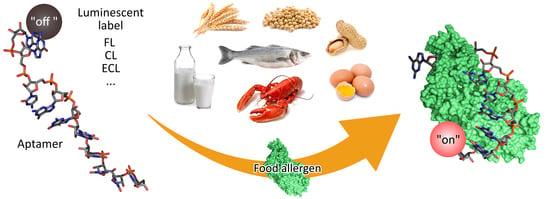
Luminescent Aptamer-Based Bioassays for Sensitive Detection of Food Allergens
Abstract
:1. Introduction
2. Food Matrices and Sample Pretreatment for Portable Biosensors
3. Luminescence-Based Aptasensors for Food Allergens
3.1. Fluorescent Aptasensors
3.2. Chemiluminescent Aptasensors
3.3. Electrochemiluminescent Aptasensors
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soon, J.M.; Brazier, A.K.M.; Wallace, C.A. Determining common contributory factors in food safety incidents—A review of global outbreaks and recalls 2008–2018. Trends Food Sci. Technol. 2020, 97, 76–87. [Google Scholar] [CrossRef]
- Littleton, P.; Walker, M.; Ward, R. Controlling cross-contamination by food allergens. Food Sci. Technol. 2021, 35, 47–51. [Google Scholar]
- Kumar, S.; Dilbaghi, N.; Barnela, M.; Bhanjana, G.; Kumar, R. Biosensors as novel platforms for detection of food pathogens and allergens. BioNanoScience 2012, 2, 196–217. [Google Scholar] [CrossRef]
- Alves, R.C.; Barroso, M.F.; González-García, M.B.; Oliveira, M.B.P.; Delerue-Matos, C. New trends in food allergens detection: Toward biosensing strategies. Crit. Rev. Food Sci. Nutr. 2016, 56, 2304–2319. [Google Scholar] [CrossRef] [PubMed]
- Aquino, A.; Conte-Junior, C.A. A systematic review of food allergy: Nanobiosensor and food allergen detection. Biosensors 2020, 10, 194. [Google Scholar] [CrossRef]
- Amaya-González, S.; De-los-Santos-Álvarez, N.; Miranda-Ordieres, A.J.; Lobo-Castañón, M.J. Aptamer-based analysis: A promising alternative for food safety control. Sensors 2013, 13, 16292–16311. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, H.; Cheng, J.H. DNA, protein and aptamer-based methods for seafood allergens detection: Principles, comparisons and updated applications. Crit. Rev. Food Sci. Nutr. 2021; in press. [Google Scholar] [CrossRef]
- Gupta, R.; Raza, N.; Bhardwaj, S.K.; Vikrant, K.; Kim, K.H.; Bhardwaj, N. Advances in nanomaterial-based electrochemical biosensors for the detection of microbial toxins, pathogenic bacteria in food matrices. J. Hazard. Mater. 2021, 401, 123379. [Google Scholar] [CrossRef]
- Riu, J.; Giussani, B. Electrochemical biosensors for the detection of pathogenic bacteria in food. Trac Trends Anal. Chem. 2020, 126, 115863. [Google Scholar] [CrossRef]
- Zangheri, M.; Di Nardo, F.; Calabria, D.; Marchegiani, E.; Anfossi, L.; Guardigli, M.; Mirasoli, M.; Baggiani, C.; Roda, A. Smartphone biosensor for point-of-need chemiluminescence detection of ochratoxin A in wine and coffee. Anal. Chim. Acta 2021, 1163, 338515. [Google Scholar] [CrossRef]
- Oliveira, I.S.; da Silva Junior, A.G.; de Andrade, C.A.S.; Oliveira, M.D.L. Biosensors for early detection of fungi spoilage and toxigenic and mycotoxins in food. Curr. Opin. Food Sci. 2019, 29, 64–79. [Google Scholar] [CrossRef]
- Xie, M.; Zhao, F.; Zhang, Y.; Xiong, Y.; Han, S. Recent advances in aptamer-based optical and electrochemical biosensors for detection of pesticides and veterinary drugs. Food Control 2022, 131, 108399. [Google Scholar] [CrossRef]
- Tao, X.; Peng, Y.; Liu, J. Nanomaterial-based fluorescent biosensors for veterinary drug detection in foods. J. Food Drug Anal. 2020, 28, 7. [Google Scholar] [CrossRef]
- Verma, M.L.; Rani, V. Biosensors for toxic metals, polychlorinated biphenyls, biological oxygen demand, endocrine disruptors, hormones, dioxin, phenolic and organophosphorus compounds: A review. Environ. Chem. Lett. 2020, 19, 1657–1666. [Google Scholar] [CrossRef]
- Lu, X.; Sun, J.; Sun, X. Recent advances in biosensors for the detection of estrogens in the environment and food. Trac Trends Anal. Chem. 2020, 127, 115882. [Google Scholar] [CrossRef]
- Pilolli, R.; Monaci, L.; Visconti, A. Advances in biosensor development based on integrating nanotechnology and applied to food-allergen management. Trac Trends Anal. Chem. 2013, 47, 12–26. [Google Scholar] [CrossRef]
- Calabria, D.; Calabretta, M.M.; Zangheri, M.; Marchegiani, E.; Trozzi, I.; Guardigli, M.; Michelini, E.; Di Nardo, F.; Anfossi, L.; Baggiani, C.; et al. Recent advancements in enzyme-based Lateral Flow Immunoassays. Sensors 2021, 21, 3358. [Google Scholar] [CrossRef]
- Calabretta, M.M.; Zangheri, M.; Calabria, D.; Lopreside, A.; Montali, L.; Marchegiani, E.; Trozzi, I.; Guardigli, M.; Mirasoli, M.; Michelini, E. Paper-based immunosensors with bio-chemiluminescence detection. Sensors 2021, 21, 4309. [Google Scholar] [CrossRef]
- Ross, G.; Salentijn, G.I.; Nielen, M.W. A critical comparison between flow-through and lateral flow immunoassay formats for visual and smartphone-based multiplex allergen detection. Biosensors 2019, 9, 143. [Google Scholar] [CrossRef]
- 3M Allergen Tests. Available online: https://www.3mcanada.ca/3M/en_CA/p/c/lab-supplies-testing/tests-indicators/allergen (accessed on 15 June 2022).
- Food Allergy/Allergen. Available online: https://www.regabio.com/food-allergen (accessed on 15 June 2022).
- Fast and Reliable Test Kits for Food Allergen Detection. Available online: https://www.romerlabs.com/en/products/test-kits/food-allergen-test-kits (accessed on 15 June 2022).
- AlerTox® Sticks. Available online: https://www.hygiena.com/food-safety-solutions/allergen-detection/alertox-sticks (accessed on 15 June 2022).
- Aller-ROSA. Available online: https://www.charm.com/products/test-and-kits/allergen-tests/aller-rosa (accessed on 15 June 2022).
- Allergens. Available online: https://www.neogen.com/en-gb/categories/allergens (accessed on 15 June 2022).
- Allergens Lateral Flow Tests. Available online: https://www.eurofins-technologies.com/products/allergens/allergen-lateral-flow-tests (accessed on 15 June 2022).
- Mishra, G.K.; Sharma, V.; Mishra, R.K. Electrochemical aptasensors for food and environmental safeguarding: A review. Biosensors 2018, 8, 28. [Google Scholar] [CrossRef]
- Li, Z.; Mohamed, M.A.; Vinu Mohan, A.M.; Zhu, Z.; Sharma, V.; Mishra, G.K.; Mishra, R.K. Application of electrochemical aptasensors toward clinical diagnostics, food, and environmental monitoring. Sensors 2019, 19, 5435. [Google Scholar] [CrossRef] [PubMed]
- Khedri, M.; Ramezani, M.; Rafatpanah, H.; Abnous, K. Detection of food-born allergens with aptamer-based biosensors. Trac Trends Anal. Chem. 2018, 103, 126–136. [Google Scholar] [CrossRef]
- Song, S.H.; Gao, Z.F.; Guo, X.; Chen, G.H. Aptamer-based detection methodology studies in food safety. Food Anal. Methods 2019, 12, 966–990. [Google Scholar] [CrossRef]
- Kaur, H.; Shorie, M. Nanomaterial based aptasensors for clinical and environmental diagnostic applications. Nanoscale Adv. 2019, 1, 2123–2138. [Google Scholar] [CrossRef]
- Kudłak, B.; Wieczerzak, M. Aptamer based tools for environmental and therapeutic monitoring: A review of developments, applications, future perspectives. Crit. Rev. Environ. Sci. Technol. 2020, 50, 816–867. [Google Scholar] [CrossRef]
- Morales, M.A.; Halpern, J.M. Guide to selecting a biorecognition element for biosensors. Bioconjug. Chem. 2018, 29, 3231–3239. [Google Scholar] [CrossRef]
- Berezovski, M.; Musheev, M.; Drabovich, A.; Krylov, S.N. Non-SELEX selection of aptamers. J. Am. Chem. Soc. 2006, 128, 1410–1411. [Google Scholar] [CrossRef]
- Manju, N.; Samiha, C.M.; Kumar, S.P.P.; Gururaj, H.L.; Flammini, F. Prediction of aptamer protein interaction using random forest algorithm. IEEE Access 2022, 10, 49677–49687. [Google Scholar] [CrossRef]
- Emami, N.; Ferdousi, R. AptaNet as a deep learning approach for aptamer-protein interaction prediction. Sci. Rep. 2021, 11, 6074. [Google Scholar] [CrossRef]
- Weng, X.; Neethirajan, S. A microfluidic biosensor using graphene oxide and aptamer functionalized quantum dots for peanut allergen detection. Biosens. Bioelectron. 2016, 68, 649–656. [Google Scholar] [CrossRef]
- Zhou, J.; Ai, R.; Weng, J.; Li, L.; Zhou, C.; Ma, A.; Fu, L.; Wang, Y. A “on-off-on” fluorescence aptasensor using carbon quantum dots and graphene oxide for ultrasensitive detection of the major shellfish allergen Arginine kinase. Microchem. J. 2020, 158, 105171. [Google Scholar] [CrossRef]
- Nadal, P.; Pinto, A.; Svobodova, M.; Canela, N.; O’Sullivan, C.K. DNA Aptamers against the Lup an 1 food allergen. PLoS ONE 2012, 7, e35253. [Google Scholar] [CrossRef] [PubMed]
- Amaya-González, S.; de-los-Santos-Álvarez, N.; Miranda-Ordieres, A.J.; Lobo-Castañón, M.J. Aptamer binding to celiac disease-triggering hydrophobic proteins: A sensitive gluten detection approach. Anal. Chem. 2014, 86, 2733–2739. [Google Scholar] [CrossRef] [PubMed]
- Eissa, S.; Zourob, M. In vitro selection of DNA aptamers targeting β-lactoglobulin and their integration in graphene-based bioassay for the detection of milk allergen. Biosens. Bioelectron. 2017, 91, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.P.; Jie, G.F.; Cui, R.J.; Zhu, J.J. DNA aptamer-based detection of lysozyme by an electrochemiluminescence assay coupled to quantum dots. Electrochem. Commun. 2009, 11, 816–818. [Google Scholar] [CrossRef]
- Cox, C.J.; Ellington, A.D. Automated selection of anti-protein aptamers. Bioorgan. Med. Chem. 2011, 9, 2525–2531. [Google Scholar] [CrossRef]
- Kirby, R.; Cho, E.J.; Gehrke, B.; Bayer, T.; Park, Y.S.; Neikirk, D.P.; McDevitt, J.T.; Ellington, A.D. Aptamer-based sensor arrays for the detection and quantitation of proteins. Anal. Chem. 2004, 76, 4066–4075. [Google Scholar] [CrossRef]
- Mishra, R.K.; Hayat, A.; Mishra, G.K.; Catanante, G.; Sharma, V.; Marty, J.L. A novel colorimetric competitive aptamer assay for lysozyme detection based on superparamagnetic nanobeads. Talanta 2017, 165, 436–441. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Zhou, J.; Qi, Q.; Fu, L. A colorimetric and fluorescent gold nanoparticle-based dual-mode aptasensor for parvalbumin detection. Microchem. J. 2020, 159, 105413. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Q.; Wei, X.; Zhang, J.; Mo, S. DNA aptamer for use in a fluorescent assay for the shrimp allergen tropomyosin. Microchim. Acta 2017, 184, 633–639. [Google Scholar] [CrossRef]
- Dong, Y.; Xu, Y.; Yong, W.; Chu, X.; Wang, D. Aptamer and its potential applications for food safety. Crit. Rev. Food Sci. Nutr. 2014, 54, 1548–1561. [Google Scholar] [CrossRef] [PubMed]
- Kurup, C.P.; Tlili, C.; Zakaria, S.N.A.; Ahmed, M.U. Recent trends in design and development of nanomaterial-based aptasensors. Biointerface Res. Appl. Chem. 2021, 11, 14057–14077. [Google Scholar]
- Yoo, H.; Jo, H.; Oh, S.S. Detection and beyond: Challenges and advances in aptamer-based biosensors. Mater. Adv. 2020, 1, 2663–2687. [Google Scholar] [CrossRef]
- Moreno, M. Sensors/Aptasensors. In Encyclopedia of Analytical Science, 3rd ed.; Worsfold, P., Townshend, A., Poole, C.F., Miró, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 9, pp. 150–153. [Google Scholar]
- Food allergen labelling and Consumer Protection Act. In Public Law; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2004; pp. 108–282.
- Immer, U. Factors affecting the effectiveness of allergen detection. In Detecting Allergens in Food; Koppelman, S.J., Hefle, S.L., Eds.; Woodhead Publishing: Cambridge, UK, 2006; pp. 330–347. [Google Scholar]
- Zangheri, M.; Calabretta, M.M.; Calabria, D.; Fiori, J.; Guardigli, M.; Michelini, E.; Melandri, S.; Maris, A.; Mirasoli, M.; Evangelisti, L. Immunological analytical techniques for cosmetics quality control and process monitoring. Processes 2021, 9, 1982. [Google Scholar] [CrossRef]
- Amaya-González, S.; de-los-Santos-Álvarez, N.; Miranda-Ordieres, A.J.; Lobo-Castañón, M.J. Sensitive gluten determination in gluten-free foods by an electrochemical aptamer-based assay. Anal. Bioanal Chem. 2015, 407, 6021–6029. [Google Scholar] [CrossRef]
- Mattarozzi, M.; Toma, L.; Bertucci, A.; Giannetto, M.; Careri, M. Aptamer-based assays: Strategies in the use of aptamers conjugated to magnetic micro-and nanobeads as recognition elements in food control. Anal. Bioanal. Chem. 2022, 414, 63–74. [Google Scholar] [CrossRef]
- Westphal, C.D.; Pereira, M.R.; Raybourne, R.B.; Williams, K.M. Evaluation of extraction buffers using the current approach of detecting multiple allergenic and nonallergenic proteins in food. J. AOAC Int. 2004, 87, 1458–1465. [Google Scholar] [CrossRef]
- Poms, R.; Emons, H.; Anklam, E. Reference materials and method validation in allergen detection. In Detecting Allergens in Food; Koppelman, S.J., Hefle, S.E., Eds.; Woodhead Publishing Ltd.: Cambridge, UK, 2006; pp. 348–356. [Google Scholar]
- Taylor, S.L.; Nordlee, J.A.; Niemann, L.M.; Lambrecht, D.M. Allergen immunoassays-considerations for use of naturally incurred standards. Anal. Bioanal. Chem. 2009, 395, 83–92. [Google Scholar] [CrossRef]
- Matsuda, R.; Yoshioka, Y.; Akiyama, H.; Aburatani, K.; Watanabe, Y.; Matsumoto, T.; Morishita, N.; Sato, H.; Mishima, T.; Gamo, R.; et al. Interlaboratory evaluation of two enzyme-linked immunosorbent assay kits for the detection of egg, milk, wheat, buckwheat, and peanut in foods. J. AOAC Int. 2006, 89, 1600–1608. [Google Scholar] [CrossRef]
- Ito, K.; Yamamoto, T.; Oyama, Y.; Tsuruma, R.; Saito, E.; Saito, Y.; Ozu, T.; Honjoh, T.; Adachi, R.; Sakai, S.; et al. Food allergen analysis for processed food using a novel extraction method to eliminate harmful reagents for both ELISA and lateral-flow tests. Anal. Bioanal. Chem. 2016, 408, 5973–5984. [Google Scholar] [CrossRef]
- Tuzimski, T.; Petruczynik, A. Review of new trends in the analysis of allergenic residues in foods and cosmetic products. J. AOAC Int. 2020, 103, 997–1028. [Google Scholar] [CrossRef] [PubMed]
- Stidham, S.; Villareal, V.; Chellappa, V.; Yoder, L.; Alley, O.; Shreffler, W.; Spergel, J.; Fleischer, D.; Sampson, H.; Gilboa-Geffen, A. Aptamer based point of care diagnostic for the detection of food allergens. Sci. Rep. 2022, 12, 1303. [Google Scholar] [CrossRef] [PubMed]
- Roda, A.; Arduini, F.; Mirasoli, M.; Zangheri, M.; Fabiani, L.; Colozza, N.; Marchegiani, E.; Simoni, P.; Moscone, D. A challenge in biosensors: Is it better to measure a photon or an electron for ultrasensitive detection? Biosens. Bioelectron. 2020, 155, 112093. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Pan, M.; Xie, X.; Liu, K.; Yang, J.; Wang, S.; Wang, S. Aptamer-based fluorescent biosensor for the rapid and sensitive detection of allergens in food matrices. Foods 2021, 10, 2598. [Google Scholar] [CrossRef]
- Zhang, G.X.; Liu, Y.L.; Yang, M.; Huang, W.S.; Xu, J.H. An aptamer-based, fluorescent and radionuclide dual-modality probe. Biochimie 2020, 171–172, 55–62. [Google Scholar] [CrossRef]
- Ma, P.F.; Guo, H.L.; Duan, N.; Ma, X.Y.; Yue, L.; Gu, Q.H.; Wang, Z.P. Label free structure-switching fluorescence polarization detection of chloramphenicol with truncated aptamer. Talanta 2021, 230, 122349. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Q.; Sun, M.; Zhang, J.; Mo, S.; Wang, J.; Wei, X.; Bai, J. Magnetic-assisted aptamer-based fluorescent assay for allergen detection in food matrix. Sens. Actuators B Chem. 2018, 263, 43–49. [Google Scholar] [CrossRef]
- Gao, L.; Li, Q.; Li, R.; Yan, L.; Zhou, Y.; Chen, K.; Shi, H. Highly sensitive detection for proteins using graphene oxide-aptamer based sensors. Nanoscale 2015, 7, 10903–10907. [Google Scholar] [CrossRef]
- Chinnappan, R.; Rahamn, A.A.; AlZabn, R.; Kamath, S.; Lopata, A.L.; Abu-Salah, K.M.; Zourob, M. Aptameric biosensor for the sensitive detection of major shrimp allergen, tropomyosin. Food Chem. 2020, 314, 126133. [Google Scholar] [CrossRef]
- Shi, M.; Cen, Y.; Sohail, M.; Xu, G.; Wei, F.; Ma, Y.; Xu, X.; Ma, Y.; Song, Y.; Hu, Q. Aptamer based fluorometric β-lactoglobulin assay based on the use of magnetic nanoparticles and carbon dots. Microchim. Acta 2018, 185, 40. [Google Scholar] [CrossRef]
- Sapkota, K.; Dhakal, S. FRET-based aptasensor for the selective and sensitive detection of lysozyme. Sensors 2020, 20, 914. [Google Scholar] [CrossRef] [PubMed]
- Mairal, T.; Nadal, P.; Svobodova, M.; O’Sullivan, C.K. FRET-based dimeric aptamer probe for selective and sensitive Lup an 1 allergen detection. Biosens. Bioelectron. 2014, 54, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Phadke, C.; Tada, S.; Kono, I.; Hiyama, A.; Takase, Y.; Gayama, S.; Aigaki, T.; Ito, Y.; Uzawa, T. Instantaneous detection of αs-casein in cow’s milk using fluorogenic peptide aptamers. Anal. Methods 2020, 12, 1368–1373. [Google Scholar] [CrossRef]
- Leung, K.H.; He, H.Z.; Chan, D.S.H.; Fu, W.C.; Leung, C.H.; Ma, D.L. An oligonucleotide-based switch-on luminescent probe for the detection of kanamycin in aqueous solution. Sens. Actuators B Chem. 2013, 177, 487–492. [Google Scholar] [CrossRef]
- Li, Y.; Lee, H.J.; Corn, R.M. Detection of protein biomarkers using RNA aptamer microarrays and enzymatically amplified surface plasmon resonance imaging. Anal. Chem. 2007, 79, 1082–1088. [Google Scholar] [CrossRef]
- Baldrich, E.; Acero, J.L.; Reekmans, G.; Laureyn, W.; O’Sullivan, C.K. Displacement enzyme linked aptamer assay. Anal. Chem. 2005, 77, 4774–4784. [Google Scholar] [CrossRef]
- Levy, M.; Cater, S.F.; Ellington, A.D. Quantum-dot aptamer beacons for the detection of proteins. ChemBioChem 2005, 6, 2163–2166. [Google Scholar] [CrossRef]
- Polsky, R.; Gill, R.; Kaganovsky, L.; Willner, I. Nucleic acid-functionalized Pt nanoparticles: Catalytic labels for the amplified electrochemical detection of biomolecules. Anal. Chem. 2006, 78, 2268–2271. [Google Scholar] [CrossRef]
- Travascio, P.; Li, Y.; Sen, D. DNA-enhanced peroxidase activity of a DNA aptamer-hemin complex. Chem. Biol. 1998, 5, 505–517. [Google Scholar] [CrossRef]
- Travascio, P.; Bennet, A.J.; Wang, D.Y.; Sen, D. A ribozyme and a catalytic DNA with peroxidase activity: Active sites versus cofactor-binding sites. Chem. Biol. 1999, 6, 779–787. [Google Scholar] [CrossRef]
- Xiao, Y.; Pavlov, V.; Gill, R.; Bourenko, T.; Willner, I. Lighting up biochemiluminescence by the surface self-assembly of DNA-hemin complexes. ChemBioChem 2004, 5, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Höbartner, C.; Silverman, S.K. Recent advances in DNA catalysis. Biopolymers 2007, 87, 279–292. [Google Scholar] [CrossRef]
- Willner, I.; Shlyahovsky, B.; Zayats, M.; Willner, B. DNAzymes for sensing, nanobiotechnology and logic gate applications. Chem. Soc. Rev. 2008, 37, 1153–1165. [Google Scholar] [CrossRef]
- Zhang, X.B.; Kong, R.M.; Lu, Y. Metal ion sensors based on DNAzymes and related DNA molecules. Annu. Rev. Anal. Chem. 2011, 4, 105–128. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, E.; Dong, S. G-quadruplex-based DNAzyme for facile colorimetric detection of thrombin. Chem. Commun. 2008, 31, 3654–3656. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, E.; Dong, S. Chemiluminescence thrombin aptasensor using high-activity DNAzyme as catalytic label. Chem. Commun. 2008, 21, 5520–5522. [Google Scholar] [CrossRef]
- Bi, S.; Li, L.; Zhang, S. Triggered polycatenated DNA scaffolds for DNA sensors and aptasensors by a combination of rolling circle amplification and DNAzyme amplification. Anal. Chem. 2010, 82, 9447–9454. [Google Scholar] [CrossRef]
- Elbaz, J.; Moshe, M.; Shlyahovsky, B.; Willner, I. Cooperative multicomponent self-assembly of nucleic acid structures for the activation of DNAzyme cascades: A paradigm for DNA sensors and aptasensors. Chem. Eur. J. 2009, 15, 3411–3418. [Google Scholar] [CrossRef]
- Li, D.; Shlyahovsky, B.; Elbaz, J.; Willner, I. Amplified analysis of low-molecular-weight substrates or proteins by the self-assembly of DNAzyme-aptamer conjugates. J. Am. Chem. Soc. 2007, 129, 5804–5805. [Google Scholar] [CrossRef]
- Liu, X.; Freeman, R.; Golub, E.; Willner, I. Chemiluminescence and chemiluminescence resonance energy transfer (CRET) aptamer sensors using catalytic hemin/G-quadruplexes. ACS Nano 2011, 5, 7648–7655. [Google Scholar] [CrossRef]
- Hao, L.; Duan, N.; Wu, S.; Xu, B.; Wang, Z. Chemiluminescent aptasensor for chloramphenicol based on N-(4-aminobutyl)-N-ethylisoluminol-functionalized flower-like gold nanostructures and magnetic nanoparticles. Anal. Bioanal. Chem. 2015, 407, 7907–7915. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Gu, H.; Duan, N.; Wu, S.; Wang, Z. A chemiluminescent aptasensor for simultaneous detection of three antibiotics in milk. Anal. Methods 2016, 8, 7929–7936. [Google Scholar] [CrossRef]
- Yang, L.; Ni, H.; Li, C.; Zhang, X.; Wen, K.; Ke, Y.; Yang, H.; Shi, W.; Zhang, S.; Shen, J.; et al. Development of a highly specific chemiluminescence aptasensor for sulfamethazine detection in milk based on in vitro selected aptamers. Sens. Actuat. B Chem. 2019, 281, 801–811. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, X.; Duan, W.; Li, F. A label-free, versatile and low-background chemiluminescence aptasensing strategy based on gold nanocluster catalysis combined with the separation of magnetic beads. Analyst 2018, 143, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Ji, Y.; Xiao, Y.; Xue, X.; Liu, J.; Li, S.; Ai, F.; Zheng, X. One-pot label-free dual-aptasensor as a chemiluminescent tool kit simultaneously detect adenosine triphosphate and chloramphenicol in foods. Talanta 2021, 229, 122226. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; He, D.; Xu, E.; Jiao, A.; Chughtai, M.F.J.; Jin, Z. Rapid detection of β-conglutin with a novel lateral flow aptasensor assisted by immunomagnetic enrichment and enzyme signal amplification. Food Chem. 2018, 269, 375–379. [Google Scholar] [CrossRef]
- Du, F.K.; Zhang, H.; Tan, X.C.; Yan, J.; Liu, M.; Chen, X.; Wu, Y.Y.; Feng, D.F.; Chen, Q.Y.; Cen, J.M.; et al. Ru(bpy)32+-Silica@Poly-L-lysine-Au as labels for electrochemiluminescence lysozyme aptasensor based on 3D graphene. Biosens. Bioelectron. 2018, 106, 50–56. [Google Scholar] [CrossRef]
- Roda, A.; Di Fusco, M.; Quintavalla, A.; Guardigli, M.; Mirasoli, M.; Lombardo, M.; Trombini, C. Dioxetane-doped silica nanoparticles as ultrasensitive reagentless thermochemiluminescent labels for bioanalytics. Anal. Chem. 2012, 84, 9913–9919. [Google Scholar] [CrossRef]
- Roda, A.; Zangheri, M.; Calabria, D.; Mirasoli, M.; Caliceti, C.; Quintavalla, A.; Lombardo, M.; Trombini, C.; Simoni, P. A simple smartphone-based thermochemiluminescent immunosensor for valproic acid detection using 1,2-dioxetane analogue-doped nanoparticles as a label. Sens. Actuators B Chem. 2019, 279, 327–333. [Google Scholar] [CrossRef]




| Commercial Name | Allergen or Food | Limit of Detection (ppm) | Company | Reference |
|---|---|---|---|---|
| 3M Rapid Kit | Almond | 2 | 3M (Saint Paul, MN, USA) | [20] |
| Cashew | 2 | |||
| Coconut | 2 | |||
| Egg | 0.5 | |||
| Fish | 1 | |||
| Gluten | 5 | |||
| Hazelnut | 2 | |||
| Milk | 3 | |||
| Peanut | 1 | |||
| Pecan | 3 | |||
| Pistachio | 2 | |||
| Soy | 2 | |||
| Walnut | 2 | |||
| Agitest | Almond | 1 | Rega Biotechnology Inc. (New Taipei City, Taiwan) | [21] |
| Buckwheat | 1 | |||
| Casein | 100 | |||
| Egg | 1 | |||
| Fish | 0.1 | |||
| Gluten | 20 | |||
| Mango | 2 | |||
| Peanut | 1 | |||
| Sesame | 0.2 | |||
| Shellfish | 1 | |||
| Soy | 10 | |||
| AgraStrip | Almond | 2 | Romer Labs GmbH (Getzersdorf, Austria) | [22] |
| B-Lactoglobulin | 0.5 | |||
| Brazil Nut | 5 | |||
| Casein | 1 | |||
| Cashew/Pistachio | 2 | |||
| Crustacean | 2 | |||
| Coconut | 10 | |||
| Gluten | 4 | |||
| Hazelnut | 5 | |||
| Lupin | 10 | |||
| Macadamia Nut | 2 | |||
| Milk | 1 | |||
| Mustard | 2 | |||
| Peanut | 1 | |||
| Sesame | 5 | |||
| Soy | 2 | |||
| Walnut | 10 | |||
| Whole Egg | ||||
| AlerTox Sticks | Almond | 20 | Hygiena LLC (Camarillo, CA, USA) | [23] |
| Β-Lactoglobulin | 2.5 | |||
| Casein | 2.5 | |||
| Crustacean | 10 | |||
| Egg | 1.25 | |||
| Fish | 5 | |||
| Hazelnut | 20 | |||
| Milk | 2.5 | |||
| Mustard | 2 | |||
| Peanut | 1 | |||
| Sesame | 3 | |||
| Soy | 10 | |||
| Walnut | 2.25 | |||
| Aller-ROSA | Milk | 2–5 | Charm Sciences Inc. (Lawrence, MA, USA) | [24] |
| Reveal/Reveal 3D | Almond | 1 | Neogen Co. (Lansing, MI, USA) | [25] |
| Coconut | 1 | |||
| Crustacean | 1–5 | |||
| Egg | 2.4 | |||
| Gliadin | 5 | |||
| Gluten | 5–10 | |||
| Hazelnut | 0.75–1.5 | |||
| Milk | 2 | |||
| Multi-Tree nuts | 1–2 | |||
| Mustard | 1.3 | |||
| Peanut | 1.3 | |||
| Sesame | 1 | |||
| Soy | 2.5 | |||
| SENSIStrip | Almond | 1 | Eurofin Technologies (Budapest, Hungary) | [26] |
| Casein | 20 | |||
| Shellfish | 1 | |||
| Egg | 1 | |||
| Fish | 1 | |||
| Peanut | 1 | |||
| Soy | 10 | |||
| Gluten | 2 |
| Allergen | Aptamer Sequence | Reference |
|---|---|---|
| Ara h 1 1 | (5’) TCG CAC ATT CCG CTT CTA CCG GGG GGG TCG AGC GAG TGA GCG AAT CTG TGG GTG GGC CGT AAG TCC GTG TGT GCG AA (3’) | [37] |
| Arginine kinase 2 | (5’) GGC GAA CAG CAG CGC GAT TCG GGT TGC GGA TAG TGA CAT A (3’) | [38] |
| β-Conglutin 3 | (5’) AGC TGA CAC AGC AGG TTG GTG GGG GCT TCC AGT TGG GTT GAC AAT ACG TAG GGA CAC GAA GTC CAA CCA CGA GTC GAG CAA TCT CGA AAT (3’) | [39] |
| Gluten 4 | (5’) CCA GTC TCC CGT TTA CCG CGC CTA CAC ATG TCT GAA TGC C (3’) | [40] |
| (5’) CTA GGC GAA ATA TAG CTA CAA CTG TCT GAA GGC ACC CAA T (3’) | [40] | |
| β-Lactoglobulin 5 | (5’) CGA CGA TCG GAC CGC AGT ACC CAC CCA CCA GCC CCA ACA TCA TGC CCA TCC GTG TGT G (3’) | [41] |
| Lysozyme 6 | (5’) ATC TAC GAA TTC ATC AGG GCT AAA GAG TGC AGA GTT ACT TAG (3’) | [42] |
| (5’) ATC AGG GCT AAA GAG TGC AGA GTT ACT TAG (3’) | [43] | |
| (5’) GGG AAT GGA TCC ACA TCT ACG AAT TCA TCA GGG CTA AAG AGT GCA GAG TTA CTT AGT TCA CTG CAG ACT TGA CGA AGC TT (3’) | [44] | |
| (5’) GCA GCT AAG CAG GCG GCT CAC AAA ACC ATT CGC ATG CGG C (3’) | [45] | |
| Parvalbumin 7 | (5’) GCC AAA GGA GGC GAG AGA TAA AAG ATT GCG AAT CCA TTC G (3’) | [46] |
| Tropomyosin 8 | (5’) TAC TAA CGG TAC AAG CTA CCA GGC CGC CAA CGT TGA CCT AGA AGC ACT GCC AGA CCC GAA CGT TGA CCT AGA AGC (3’) | [47] |
| Detection Method | Mechanism | Label | Analyte | Detection Limit | Ref. |
|---|---|---|---|---|---|
| Fluorescence Advantages:
| The capture aptamer was conjugated on the surface of MNPs. When the aptamer interacts with target analytes, it was released from the surface of MNPs, thus producing a fluorescent signal by adding the OliGreen dye, which is able to enhance its fluorescence upon binding to ssDNA. | Label-free | Tropomyosin | 0.077 µg mL−1 | [68] |
| Label-free fluorescent approach was exploited by utilizing the OliGreen ssDNA reagent to quantitatively detect the aptamers bound to analyte in solution with the aid of the adsorption of unfolded aptamers by GO. | Label-free | Tropomyosin | 0.15 μg mL−1 | [69] | |
| A fluorescein dye-labeled GO quenches the truncated DNA aptamer. After the addition of the target analyte, the fluorescence was restored due to the competitive binding of the aptamer to GO. | Fluorescein dye | Tropomyosin | 2.5 nmol L−1 | [70] | |
| The formation of QD-DNA aptamer–GO complexes as probes is able to undergo conformational change upon interaction with the target analytes, resulting in fluorescence changes: fluorescence is quenched or recovered depending on the adsorption and desorption of aptamer-QDs on GO. | QDs | Ara h 1 | 56 ng mL−1 | [37] | |
| The aptamer was immoblized on MNPs, and the C-dots served as a label for the cDNA. The aptamer preferentially binds the target analyte, leading to a partial release of the C-dots-cDNA into the solution. After magnetic separation, the solution contained the released C-dots-cDNA, which are quantified by fluorescence. | C-dots | β-lactoglobulin | 37 pg mL−1 | [71] | |
| QDs-DNA aptamer probe and GO were self-assembled to effectively quench the fluorescence of the Qdots. Upon adding the target analyte, the QDs-aptamer was released from the GO surface and formed the QDs–aptamers–analyte complex, leading to a fluorescent signal. | QDs | Arginine kinase | 0.14 ng mL−1 | [38] | |
| The aptamer is composed of two partially cDNA arms, each labeled with either a donor (Cy3) or an acceptor (Cy5) fluorophore to enable FRET when the complementary arms hybridize to one another. | Donor (Cy3) and acceptor (Cy5) fluorophore to enable FRET | Lysozyme | 30 nmol L−1 | [72] | |
| The probe was represented by a dimeric aptamer, with each monomeric aptamer being flanked by donor/acceptor moieties. Upon addition of target analyte, the specific interaction induces a change in the biaptameric structure, resulting in an increase in fluorescence emission. | Donor (Alexa Fluor 488) and acceptor (Alexa Fluor 555) fluorophore to enable FRET | β-conglutin | 150 pmol L−1 | [73] | |
| The assay was based on the use of two fluorogenic peptide aptamers that instantaneously enhance their fluorescence upon binding to a target molecule. | Label free | αs-casein | 0.04 μmol L−1 | [74] | |
| The aptasensor was based on hybridization of the DNA aptamer-modified AuNP, the complementary short chain-modified gold nanoparticles and the fluorescent dye-labeled complementary short chain. The presence of target analyte led to a competition, which allows to observe a change in the solution color of the AuNPs and a recovery of the fluorescence signals of FAM-CS2. | AuNP (colorimetric detection) and Fluorescent dye (fluorescent detection) | Parvalbumin | 0.72 μg mL−1 | [46] | |
| The assay was based on a square–planar luminescent platinum(II) complex and the DNA aptamer. Upon the addition of the target analyte, the aptamer changes from a random-coiled structure into a specific conformation containing a hairpin region, allowing the intercalation of the platinum(II) complex into the bound aptamer and enhancing the luminescence signal. | Label-free | Kanamycin | 140 nmol L−1 | [75] | |
| Chemiluminescence Advantages:
| The assay employed a capture probe, obtained by immobilizing a biotinylated chloramphenicol-specific aptamer on avidin-modified MNPs, and a detection probe consisting of c-DNA sequence-conjugated ABEI-functionalized AuNFs. The analyte and the detection probe compete for binding to the capture probe, followed by magnetic separation of the capture probe and the addition of CL substrate to trigger the CL reaction. | ABEI-AuNFs | Chloramphenicol | 0.01 ng mL−1 | [92] |
| Aptamers specific for the target analytes, acting as capture probes, were immobilized in the wells of a microtiter plate; then, the sample was added to the wells together with detection probes consisting of c-DNA modified with ABEI-functionalized AuNFs. After the competition of the analytes and the detection probes for binding to the immobilized aptamers, the bound detection probes were detected by CL. | ABEI-AuNFs | Oxytetracyclin, tetracycline and kanamycin | 0.02 ng mL−1 (oxytetracycline), 0.02 ng mL−1 (tetracycline) and 0.002 ng mL−1 (kanamycin). | [93] | |
| The assay was performed in a streptavidinated microtiter plate coated with a biotin-functionalized capture DNA aptamer and was based on the competition between the analyte in the sample and a tracer (a sulfamethazine ana-log conjugated to the CL enzyme HRP) for binding to the capture aptamer, followed by CL detection of the bound tracer. | HRP | Sulfamethazine | 0.92 ng mL−1 | [94] | |
| DNA aptamers specific for the target analyte were immobilized on MBs and hybridized with a complementary oligonucleotide sequence labeled with AuNC. In the presence of the target analyte, its interaction with the aptamer resulted in the release of the AuNC-labeled oligonucleotide sequences. The MBs were removed by magnetic separation; then, the released oligonucleotide sequences were detected. | AuNCs | Kanamycin | 0.035 nmol L−1 | [95] | |
| Oligonucleotide capture probes for ATP- and chloramphenicol-binding aptamers were immobilized on polystyrene and magnetic microspheres, respectively. The competition between the analytes and the immobilized capture probes for binding to the aptamers resulted in amounts of aptamer bound to the microspheres that are inversely proportional to the analyte concentrations. The bound aptamers were detected thanks to the CL reaction of the guanine DNA nucleobase with phenylglyoxal and N,N-dimethylformamide. | Label free | ATP and chloramphenicol | 37.6 nmol L−1 (ATP) and 24.8 nmol L−1 (chloramphenicol) | [96] | |
| Electrochemiluminescence Advantages:
| A 3D graphene-modified electrode was coated with AuNPs, then functionalized with a lysozyme binding aptamer hybridized with a complementary single-stranded DNA sequence labeled by RuSiNPs@PLL-Au, which acted as an ECL signal amplifier. In the presence of lysozyme, the cDNA sequence of the duplex was displaced by lysozyme, resulting in weaker ECL emission. | RuSiNPs@ PLL-Au | Lysozyme | 7.5 × 10−13 mol L−1 | [98] |
| Sample was incubated with probes immobilized at Au electrode in order to form the aptamer–lysozyme bioaffinity complexes, and the free probes were hybridized with the biotin modified cDNA oligonucleotides to form double-stranded DNA (ds-DNA) oligonucleotides. Avidin-QDs were bound to these hybridized cDNA through the biotin–avidin system. The ECL signal of the biosensor was responsive to the amount of QDs bonded to the cDNA oligonucleotides, which was inverse proportional to the combined target protein. | QDs | Lysozyme | Not reported | [42] |
| Food Allergen | Aptasensor | LFIA | ||
|---|---|---|---|---|
| LOD 1 | Ref. | LOD 2 | Ref. | |
| Ara h 1 | 56 µg L−1 | [37] | 0.5 mg L−1 (detects Ara h 1, Ara h 2, and Ara h 3) | [23] |
| Casein | 1 mg L−1 | [74] | 0.3 mg L−1 | [21] |
| 0.25 mg L−1 | [23] | |||
| 1.8 mg L−1 | [25] | |||
| 0.03 mg L−1 | [26] | |||
| β-Lactoglobulin | 37 ng L−1 | [71] | 0.25 mg L−1 | [23] |
| Tropomyosin | 77 µg L−1 | [68] | 1.7 µg L−1 | [26] |
| 0.15 mg L−1 | [69] | |||
| 90 µg L−1 | [70] | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calabria, D.; Zangheri, M.; Pour, S.R.S.; Trozzi, I.; Pace, A.; Lazzarini, E.; Calabretta, M.M.; Mirasoli, M.; Guardigli, M. Luminescent Aptamer-Based Bioassays for Sensitive Detection of Food Allergens. Biosensors 2022, 12, 644. https://doi.org/10.3390/bios12080644
Calabria D, Zangheri M, Pour SRS, Trozzi I, Pace A, Lazzarini E, Calabretta MM, Mirasoli M, Guardigli M. Luminescent Aptamer-Based Bioassays for Sensitive Detection of Food Allergens. Biosensors. 2022; 12(8):644. https://doi.org/10.3390/bios12080644
Chicago/Turabian StyleCalabria, Donato, Martina Zangheri, Seyedeh Rojin Shariati Pour, Ilaria Trozzi, Andrea Pace, Elisa Lazzarini, Maria Maddalena Calabretta, Mara Mirasoli, and Massimo Guardigli. 2022. "Luminescent Aptamer-Based Bioassays for Sensitive Detection of Food Allergens" Biosensors 12, no. 8: 644. https://doi.org/10.3390/bios12080644
APA StyleCalabria, D., Zangheri, M., Pour, S. R. S., Trozzi, I., Pace, A., Lazzarini, E., Calabretta, M. M., Mirasoli, M., & Guardigli, M. (2022). Luminescent Aptamer-Based Bioassays for Sensitive Detection of Food Allergens. Biosensors, 12(8), 644. https://doi.org/10.3390/bios12080644