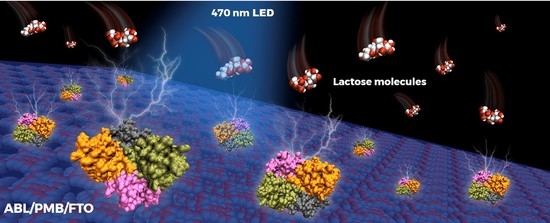
Agaricus bisporus Wild Mushroom Extract as Lectin Source for Engineering a Lactose Photoelectrochemical Biosensor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Protein Extraction and Purification
2.3. Protein Activity Tests
2.4. Characterization of (Bio)materials and Analytical Devices
2.5. Semi-Empirical Studies and Molecular Modeling
2.6. Biosensor Construction
2.7. (Photo)electrochemical Assays and Data Handling
2.8. Real Samples Analysis
3. Results and Discussion
3.1. Lectin Extraction and Characterization
3.2. Lectin Activity Threshold
3.3. Biosensor Manufacturing and Operation
3.4. Biosensor Analytical Performance
3.5. Real Application and Validation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, R.S.; Walia, A.K.; Kennedy, J.F. Mushroom Lectins in Biomedical Research and Development. Int. J. Biol. Macromol. 2020, 151, 1340–1350. [Google Scholar] [CrossRef] [PubMed]
- Abrantes-Coutinho, V.E.; Santos, A.O.; Moura, R.B.; Pereira-Junior, F.N.; Mascaro, L.H.; Morais, S.; Oliveira, T.M.B.F. Systematic Review on Lectin-Based Electrochemical Biosensors for Clinically Relevant Carbohydrates and Glycoconjugates. Colloids Surf. B Biointerfaces 2021, 208, 112148. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.S.; Bhari, R.; Kaur, H.P. Mushroom Lectins: Current Status and Future Perspectives. Crit. Rev. Biotechnol. 2010, 30, 99–126. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Wang, H.; Chan, Y.; Pan, W.; Dan, X.; Yin, C.; Akkouh, O.; Ng, T. Lectins from Edible Mushrooms. Molecules 2014, 20, 446–469. [Google Scholar] [CrossRef] [PubMed]
- Nikitina, V.; Loshchinina, E.; Vetchinkina, E. Lectins from Mycelia of Basidiomycetes. Int. J. Mol. Sci. 2017, 18, 1334. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Khan, M.I. Fungal Lectins: Current Molecular and Biochemical Perspectives. Int. J. Biol. Chem. 2010, 5, 1–20. [Google Scholar] [CrossRef]
- Lam, S.K.; Ng, T.B. Lectins: Production and Practical Applications. Appl. Microbiol. Biotechnol. 2011, 89, 45–55. [Google Scholar] [CrossRef]
- Kampmann, M.; Boll, S.; Kossuch, J.; Bielecki, J.; Uhl, S.; Kleiner, B.; Wichmann, R. Efficient Immobilization of Mushroom Tyrosinase Utilizing Whole Cells from Agaricus bisporus and Its Application for Degradation of Bisphenol A. Water Res. 2014, 57, 295–303. [Google Scholar] [CrossRef]
- Mohana, S.; Sumathi, S. Multi-Functional Biological Effects of Palladium Nanoparticles Synthesized Using Agaricus bisporus. J. Clust. Sci. 2020, 31, 391–400. [Google Scholar] [CrossRef]
- Morosanova, M.A.; Fedorova, T.V.; Polyakova, A.S.; Morosanova, E.I. Agaricus bisporus Crude Extract: Characterization and Analytical Application. Molecules 2020, 25, 5996. [Google Scholar] [CrossRef]
- Usman, M.; Murtaza, G.; Ditta, A. Nutritional, Medicinal, and Cosmetic Value of Bioactive Compounds in Button Mushroom (Agaricus bisporus): A Review. Appl. Sci. 2021, 11, 5943. [Google Scholar] [CrossRef]
- Tirta Ismaya, W.; Tjandrawinata, R.R.; Rachmawati, H. Lectins from the Edible Mushroom Agaricus bisporus and Their Therapeutic Potentials. Molecules 2020, 25, 2368. [Google Scholar] [CrossRef]
- Crenshaw, R.W.; Harper, S.N.; Moyer, M.; Privalle, L.S. Isolation and Characterization of a CDNA Clone Encoding a Lectin Gene from Agaricus bisporus. Plant Physiol. 1995, 107, 1465–1466. [Google Scholar] [CrossRef]
- Boraston, A.B.; Tomme, P.; Amandoron, E.A.; Kilburn, D.G. A Novel Mechanism of Xylan Binding by a Lectin-like Module from Streptomyces Lividans Xylanase 10A. Biochem. J. 2000, 350, 933. [Google Scholar] [CrossRef]
- Irazoqui, F.J.; Vides, M.A.; Nores, G.A. Structural Requirements of Carbohydrates to Bind Agaricus bisporus Lectin. Glycobiology 1999, 9, 59–64. [Google Scholar] [CrossRef]
- Trigueros, V.; Lougarre, A.; Ali-Ahmed, D.; Rahbé, Y.; Guillot, J.; Chavant, L.; Fournier, D.; Paquereau, L. Xerocomus Chrysenteron Lectin: Identification of a New Pesticidal Protein. Biochim. Biophys. Acta Gen. Subj. 2003, 1621, 292–298. [Google Scholar] [CrossRef]
- Facioni, M.S.; Raspini, B.; Pivari, F.; Dogliotti, E.; Cena, H. Nutritional Management of Lactose Intolerance: The Importance of Diet and Food Labelling. J. Transl. Med. 2020, 18, 260. [Google Scholar] [CrossRef]
- Enko, D.; Rezanka, E.; Stolba, R.; Halwachs-Baumann, G. Lactose Malabsorption Testing in Daily Clinical Practice: A Critical Retrospective Analysis and Comparison of the Hydrogen/Methane Breath Test and Genetic Test (C/T−13910 Polymorphism) Results. Gastroenterol. Res. Pract. 2014, 2014, 464382. [Google Scholar] [CrossRef]
- Ivory, R.; Mangan, D.; McCleary, B.V. Lactose Concentration in Low-Lactose and Lactose-Free Milk, Milk Products, and Products Containing Dairy Ingredients by High Sensitivity Enzymatic Method (K-LOLAC), Collaborative Study: Final Action 2020.08. J. AOAC Int. 2022, 105, 1617–1624. [Google Scholar] [CrossRef]
- Churakova, E.; Peri, K.; Vis, J.S.; Smith, D.W.; Beam, J.M.; Vijverberg, M.P.; Stor, M.C.; Winter, R.T. Accurate Analysis of Residual Lactose in Low-Lactose Milk: Comparing a Variety of Analytical Techniques. Int. Dairy J. 2019, 96, 126–131. [Google Scholar] [CrossRef]
- Gille, D.; Walther, B.; Badertscher, R.; Bosshart, A.; Brügger, C.; Brühlhart, M.; Gauch, R.; Noth, P.; Vergères, G.; Egger, L. Detection of Lactose in Products with Low Lactose Content. Int. Dairy J. 2018, 83, 17–19. [Google Scholar] [CrossRef]
- Rao, P.S.; Singh, P.; Sharma, V.; Arora, S. Traditional Analytical Approaches for Lactose Residues Determination in Lactose Hydrolysed Milks: A Review. LWT 2021, 151, 112069. [Google Scholar] [CrossRef]
- Trani, A.; Gambacorta, G.; Loizzo, P.; Cassone, A.; Fasciano, C.; Zambrini, A.V.; Faccia, M. Comparison of HPLC-RI, LC/MS-MS and Enzymatic Assays for the Analysis of Residual Lactose in Lactose-Free Milk. Food Chem. 2017, 233, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Khanam, Z.; Gupta, S.; Verma, A. Endophytic Fungi-Based Biosensors for Environmental Contaminants—A Perspective. S. Afr. J. Bot. 2020, 134, 401–406. [Google Scholar] [CrossRef]
- Upadhyay, L.S.B.; Verma, N. Role of Biosensors in Environmental Monitoring. In Environmental Microbial Biotechnology; Soil Biology; Sukla, L.B., Pradhan, N., Panda, S., Mishra, B.K., Eds.; Springer: Cham, Switzerland, 2015; pp. 77–90. [Google Scholar]
- Shu, J.; Tang, D. Recent Advances in Photoelectrochemical Sensing: From Engineered Photoactive Materials to Sensing Devices and Detection Modes. Anal. Chem. 2020, 92, 363–377. [Google Scholar] [CrossRef]
- Hichem, H.; Djamila, A.; Hania, A. Optical, Electrical and Photoelectrochemical Characterization of Electropolymerized Poly Methylene Blue on Fluorine Doped Tin Oxide Conducting Glass. Electrochim. Acta 2013, 106, 69–74. [Google Scholar] [CrossRef]
- Çakıroğlu, B.; Demirci, Y.C.; Gökgöz, E.; Özacar, M. A Photoelectrochemical Glucose and Lactose Biosensor Consisting of Gold Nanoparticles, MnO2 and g-C3N4 Decorated TiO2. Sens. Actuators B Chem. 2019, 282, 282–289. [Google Scholar] [CrossRef]
- Moura, J.V.B.; Souza, A.A.G.; Freire, P.T.C.; Luz-Lima, C.; Oliveira, T.M.B.F. Blue-light-excited NaCe(MoO4)2 Microcrystals for Photoelectrochemical Water Splitting. Int. J. Appl. Ceram. Technol. 2021, 18, 615–621. [Google Scholar] [CrossRef]
- Zor, T.; Selinger, Z. Linearization of the Bradford Protein Assay Increases Its Sensitivity: Theoretical and Experimental Studies. Anal. Biochem. 1996, 236, 302–308. [Google Scholar] [CrossRef]
- Kumaran, S.; Pandurangan, A.K.; Shenbhagaraman, R.; Esa, N.M. Isolation and Characterization of Lectin from the Artist’s Conk Medicinal Mushroom, Ganoderma applanatum (Agaricomycetes), and Evaluation of Its Antiproliferative Activity in HT-29 Colon Cancer Cells. Int. J. Med. Mushrooms 2017, 19, 675–684. [Google Scholar] [CrossRef]
- Jurrus, E.; Engel, D.; Star, K.; Monson, K.; Brandi, J.; Felberg, L.E.; Brookes, D.H.; Wilson, L.; Chen, J.; Liles, K.; et al. Improvements to the APBS Biomolecular Solvation Software Suite. Protein Sci. 2018, 27, 112–128. [Google Scholar] [CrossRef] [PubMed]
- Pahari, S.; Sun, L.; Basu, S.; Alexov, E. DelPhiPKa: Including Salt in the Calculations and Enabling Polar Residues to Titrate. Proteins Struct. Funct. Bioinform. 2018, 86, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Petukh, M.; Li, L.; Alexov, E. Continuous Development of Schemes for Parallel Computing of the Electrostatics in Biological Systems: Implementation in DelPhi. J. Comput. Chem. 2013, 34, 1949–1960. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Kaplan, İ.H.; Dağcı, K.; Alanyalıoğlu, M. Nucleation and Growth Mechanism of Electropolymerization of Methylene Blue: The Effect of Preparation Potential on Poly(Methylene Blue) Structure. Electroanalysis 2010, 22, 2694–2701. [Google Scholar] [CrossRef]
- Kurganov, B.; Lobanov, A.; Borisov, I.; Reshetilov, A. Criterion for Hill Equation Validity for Description of Biosensor Calibration Curves. Anal. Chim. Acta 2001, 427, 11–19. [Google Scholar] [CrossRef]
- Miller, J.N.; Miller, J.C. Statistics and Chemometrics for Analytical Chemistry, 7th ed.; Pearson: Harlow, UK, 2018; ISBN 1292186712. [Google Scholar]
- Jain, A.; Jain, R.; Jain, S. Quantitative Analysis of Reducing Sugars by 3, 5-Dinitrosalicylic Acid (DNSA Method). In Basic Techniques in Biochemistry, Microbiology and Molecular Biology; Humana: New York, NY, USA, 2020; pp. 181–183. [Google Scholar]
- Dogra, P.; Roy, S.S.; Joshi, A.; Mukhopadhyay, S. Hofmeister Ions Modulate the Autocatalytic Amyloidogenesis of an Intrinsically Disordered Functional Amyloid Domain via Unusual Biphasic Kinetics. J. Mol. Biol. 2020, 432, 6173–6186. [Google Scholar] [CrossRef]
- Sachs, J.N.; Woolf, T.B. Understanding the Hofmeister Effect in Interactions between Chaotropic Anions and Lipid Bilayers: Molecular Dynamics Simulations. J. Am. Chem. Soc. 2003, 125, 8742–8743. [Google Scholar] [CrossRef]
- Kang, B.; Tang, H.; Zhao, Z.; Song, S. Hofmeister Series: Insights of Ion Specificity from Amphiphilic Assembly and Interface Property. ACS Omega 2020, 5, 6229–6239. [Google Scholar] [CrossRef]
- Mahmood, R.I.; Abbass, A.K.; Al-Saffar, A.Z.; Al-Obaidi, J.R. An in Vitro Cytotoxicity of a Novel PH-Sensitive Lectin Loaded-Cockle Shell-Derived Calcium Carbonate Nanoparticles against MCF-7 Breast Tumour Cell. J. Drug Deliv. Sci. Technol. 2021, 61, 102230. [Google Scholar] [CrossRef]
- Zhao, J.K.; Zhao, Y.C.; Li, S.H.; Wang, H.X.; Ng, T.B. Isolation and Characterization of a Novel Thermostable Lectin from the Wild Edible Mushroom Agaricus Arvensis. J. Basic Microbiol. 2011, 51, 304–311. [Google Scholar] [CrossRef]
- Islam, M.R.; Tudryn, G.; Bucinell, R.; Schadler, L.; Picu, R.C. Morphology and Mechanics of Fungal Mycelium. Sci. Rep. 2017, 7, 13070. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, B.; Shao, J.; Jia, J.; Tian, Y.; Shu, X.; Ren, X.; Guan, Y. Extraction, Purification and Physicochemical Properties of a Novel Lectin from Laetiporus Sulphureus Mushroom. LWT 2018, 91, 151–159. [Google Scholar] [CrossRef]
- Bhowal, J.; Guha, A.K.; Chatterjee, B.P. Purification and Molecular Characterization of a Sialic Acid Specific Lectin from the Phytopathogenic Fungus Macrophomina Phaseolina. Carbohydr. Res. 2005, 340, 1973–1982. [Google Scholar] [CrossRef]
- Wang, H.X.; Ng, T.B.; Liu, W.K.; Oou, V.E.C.; Chang, S.T. Isolation and Characterization of Two Distinct Lectins with Antiproliferative Activity from the Cultured Mycelium of the Edible Mushroom Tricholoma mongolicum. Int. J. Pept. Protein Res. 2009, 46, 508–513. [Google Scholar] [CrossRef]
- Paul, T.J.; Strzelczyk, A.K.; Feldhof, M.I.; Schmidt, S. Temperature-Switchable Glycopolymers and Their Conformation-Dependent Binding to Receptor Targets. Biomacromolecules 2020, 21, 2913–2921. [Google Scholar] [CrossRef]
- González-Cruz, L.; Valadez-Vega, C.; Juárez-Goiz, J.M.S.; Flores-Martínez, N.L.; Montañez-Soto, J.L.; Bernardino-Nicanor, A. Partial Purification and Characterization of the Lectins of Two Varieties of Phaseolus coccineus (Ayocote Bean). Agronomy 2022, 12, 716. [Google Scholar] [CrossRef]
- Silber, A.; Hampp, N.; Schuhmann, W. Poly(Methylene Blue)-Modified Thick-Film Gold Electrodes for the Electrocatalytic Oxidation of NADH and Their Application in Glucose Biosensors. Biosens. Bioelectron. 1996, 11, 215–223. [Google Scholar] [CrossRef]
- Pfaffen, V.; Ortiz, P.I.; Córdoba de Torresi, S.I.; Torresi, R.M. On the PH Dependence of Electroactivity of Poly(Methylene Blue) Films. Electrochim. Acta 2010, 55, 1766–1771. [Google Scholar] [CrossRef]
- Bollella, P.; Schulz, C.; Favero, G.; Mazzei, F.; Ludwig, R.; Gorton, L.; Antiochia, R. Green Synthesis and Characterization of Gold and Silver Nanoparticles and Their Application for Development of a Third Generation Lactose Biosensor. Electroanalysis 2017, 29, 77–86. [Google Scholar] [CrossRef] [Green Version]
- De Brito, A.R.; dos Santos Reis, N.; Oliveira, P.C.; Rezende, D.V.B.; Monteiro, G.P.; Soares, G.A.; de Jesus, R.S.; Santos, A.S.; Salay, L.C.; de Oliveira, J.R.; et al. Development of Amperometric Biosensor in Modified Carbon Paste with Enzymatic Preparation Based on Lactase Immobilized on Carbon Nanotubes. J. Food Sci. Technol. 2020, 57, 1342–1350. [Google Scholar] [CrossRef] [PubMed]
- Conzuelo, F.; Gamella, M.; Campuzano, S.; Ruiz, M.A.; Reviejo, A.J.; Pingarrón, J.M. An Integrated Amperometric Biosensor for the Determination of Lactose in Milk and Dairy Products. J. Agric. Food Chem. 2010, 58, 7141–7148. [Google Scholar] [CrossRef] [PubMed]







| pH | Hemagglutinating Activity (HU) | |||||
|---|---|---|---|---|---|---|
| Control * | 4 | 8 | 16 | 32 | 64 | |
| 4 |  |  |  |  |  |  |
| − | + | + | + | + | + | |
| 6 |  |  |  |  |  |  |
| − | + | + | + | + | + | |
| 7 |  |  |  |  |  |  |
| − | + | + | + | + | + | |
| 8 |  |  |  |  |  |  |
| − | + | + | + | + | + | |
| 10 |  |  |  |  |  |  |
| − | + | + | + | − | − | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, A.O.; Abrantes-Coutinho, V.E.; Morais, S.; Oliveira, T.M.B.F. Agaricus bisporus Wild Mushroom Extract as Lectin Source for Engineering a Lactose Photoelectrochemical Biosensor. Biosensors 2023, 13, 224. https://doi.org/10.3390/bios13020224
Santos AO, Abrantes-Coutinho VE, Morais S, Oliveira TMBF. Agaricus bisporus Wild Mushroom Extract as Lectin Source for Engineering a Lactose Photoelectrochemical Biosensor. Biosensors. 2023; 13(2):224. https://doi.org/10.3390/bios13020224
Chicago/Turabian StyleSantos, André O., Vanessa E. Abrantes-Coutinho, Simone Morais, and Thiago M. B. F. Oliveira. 2023. "Agaricus bisporus Wild Mushroom Extract as Lectin Source for Engineering a Lactose Photoelectrochemical Biosensor" Biosensors 13, no. 2: 224. https://doi.org/10.3390/bios13020224
APA StyleSantos, A. O., Abrantes-Coutinho, V. E., Morais, S., & Oliveira, T. M. B. F. (2023). Agaricus bisporus Wild Mushroom Extract as Lectin Source for Engineering a Lactose Photoelectrochemical Biosensor. Biosensors, 13(2), 224. https://doi.org/10.3390/bios13020224