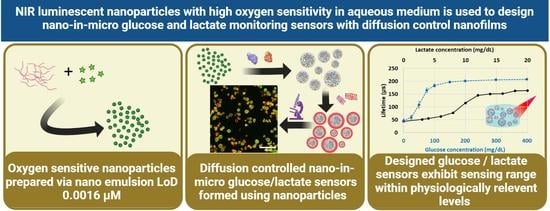
NIR Luminescent Oxygen-Sensing Nanoparticles for Continuous Glucose and Lactate Monitoring
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Measurements and Instrumentation
2.3. Synthesis of NPs Containing Oxygen-Sensitive Phosphor
2.4. Preparation of Calcium Alginate Hydrogel Slab with Oxygen-Sensitive NPs
2.5. Preparation of Poly(2-methacryloyloxyethylphosphorylcholine) (MPC) Hydrogels with NPs
2.6. Preparation of Poly(2-hydroxyethylmethacrylate) (HEMA) Hydrogels with NPs
2.7. Preparation of Poly [2-(methacryloyloxy)ethyl]trimethyl-ammonium (TMA) Chloride Hydrogel with NPs
2.8. Preparation of Alginate Microspheres Containing Oxidase Enzyme and Oxygen-Sensitive NPs
2.9. Preparation of Glucose- and Lactate-Sensing Alginate Hydrogels
2.10. Characterization for Oxygen Sensitivity
2.11. Glucose and Lactate-Sensing Experiments
2.12. Cytotoxicity Studies of NPs and Alginate Microparticles
3. Results and Discussion
3.1. Physical Characteristics of NPs
3.2. Oxygen Sensing Property of NPs
3.3. Effect of Dispersion Medium on Performance of Oxygen-Sensing Nanoparticle
3.4. Cytotoxicity Studies of Non-Encapsulated NPs and Encapsulated NPs within Alginate Microspheres
3.5. Design of NIMs Biosensors Using NPs Encapsulated Microdomains
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brown, J.Q.; Srivastava, R.; McShane, M.J. Encapsulation of glucose oxidase and an oxygen-quenched fluorophore in polyelectrolyte-coated calcium alginate microspheres as optical glucose sensor systems. Biosens. Bioelectron. 2005, 21, 212–216. [Google Scholar] [CrossRef]
- Jeevarathinam, A.S.; Guo, F.; Williams, T.; Smolen, J.A.; Hyde, J.A.; McShane, M.J.; de Figueiredo, P.; Alge, D.L. Enzyme functionalized microgels enable precise regulation of dissolved oxygen and anaerobe culture. Mater. Today Bio 2021, 9, 100092. [Google Scholar] [CrossRef] [PubMed]
- Tric, M.; Lederle, M.; Neuner, L.; Dolgowjasow, I.; Wiedemann, P.; Wölfl, S.; Werner, T. Optical biosensor optimized for continuous in-line glucose monitoring in animal cell culture. Anal. Bioanal. Chem. 2017, 409, 5711–5721. [Google Scholar] [CrossRef]
- Tanno, K. An Automatic Recording Analyzer for the Determination of Dissolved Oxygen in Boiler Feed Water. Bull. Chem. Soc. Jpn. 1964, 37, 804–810. [Google Scholar] [CrossRef] [Green Version]
- Nakano, T.; Hoshi, K.I.; Baba, S. Effect of background gas environment on oxygen incorporation in TiN films deposited using UHV reactive magnetron sputtering. Vacuum 2008, 83, 467–469. [Google Scholar] [CrossRef]
- Berger, H. Contamination due to process gases. Microelectron. Eng. 1991, 10, 259–267. [Google Scholar] [CrossRef]
- Stolper, D.A.; Revsbech, N.P.; Canfield, D.E. Aerobic growth at nanomolar oxygen concentrations. Proc. Natl. Acad. Sci. USA 2010, 107, 18755–18760. [Google Scholar] [CrossRef] [Green Version]
- Shevela, D.; Beckmann, K.; Clausen, J.; Junge, W.; Messinger, J. Membrane-inlet mass spectrometry reveals a high driving force for oxygen production by photosystem II. Proc. Natl. Acad. Sci. USA 2011, 108, 3602–3607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karunakaran, C.; Madasamy, T.; Sethy, N.K. Enzymatic Biosensors. In Biosensors and Bioelectronics; Karunakaran, C., Bhargava, K., Benjamin, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; Chapter 3; pp. 133–204. [Google Scholar]
- Joseph, J.I. Review of the Long-Term Implantable Senseonics Continuous Glucose Monitoring System and Other Continuous Glucose Monitoring Systems. J. Diabetes Sci. Technol. 2020, 15, 167–173. [Google Scholar] [CrossRef]
- Oppel, E.; Kamann, S.; Reichl, F.-X.; Högg, C. The Dexcom glucose monitoring system—An isobornyl acrylate-free alternative for diabetic patients. Contact Dermat. 2019, 81, 32–36. [Google Scholar] [CrossRef]
- Khosravi Ardakani, H.; Gerami, M.; Chashmpoosh, M.; Omidifar, N.; Gholami, A. Recent Progress in Nanobiosensors for Precise Detection of Blood Glucose Level. Biochem. Res. Int. 2022, 2022, 2964705. [Google Scholar] [CrossRef]
- Wisniewski, N.A.; Nichols, S.P.; Gamsey, S.J.; Pullins, S.; Au-Yeung, K.Y.; Klitzman, B.; Helton, K.L. Tissue-Integrating Oxygen Sensors: Continuous Tracking of Tissue Hypoxia. In Oxygen Transport to Tissue XXXIX; Halpern, H.J., LaManna, J.C., Harrison, D.K., Epel, B., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 377–383. [Google Scholar]
- Nichols, S.P.; Balaconis, M.K.; Gant, R.M.; Au-Yeung, K.Y.; Wisniewski, N.A. Long-Term In Vivo Oxygen Sensors for Peripheral Artery Disease Monitoring. In Oxygen Transport to Tissue XL; Thews, O., LaManna, J.C., Harrison, D.K., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 351–356. [Google Scholar]
- Koo Lee, Y.-E.; Ulbrich, E.E.; Kim, G.; Hah, H.; Strollo, C.; Fan, W.; Gurjar, R.; Koo, S.; Kopelman, R. Near Infrared Luminescent Oxygen Nanosensors with Nanoparticle Matrix Tailored Sensitivity. Anal. Chem. 2010, 82, 8446–8455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horka, M.; Sun, S.; Ruszczak, A.; Garstecki, P.; Mayr, T. Lifetime of Phosphorescence from Nanoparticles Yields Accurate Measurement of Concentration of Oxygen in Microdroplets, Allowing One To Monitor the Metabolism of Bacteria. Anal. Chem. 2016, 88, 12006–12012. [Google Scholar] [CrossRef] [PubMed]
- Gkika, K.S.; Kargaard, A.; Burke, C.S.; Dolan, C.; Heise, A.; Keyes, T.E. Ru(II)/BODIPY core co-encapsulated ratiometric nanotools for intracellular O2 sensing in live cancer cells. RSC Chem. Biol. 2021, 2, 1520–1533. [Google Scholar] [CrossRef]
- Haghighi, B.; Bozorgzadeh, S. Enhanced electrochemiluminescence from luminol at multi-walled carbon nanotubes decorated with palladium nanoparticles: A novel route for the fabrication of an oxygen sensor and a glucose biosensor. Anal. Chim. Acta 2011, 697, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Quaranta, M.; Borisov, S.M.; Klimant, I. Indicators for optical oxygen sensors. Bioanal. Rev. 2012, 4, 115–157. [Google Scholar] [CrossRef] [Green Version]
- Neugebauer, U.; Pellegrin, Y.; Devocelle, M.; Forster, R.J.; Signac, W.; Moran, N.; Keyes, T.E. Ruthenium polypyridyl peptide conjugates: Membrane permeable probes for cellular imaging. Chem. Commun. 2008, 42, 5307–5309. [Google Scholar] [CrossRef] [Green Version]
- Szmacinski, H.; Lakowicz, J.R. Fluorescence lifetime-based sensing and imaging. Sens. Actuators B Chem. 1995, 29, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Patrycja, N.-S.; Eddy, F.; Hubert van den, B.; Georges, W. In vitro and in vivo studies of new photoluminescent oxygen sensors for non-invasive intravascular pO2 measurements. In Proceedings of the Society of Photo-Optical Instrumentation Engineers, San Jose, California, USA, 22–27 February 2009. [Google Scholar]
- Satija, J.; Sai, V.V.R.; Mukherji, S. Dendrimers in biosensors: Concept and applications. J. Mater. Chem. 2011, 21, 14367–14386. [Google Scholar] [CrossRef]
- Dunphy, I.; Vinogradov, S.A.; Wilson, D.F. Oxyphor R2 and G2: Phosphors for measuring oxygen by oxygen-dependent quenching of phosphorescence. Anal. Biochem. 2002, 310, 191–198. [Google Scholar] [CrossRef]
- Maisuls, I.; Wang, C.; Gutierrez Suburu, M.E.; Wilde, S.; Daniliuc, C.-G.; Brünink, D.; Doltsinis, N.L.; Ostendorp, S.; Wilde, G.; Kösters, J.; et al. Ligand-controlled and nanoconfinement-boosted luminescence employing Pt(ii) and Pd(ii) complexes: From color-tunable aggregation-enhanced dual emitters towards self-referenced oxygen reporters. Chem. Sci. 2021, 12, 3270–3281. [Google Scholar] [CrossRef]
- Eltermann, M.; Utt, K.; Lange, S.; Jaaniso, R. Sm3+ doped TiO2 as optical oxygen sensor material. Opt. Mater. 2016, 51, 24–30. [Google Scholar] [CrossRef]
- Pandey, G.; Chaudhari, R.; Joshi, B.; Choudhary, S.; Kaur, J.; Joshi, A. Fluorescent Biocompatible Platinum-Porphyrin—Doped Polymeric Hybrid Particles for Oxygen and Glucose Biosensing. Sci. Rep. 2019, 9, 5029. [Google Scholar] [CrossRef] [Green Version]
- Andrus, L.P.; Unruh, R.; Wisniewski, N.A.; McShane, M.J. Characterization of Lactate Sensors Based on Lactate Oxidase and Palladium Benzoporphyrin Immobilized in Hydrogels. Biosensors 2015, 5, 398–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zavareh, A.T.; Ko, B.; Roberts, J.; Elahi, S.; McShane, M.J. A Versatile Multichannel Instrument for Measurement of Ratiometric Fluorescence Intensity and Phosphorescence Lifetime. IEEE Access 2021, 9, 103835–103849. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.Q.; Srivastava, R.; Zhu, H.; McShane, M.J. Enzymatic fluorescent microsphere glucose sensors:evaluation of response under dynamic conditions. Diabetes Technol. Ther. 2006, 8, 288–295. [Google Scholar] [CrossRef] [Green Version]
- Zach, P.W.; Hofmann, O.T.; Klimant, I.; Borisov, S.M. NIR Phosphorescent Intramolecularly Bridged Benzoporphyrins and Their Application in Oxygen-Compensated Glucose Optode. Anal. Chem. 2018, 90, 2741–2748. [Google Scholar] [CrossRef]
- Hiltebrand, L.B.; Kaiser, H.A.; Niedhart, D.J.; Pestel, G.; Kurz, A. Subcutaneous Oxygen Pressure in Spontaneously Breathing Lean and Obese Volunteers: A Pilot Study. Obes. Surg. 2008, 18, 77–83. [Google Scholar] [CrossRef]
- Gehlen, M.H. The centenary of the Stern-Volmer equation of fluorescence quenching: From the single line plot to the SV quenching map. J. Photochem. Photobiol. C Photochem. Rev. 2020, 42, 100338. [Google Scholar] [CrossRef]
- Biring, S.; Sadhu, A.S.; Deb, M. An Effective Optical Dual Gas Sensor for Simultaneous Detection of Oxygen and Ammonia. Sensors 2019, 19, 5124. [Google Scholar] [CrossRef]
- Wasilewska, K.; Winnicka, K. Ethylcellulose—A Pharmaceutical Excipient with Multidirectional Application in Drug Dosage Forms Development. Materials 2019, 12, 3386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Wang, L.; Li, X.; Hu, X.; Han, Y.; Luo, Y.; Wang, Z.; Li, Q.; Aldalbahi, A.; Wang, L.; et al. Size-Dependent Regulation of Intracellular Trafficking of Polystyrene Nanoparticle-Based Drug-Delivery Systems. ACS Appl. Mater. Interfaces 2017, 9, 18619–18625. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, J.; Tanimura, S.I.; Shiragami, T.; Yasuda, M. Concentration-dependent aggregation of water-soluble phosphorus porphyrin in an aqueous solution. J. Porphyr. Phthalocyanines 2012, 16, 210–217. [Google Scholar] [CrossRef]
- Pasternack, R.F.; Fleming, C.; Herring, S.; Collings, P.J.; dePaula, J.; DeCastro, G.; Gibbs, E.J. Aggregation Kinetics of Extended Porphyrin and Cyanine Dye Assemblies. Biophys. J. 2000, 79, 550–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitra, S.; Foster, T.H. Photochemical Oxygen Consumption Sensitized by a Porphyrin Phosphorescent Probe in Two Model Systems. Biophys. J. 2000, 78, 2597–2605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pyrz, W.D.; Buttrey, D.J. Particle Size Determination Using TEM: A Discussion of Image Acquisition and Analysis for the Novice Microscopist. Langmuir 2008, 24, 11350–11360. [Google Scholar] [CrossRef]
- Kestens, V.; Bozatzidis, V.; De Temmerman, P.-J.; Ramaye, Y.; Roebben, G. Validation of a particle tracking analysis method for the size determination of nano- and microparticles. J. Nanoparticle Res. 2017, 19, 271. [Google Scholar] [CrossRef] [Green Version]
- Mills, A.; Hazafy, D. A solvent-based intelligence ink for oxygen. Analyst 2008, 133, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Kochmann, S.; Baleizão, C.; Berberan-Santos, M.N.; Wolfbeis, O.S. Sensing and Imaging of Oxygen with Parts per Billion Limits of Detection and Based on the Quenching of the Delayed Fluorescence of 13C70 Fullerene in Polymer Hosts. Anal. Chem. 2013, 85, 1300–1304. [Google Scholar] [CrossRef]
- Borisov, S.M.; Nuss, G.; Haas, W.; Saf, R.; Schmuck, M.; Klimant, I. New NIR-emitting complexes of platinum (II) and palladium (II) with fluorinated benzoporphyrins. J. Photochem. Photobiol. A Chem. 2009, 201, 128–135. [Google Scholar] [CrossRef]
- Zhang, Y.; Newton, B.; Lewis, E.; Fu, P.P.; Kafoury, R.; Ray, P.C.; Yu, H. Cytotoxicity of organic surface coating agents used for nanoparticles synthesis and stability. Toxicol Vitr. 2015, 29, 762–768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; McShane, M. Enhancing the longevity of microparticle-based glucose sensors towards 1 month continuous operation. Biosens. Bioelectron. 2010, 25, 1075–1081. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-S.; Johnson, P.J.; Robbins, P.T.; Bridson, R.H. Production of nanoparticles-in-microparticles by a double emulsion method: A comprehensive study. Eur. J. Pharm. Biopharm. 2013, 83, 168–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, R.J.; Pishko, M.V.; Gefrides, C.C.; McShane, M.J.; Cote, G.L. A fluorescence-based glucose biosensor using concanavalin A and dextran encapsulated in a poly (ethylene glycol) hydrogel. Anal. Chem. 1999, 71, 3126–3132. [Google Scholar] [CrossRef] [PubMed]
- Unruh, R.M.; Roberts, J.R.; Nichols, S.P.; Gamsey, S.; Wisniewski, N.A.; McShane, M.J. Preclinical Evaluation of Poly (HEMA-co-acrylamide) Hydrogels Encapsulating Glucose Oxidase and Palladium Benzoporphyrin as Fully Implantable Glucose Sensors. J. Diabetes Sci. Technol. 2015, 9, 985–992. [Google Scholar] [CrossRef] [Green Version]
- Saxl, T.; Khan, F.; Ferla, M.; Birch, D.; Pickup, J. A fluorescence lifetime-based fibre-optic glucose sensor using glucose/galactose-binding protein. Analyst 2011, 136, 968–972. [Google Scholar] [CrossRef] [Green Version]
- Roberts, J.R.; Park, J.; Helton, K.; Wisniewski, N.; McShane, M.J. Biofouling of Polymer Hydrogel Materials and its Effect on Diffusion and Enzyme-Based Luminescent Glucose Sensor Functional Characteristics. J. Diabetes Sci. Technol. 2012, 6, 1267–1275. [Google Scholar] [CrossRef] [Green Version]
- Bornhoeft, L.R.; Biswas, A.; McShane, M.J. Composite Hydrogels with Engineered Microdomains for Optical Glucose Sensing at Low Oxygen Conditions. Biosensors 2017, 7, 8. [Google Scholar] [CrossRef] [Green Version]
- Biswas, A.; Nagaraja, A.T.; You, Y.-H.; Roberts, J.R.; McShane, M.J. Cross-linked nanofilms for tunable permeability control in a composite microdomain system. RSC Adv. 2016, 6, 71781–71790. [Google Scholar] [CrossRef]
- Park, J.; McShane, M.J. Dual-Function Nanofilm Coatings with Diffusion Control and Protein Resistance. ACS Appl. Mater. Interfaces 2010, 2, 991–997. [Google Scholar] [CrossRef]
- Biswas, A.; Bornhoeft, L.R.; Banerjee, S.; You, Y.-H.; McShane, M.J. Composite Hydrogels Containing Bioactive Microreactors for Optical Enzymatic Lactate Sensing. ACS Sens. 2017, 2, 1584–1588. [Google Scholar] [CrossRef] [PubMed]
- Elistratova, A.A.; Kritchenkov, I.S.; Lezov, A.A.; Gubarev, A.S.; Solomatina, A.I.; Kachkin, D.V.; Shcherbina, N.A.; Liao, Y.-C.; Liu, Y.-C.; Yang, Y.-Y.; et al. Lifetime oxygen sensors based on block copolymer micelles and non-covalent human serum albumin adducts bearing phosphorescent near-infrared iridium(III) complex. Eur. Polym. J. 2021, 159, 110761. [Google Scholar] [CrossRef]
- Liu, H.; Yang, H.; Hao, X.; Xu, H.; Lv, Y.; Xiao, D.; Wang, H.; Tian, Z. Development of Polymeric Nanoprobes with Improved Lifetime Dynamic Range and Stability for Intracellular Oxygen Sensing. Small 2013, 9, 2639–2648. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Bull, B.; Christensen, K.; McNeill, J. Ratiometric Single-Nanoparticle Oxygen Sensors for Biological Imaging. Angew. Chem. Int. Ed. 2009, 48, 2741–2745. [Google Scholar] [CrossRef] [PubMed]
- Pajusalu, M.; Borlina, C.S.; Seager, S.; Ono, S.; Bosak, T. Open-source sensor for measuring oxygen partial pressures below 100 microbars. PLoS ONE 2018, 13, e0206678. [Google Scholar] [CrossRef] [Green Version]
- Baleizão, C.; Nagl, S.; Schäferling, M.; Berberan-Santos, M.N.; Wolfbeis, O.S. Dual Fluorescence Sensor for Trace Oxygen and Temperature with Unmatched Range and Sensitivity. Anal. Chem. 2008, 80, 6449–6457. [Google Scholar] [CrossRef]
- Nagl, S.; Baleizão, C.; Borisov, S.M.; Schäferling, M.; Berberan-Santos, M.N.; Wolfbeis, O.S. Optical Sensing and Imaging of Trace Oxygen with Record Response. Angew. Chem. Int. Ed. 2007, 46, 2317–2319. [Google Scholar] [CrossRef]
- Müller, B.J.; Burger, T.; Borisov, S.M.; Klimant, I. High performance optical trace oxygen sensors based on NIR-emitting benzoporphyrins covalently coupled to silicone matrixes. Sens. Actuators B Chem. 2015, 216, 527–534. [Google Scholar] [CrossRef]
- Burger, T.; Winkler, C.; Dalfen, I.; Slugovc, C.; Borisov, S.M. Porphyrin based metal–organic frameworks: Highly sensitive materials for optical sensing of oxygen in gas phase. J. Mater. Chem. C 2021, 9, 17099–17112. [Google Scholar] [CrossRef]
- Christopher, D.F.; Robert, E.T. Lactic Acidosis. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470202/ (accessed on 5 August 2022).
- Mihir, N.N.; Robert, H.W.; Fatima, A. Physiology, Glucose Metabolism. Available online: https://www.ncbi.nlm.nih.gov/books/NBK560599/ (accessed on 5 August 2022).








| Surfactant | pHEMA a (τ21% (µs)/τ0% (µs)/Ksv (µM−1)) | AnA a (τ21% (µs)/τ0% (µs)/Ksv (µM−1)) | pMTMA a (τ21% (µs)/τ0% (µs)/Ksv (µM−1)) | pMPC a (τ21% (µs)/τ0% (µs)/Ksv (µM−1)) | Average Ksv ± S.D. (µM−1) |
|---|---|---|---|---|---|
| SDS b | 24.1 ± 1.9/192.7 ± 7.2/0.028 | 23.2 ± 0.6/244.0 ± 3.5/0.041 | 21.5 ± 0.3/236.5 ± 1.4/0.041 | 23.3 ± 2.1/256.6 ± 5.1/0.041 | 0.038 ± 0.006 |
| CTAB b | 11.3 ± 0.3/153.4 ± 2.0/0.050 | 17.3 ± 0.2/224.6 ± 11.6/0.052 | 24.9 ± 0.9/283.7 ± 4.3/0.043 | 21.9 ± 4.3/236.7 ± 2.4/0.046 | 0.048 ± 0.004 |
| PF-68 b | 18.6 ± 0.3/180.0 ± 3.3/0.035 | 20.9 ± 0.1/221.0 ± 5.8/0.047 | 23.0 ± 0.4/257.2 ± 10.6/0.042 | 19.7 ± 0.4/244.3 ± 6.6/0.048 | 0.043 ± 0.006 |
| PDMS-PEG b | 19.3 ± 0.2/173.3 ± 4.3/0.031 | 20.0 ± 0.6/236.3 ± 4.1/0.042 | 21.5 ± 0.1/269.7 ± 5.3/0.047 | 20.2 ± 0.2/246.1 ± 3.5/0.046 | 0.041 ± 0.007 |
| Free PdBP | 39.5 ± 0.4/220.6 ± 37.9/0.019 | 41.0 ± 6.3/ 235.9 ± 7.1/0.020 | 24.9 ± 1.3/150.2 ± 3.5/0.022 | 31.2 ± 12.6/225.6 ± 13.9/0.025 | 0.021 ± 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soundaram Jeevarathinam, A.; Saleem, W.; Martin, N.; Hu, C.; McShane, M.J. NIR Luminescent Oxygen-Sensing Nanoparticles for Continuous Glucose and Lactate Monitoring. Biosensors 2023, 13, 141. https://doi.org/10.3390/bios13010141
Soundaram Jeevarathinam A, Saleem W, Martin N, Hu C, McShane MJ. NIR Luminescent Oxygen-Sensing Nanoparticles for Continuous Glucose and Lactate Monitoring. Biosensors. 2023; 13(1):141. https://doi.org/10.3390/bios13010141
Chicago/Turabian StyleSoundaram Jeevarathinam, Ananthakrishnan, Waqas Saleem, Nya Martin, Connie Hu, and Michael J. McShane. 2023. "NIR Luminescent Oxygen-Sensing Nanoparticles for Continuous Glucose and Lactate Monitoring" Biosensors 13, no. 1: 141. https://doi.org/10.3390/bios13010141
APA StyleSoundaram Jeevarathinam, A., Saleem, W., Martin, N., Hu, C., & McShane, M. J. (2023). NIR Luminescent Oxygen-Sensing Nanoparticles for Continuous Glucose and Lactate Monitoring. Biosensors, 13(1), 141. https://doi.org/10.3390/bios13010141