 100 | 200 | 72.4 | 4.1 |
| Yoghurt | 100 | 200 | 87.5 | 7.9 |
| 10 | 200 | 84.4 | 3.9 | |
| Cheese | 100 | 200 | 91.8 | 6.1 |
| 10 | 200 | 102.7 | 4.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burkin, M.A.; Moshcheva, A.G.; Galvidis, I.A. Immunoassay for Natamycin Trace Screening: Bread, Wine and Other Edibles Analysis. Biosensors 2022, 12, 493. https://doi.org/10.3390/bios12070493
Burkin MA, Moshcheva AG, Galvidis IA. Immunoassay for Natamycin Trace Screening: Bread, Wine and Other Edibles Analysis. Biosensors. 2022; 12(7):493. https://doi.org/10.3390/bios12070493
Chicago/Turabian StyleBurkin, Maksim A., Anastasia G. Moshcheva, and Inna A. Galvidis. 2022. "Immunoassay for Natamycin Trace Screening: Bread, Wine and Other Edibles Analysis" Biosensors 12, no. 7: 493. https://doi.org/10.3390/bios12070493
APA StyleBurkin, M. A., Moshcheva, A. G., & Galvidis, I. A. (2022). Immunoassay for Natamycin Trace Screening: Bread, Wine and Other Edibles Analysis. Biosensors, 12(7), 493. https://doi.org/10.3390/bios12070493