Optical Biosensors and Their Applications for the Detection of Water Pollutants
Abstract
:1. Introduction
2. Methods
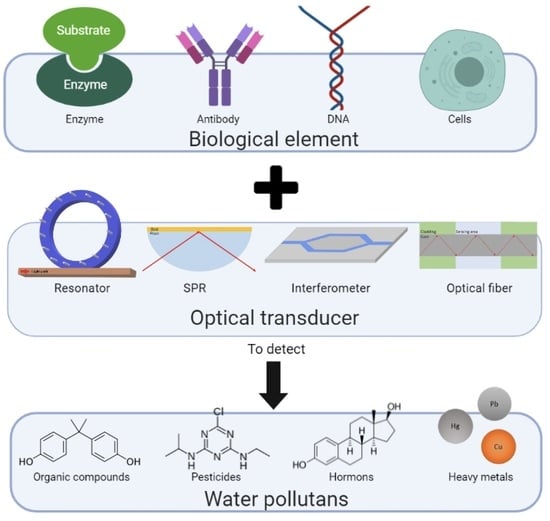
3. Main Optical Biosensor Components
3.1. Biological Recognition Element
3.1.1. Enzymes
3.1.2. Antibodies
3.1.3. DNA
3.1.4. Other
3.1.5. Comparison of Biological Elements for Optical Sensors Monitoring Water Quality
3.2. Optical Transducer
3.2.1. Interferometer
3.2.2. SPR and LSPR
3.2.3. Optical Resonators
3.2.4. Gratings
3.2.5. Fiber Optic
3.2.6. Fluorescence
3.2.7. Comparison of Transducers Used in Optical Sensors for Monitoring Water Quality
4. Detection of Selected Water Pollutants
4.1. Pesticides

4.2. Pharmaceuticals
4.3. Other Organic Compounds
4.4. Microorganisms and Toxins
4.5. Heavy Metals
5. Discussion
6. Key Trends and Future Perspective
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Richardson, S.D.; Ternes, T.A. Water Analysis: Emerging Contaminants and Current Issues. Anal. Chem. 2022, 94, 382–416. [Google Scholar] [CrossRef] [PubMed]
- Rathi, B.S.; Kumar, P.S.; Vo, D.V.N. Critical Review on Hazardous Pollutants in Water Environment: Occurrence, Monitoring, Fate, Removal Technologies and Risk Assessment. Sci. Total Environ. 2021, 797, 149134. [Google Scholar] [CrossRef] [PubMed]
- Biosensors Market Size, Global Forecast, Growth Drivers, Opportunities 2030. Available online: https://www.marketsandmarkets.com/Market-Reports/biosensors-market-798.html (accessed on 15 October 2022).
- Monosik, R.; Stredansky, M.; Tkac, J.; Sturdik, E. Application of Enzyme Biosensors in Analysis of Food and Beverages. Food Anal. Methods 2012, 5, 40–53. [Google Scholar] [CrossRef]
- Mohsin, M.; Ahmad, A. Genetically-Encoded Nanosensor for Quantitative Monitoring of Methionine in Bacterial and Yeast Cells. Biosens. Bioelectron. 2014, 59, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Generelli, S.; Heitger, F. Online Monitoring the Water Contaminations with Optical Biosensor. Proceedings 2017, 1, 522. [Google Scholar] [CrossRef] [Green Version]
- Turner, A.P.F. Biosensors–Sense and Sensitivity. Science 2000, 290, 1315–1317. [Google Scholar] [CrossRef]
- Velasco-Garcia, M.N. Optical Biosensors for Probing at the Cellular Level: A Review of Recent Progress and Future Prospects. Semin. Cell Dev. Biol. 2009, 20, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Y.; Huang, Q.; Canady, T.D.; Barya, P.; Liu, S.; Arogundade, O.H.; Race, C.M.; Che, C.; Wang, X.; Zhou, L.; et al. Photonic Crystal Enhanced Fluorescence Emission and Blinking Suppression for Single Quantum Dot Digital Resolution Biosensing. Nat. Commun. 2022, 13, 4647. [Google Scholar] [CrossRef]
- Sansone, L.; Macchia, E.; Taddei, C.; Torsi, L.; Giordano, M. Label-Free Optical Biosensing at Femtomolar Detection Limit. Sens. Actuators B Chem. 2018, 255, 1097–1104. [Google Scholar] [CrossRef]
- Jason-Moller, L.; Murphy, M.; Bruno, J.A. Overview of Biacore Systems and Their Applications. Curr. Protoc. Protein Sci. 2006, 19, 19.13.1–19.13.14. [Google Scholar] [CrossRef]
- Xia, Y.; Hu, J.; Zhao, S.; Tao, L.; Li, Z.; Yue, T.; Kong, J. Build-in Sensors and Analysis Algorithms Aided Smartphone-Based Sensors for Point-of-Care Tests. Biosens. Bioelectron. X 2022, 11, 100195. [Google Scholar] [CrossRef]
- Di Nonno, S.; Ulber, R. Smartphone-Based Optical Analysis Systems. Analyst 2021, 146, 2749–2768. [Google Scholar] [CrossRef] [PubMed]
- Doǧan, V.; Isık, T.; Kılıç, V.; Horzum, N. A Field-Deployable Water Quality Monitoring with Machine Learning-Based Smartphone Colorimetry. Anal. Methods 2022, 14, 3458–3466. [Google Scholar] [CrossRef] [PubMed]
- Roda, A.; Zangheri, M.; Calabria, D.; Mirasoli, M.; Caliceti, C.; Quintavalla, A.; Lombardo, M.; Trombini, C.; Simoni, P. A Simple Smartphone-Based Thermochemiluminescent Immunosensor for Valproic Acid Detection Using 1,2-Dioxetane Analogue-Doped Nanoparticles as a Label. Sens. Actuators B Chem. 2019, 279, 327–333. [Google Scholar] [CrossRef]
- Freund, B.; Tatum, W.O. Pitfalls Using Smartphones Videos in Diagnosing Functional Seizures. Epilepsy Behav. Rep. 2021, 16, 100497. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Gong, H.; Li, M.; Tang, D. Hollow Prussian Blue Nanozyme-Richened Liposome for Artificial Neural Network-Assisted Multimodal Colorimetric-Photothermal Immunoassay on Smartphone. Biosens. Bioelectron. 2022, 218, 114751. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Wang, H.; Zhuo, Y.; Song, D.; Li, C.; Zhu, A.; Long, F. Reusable Smartphone-Facilitated Mobile Fluorescence Biosensor for Rapid and Sensitive on-Site Quantitative Detection of Trace Pollutants. Biosens. Bioelectron. 2022, 199, 113863. [Google Scholar] [CrossRef]
- Ramirez, J.C.; Grajales García, D.; Maldonado, J.; Fernández-Gavela, A. Current Trends in Photonic Biosensors: Advances towards Multiplexed Integration. Chemosensors 2022, 10, 398. [Google Scholar] [CrossRef]
- Sohrabi, H.; Hemmati, A.; Majidi, M.R.; Eyvazi, S.; Jahanban-Esfahlan, A.; Baradaran, B.; Adlpour-Azar, R.; Mokhtarzadeh, A.; de la Guardia, M. Recent Advances on Portable Sensing and Biosensing Assays Applied for Detection of Main Chemical and Biological Pollutant Agents in Water Samples: A Critical Review. TrAC Trends Anal. Chem. 2021, 143, 116344. [Google Scholar] [CrossRef]
- Erdem, A.; Senturk, H.; Yildiz, E. Recent Progress on Biosensors Developed for Detecting Environmental Pollutants. In Biosensors: Fundamentals, Emerging Technologies, and Application, 1st ed.; CRC Press: Boca Raton, FL, USA, 2022; pp. 213–231. [Google Scholar] [CrossRef]
- Nehra, M.; Dilbaghi, N.; Kumar, R.; Kumar, S. Trends in Point-of-Care Optical Biosensors for Antibiotics Detection in Aqueous Media. Mater. Lett. 2022, 308, 131235. [Google Scholar] [CrossRef]
- Sarkar, A.; Sarkar, K.D.; Amrutha, V.; Dutta, K. An Overview of Enzyme-Based Biosensors for Environmental Monitoring. In Tools, Techniques and Protocols for Monitoring Environmental Contaminants, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 307–329. ISBN 9780128146798. [Google Scholar]
- Zhu, Y.C.; Mei, L.P.; Ruan, Y.F.; Zhang, N.; Zhao, W.W.; Xu, J.J.; Chen, H.Y. Enzyme-Based Biosensors and Their Applications, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780444641144. [Google Scholar]
- Zhang, Z.; Zeng, K.; Liu, J. Immunochemical Detection of Emerging Organic Contaminants in Environmental Waters. TrAC Trends Anal. Chem. 2017, 87, 49–57. [Google Scholar] [CrossRef]
- Singh, K.V.; Kaur, J.; Varshney, G.C.; Raje, M.; Suri, C.R. Synthesis and Characterization of Hapten-Protein Conjugates for Antibody Production against Small Molecules. Bioconjug. Chem. 2004, 15, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ju, H.X. Clinical Immunoassays and Immunosensing. In Comprehensive Sampling and Sample Preparation; Academic Press: Cambridge, MA, USA, 2012; Volume 3, pp. 143–167. [Google Scholar] [CrossRef]
- Pohanka, M. Overview of Piezoelectric Biosensors, Immunosensors and DNA Sensors and Their Applications. Materials 2018, 11, 448. [Google Scholar] [CrossRef] [Green Version]
- P Gautam A Review on Recent Advances in Biosensors for Detection of Water. Int. J. Environ. Sci. 2012, 2, 1565–1574. [CrossRef] [Green Version]
- Oliveira Brett, A.M. Chapter 4 DNA-Based Biosensors. Compr. Anal. Chem. 2005, 44, 179–208. [Google Scholar]
- Borisov, S.M.; Wolfbeis, O.S. Optical Biosensors. Chem. Rev. 2008, 108, 423–461. [Google Scholar] [CrossRef] [PubMed]
- Sciuto, E.L.; Coniglio, M.A.; Corso, D.; van der Meer, J.R.; Acerbi, F.; Gola, A.; Libertino, S. Biosensors in Monitoring Water Quality and Safety: An Example of a Miniaturizable Whole-Cell Based Sensor for Hg2+ Optical Detection in Water. Water 2019, 11, 1986. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Zhou, X.; Lu, Y.; Shi, H.; Ma, M.; Yu, T. Triple Functional Small-Molecule-Protein Conjugate Mediated Optical Biosensor for Quantification of Estrogenic Activities in Water Samples. Environ. Int. 2019, 132, 105091. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, X.; Zhu, Q.; Li, K.; Lu, Y.; Zhou, X.; Guo, T. Ultrasensitive Detection of Endocrine Disruptors via Superfine Plasmonic Spectral Combs. Light Sci. Appl. 2021, 10, 181. [Google Scholar] [CrossRef]
- Yaghoubi, M.; Rahimi, F.; Negahdari, B.; Rezayan, A.H.; Shafiekhani, A. A Lectin-Coupled Porous Silicon-Based Biosensor: Label-Free Optical Detection of Bacteria in a Real-Time Mode. Sci. Rep. 2020, 10, 16017. [Google Scholar] [CrossRef]
- Duan, R.; Li, Y.; Li, H.; Yang, J. Detection of Heavy Metal Ions Using Whispering Gallery Mode Lasing in Functionalized Liquid Crystal Microdroplets. Biomed. Opt. Express 2019, 10, 6073. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Song, Y.; Liu, Z.; Huang, W.E.; Li, G.; Deng, S.; Xing, Y.; Zhang, D. Whole-Cell Bioreporters for Evaluating Petroleum Hydrocarbon Contamination. Crit. Rev. Environ. Sci. Technol. 2021, 51, 272–322. [Google Scholar] [CrossRef]
- Van Der Meer, J.R.; Tropel, D.; Jaspers, M. Illuminating the Detection Chain of Bacterial Bioreporters. Environ. Microbiol. 2004, 6, 1005–1020. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Elcin, E.; Jiang, M.; Li, B.; Wang, H.; Zhang, X.; Wang, Z. Use of Whole-Cell Bioreporters to Assess Bioavailability of Contaminants in Aquatic Systems. Front. Chem. 2022, 10, 1018124. [Google Scholar] [CrossRef] [PubMed]
- Haupt, K.; Mosbach, K. Molecularly Imprinted Polymers and Their Use in Biomimetic Sensors. Chem. Rev. 2000, 100, 2495–2504. [Google Scholar] [CrossRef]
- Arjmand, M.; Saghafifar, H.; Alijanianzadeh, M.; Soltanolkotabi, M. A Sensitive Tapered-Fiber Optic Biosensor for the Label-Free Detection of Organophosphate Pesticides. Sens. Actuators B Chem. 2017, 249, 523–532. [Google Scholar] [CrossRef]
- Duan, R.; Hao, X.; Li, Y.; Li, H. Detection of Acetylcholinesterase and Its Inhibitors by Liquid Crystal Biosensor Based on Whispering Gallery Mode. Sens. Actuators B Chem. 2020, 308, 127672. [Google Scholar] [CrossRef]
- Bidmanova, S.; Kotlanova, M.; Rataj, T.; Damborsky, J.; Trtilek, M.; Prokop, Z. Fluorescence-Based Biosensor for Monitoring of Environmental Pollutants: From Concept to Field Application. Biosens. Bioelectron. 2016, 84, 97–105. [Google Scholar] [CrossRef]
- Shahar, H.; Tan, L.L.; Ta, G.C.; Heng, L.Y. Optical Enzymatic Biosensor Membrane for Rapid in Situ Detection of Organohalide in Water Samples. Microchem. J. 2019, 146, 41–48. [Google Scholar] [CrossRef]
- Tagad, C.K.; Kulkarni, A.; Aiyer, R.C.; Patil, D.; Sabharwal, S.G. A Miniaturized Optical Biosensor for the Detection of Hg2+ Based on Acid Phosphatase Inhibition. Optik 2016, 127, 8807–8811. [Google Scholar] [CrossRef]
- Ramirez-Priego, P.; Estévez, M.C.; Díaz-Luisravelo, H.J.; Manclús, J.J.; Montoya, Á.; Lechuga, L.M. Real-Time Monitoring of Fenitrothion in Water Samples Using a Silicon Nanophotonic Biosensor. Anal. Chim. Acta 2021, 1152, 338276. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, R.S.; Franco, D.F.; Nalin, M.; de Lima Gomes, P.C.F.; Messaddeq, Y. Label-Free Ultrasensitive and Environment-Friendly Immunosensor Based on a Silica Optical Fiber for the Determination of Ciprofloxacin in Wastewater Samples. Anal. Chem. 2020, 92, 14415–14422. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.S.; Erika, G.; Jiří, H. Surface Plasmon Resonance Biosensor for the Ultrasensitive Detection of Bisphenol A. Anal. Bioanal. Chem. 2019, 411, 5655–5658. [Google Scholar] [CrossRef] [PubMed]
- Cennamo, N.; Zeni, L.; Ricca, E.; Isticato, R.; Marzullo, V.M.; Capo, A.; Staiano, M.; D’Auria, S.; Varriale, A. Detection of Naphthalene in Sea-Water by a Label-Free Plasmonic Optical Fiber Biosensor. Talanta 2019, 194, 289–297. [Google Scholar] [CrossRef]
- Yang, F.; Chang, T.L.; Liu, T.; Wu, D.; Du, H.; Liang, J.; Tian, F. Label-Free Detection of Staphylococcus Aureus Bacteria Using Long-Period Fiber Gratings with Functional Polyelectrolyte Coatings. Biosens. Bioelectron. 2019, 133, 147–153. [Google Scholar] [CrossRef]
- Al-Jawdah, A.; Nabok, A.; Jarrah, R.; Holloway, A.; Tsargorodska, A.; Takacs, E.; Szekacs, A. Mycotoxin Biosensor Based on Optical Planar Waveguide. Toxins 2018, 10, 272. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Wood, R. Development of FRET Biosensor Based on Aptamer/Functionalized Graphene for Ultrasensitive Detection of Bisphenol A and Discrimination from Analogs. Nano-Struct. Nano-Objects 2017, 10, 131–140. [Google Scholar] [CrossRef]
- Kim, S.; Lee, H.J. Gold Nanostar Enhanced Surface Plasmon Resonance Detection of an Antibiotic at Attomolar Concentrations via an Aptamer-Antibody Sandwich Assay. Anal. Chem. 2017, 89, 6624–6630. [Google Scholar] [CrossRef]
- Schirmer, C.; Posseckardt, J.; Schröder, M.; Gläser, M.; Howitz, S.; Scharff, W.; Mertig, M. Portable and Low-Cost Biosensor towards on-Site Detection of Diclofenac in Wastewater. Talanta 2019, 203, 242–247. [Google Scholar] [CrossRef]
- Gosset, A.; Oestreicher, V.; Perullini, M.; Bilmes, S.A.; Jobbágy, M.; Dulhoste, S.; Bayard, R.; Durrieu, C. Optimization of Sensors Based on Encapsulated Algae for Pesticide Detection in Water. Anal. Methods 2019, 11, 6193–6203. [Google Scholar] [CrossRef]
- Halkare, P.; Punjabi, N.; Wangchuk, J.; Nair, A.; Kondabagil, K.; Mukherji, S. Bacteria Functionalized Gold Nanoparticle Matrix Based Fiber-Optic Sensor for Monitoring Heavy Metal Pollution in Water. Sens. Actuators B Chem. 2019, 281, 643–651. [Google Scholar] [CrossRef]
- Shrivastav, A.M.; Mishra, S.K.; Gupta, B.D. Localized and Propagating Surface Plasmon Resonance Based Fiber Optic Sensor for the Detection of Tetracycline Using Molecular Imprinting. Mater. Res. Express 2015, 2, 35007. [Google Scholar] [CrossRef]
- Huang, Q.D.; Lv, C.H.; Yuan, X.L.; He, M.; Lai, J.P.; Sun, H. A Novel Fluorescent Optical Fiber Sensor for Highly Selective Detection of Antibiotic Ciprofloxacin Based on Replaceable Molecularly Imprinted Nanoparticles Composite Hydrogel Detector. Sens. Actuators B Chem. 2021, 328, 129000. [Google Scholar] [CrossRef]
- Malacara, D. Óptica Básica, 3rd ed.; FCE—Fondo de Cultura Económica: Mexico City, Mexico, 2015; ISBN 9786071634139. [Google Scholar]
- Hariharan, P. Optical Interferometry, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2003; ISBN 9780123116307. [Google Scholar]
- Islam, M.S.; Kouzani, A.Z.; Dai, X.J.; Michalski, W.P.; Gholamhosseini, H. Comparison of Performance Parameters for Conventional and Localized Surface Plasmon Resonance Graphene Biosensors. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA; 2011; pp. 1851–1854. [Google Scholar] [CrossRef]
- Estevez, M.C.; Otte, M.A.; Sepulveda, B.; Lechuga, L.M. Trends and Challenges of Refractometric Nanoplasmonic Biosensors: A Review. Anal. Chim. Acta 2014, 806, 55–73. [Google Scholar] [CrossRef] [Green Version]
- Schasfoort, R.B.M.; McWhirter, A. Chapter 3: SPR Instrumentation. In Handbook of Surface Plasmon Resonance, 1st ed; Royal Society of Chemistry: Cambridge, UK, 2010; pp. 35–80. [Google Scholar]
- Chen, Y.; Yu, Y.; Li, X.; Tan, Z.; Geng, Y. Experimental Comparison of Fiber-Optic Surface Plasmon Resonance Sensors with Multi Metal Layers and Single Silver or Gold Layer. Plasmonics 2015, 10, 1801–1808. [Google Scholar] [CrossRef]
- Kamaruddin, N.H.; Bakar, A.A.A.; Yaacob, M.H.; Mahdi, M.A.; Zan, M.S.D.; Shaari, S. Enhancement of Chitosan-Graphene Oxide SPR Sensor with a Multi-Metallic Layers of Au–Ag–Au Nanostructure for Lead(II) Ion Detection. Appl. Surf. Sci. 2016, 361, 177–184. [Google Scholar] [CrossRef]
- Schasfoort, R.B.M. History and Physics of Surface Plasmon Resonance. In Handbook of Surface Plasmon Resonance, 2nd ed; Royal Society of Chemistry: Cambridge, UK, 2017; pp. 27–59. [Google Scholar]
- Thakur, A.; Qiu, G.; NG, S.P.; Guan, J.; Yue, J.; Lee, Y.; Wu, C.M.L. Direct Detection of Two Different Tumor-Derived Extracellular Vesicles by SAM-AuNIs LSPR Biosensor. Biosens. Bioelectron. 2017, 94, 400–407. [Google Scholar] [CrossRef]
- Morales-Luna, G.; Herrera-Domínguez, M.; Pisano, E.; Balderas-Elizalde, A.; Hernandez-Aranda, R.I.; Ornelas-Soto, N. Plasmonic Biosensor Based on an Effective Medium Theory as a Simple Tool to Predict and Analyze Refractive Index Changes. Opt. Laser Technol. 2020, 131, 106332. [Google Scholar] [CrossRef]
- Hossain, M.B.; Rana, M.M. DNA Hybridization Detection Based on Resonance Frequency Readout in Graphene on Au SPR Biosensor. J. Sens. 2016, 2016, 6070742. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Jia, Y.; Jiang, L.; Guo, J.; Dai, X.; Xiang, Y.; Fan, D. Sensitivity Improved SPR Biosensor Based on the MoS2/Graphene-Aluminum Hybrid Structure. J. Light. Technol. 2017, 35, 82–87. [Google Scholar] [CrossRef]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The Optical Properties of Metal Nanoparticles: The Influence of Size, Shape, and Dielectric Environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Fathi, F.; Rashidi, M.R.; Omidi, Y. Ultra-Sensitive Detection by Metal Nanoparticles-Mediated Enhanced SPR Biosensors. Talanta 2019, 192, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Hao, F.; Nehl, C.L.; Hafner, J.H.; Nordlander, P. Plasmon Resonances of a Gold Nanostar. Nano Lett. 2007, 7, 729–732. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.T.; Chen, M.; Weng, Y.H.; Xie, K.X.; Wang, J.; Cao, S.H.; Li, Y.Q. Label-Free Fluorescent Nanofilm Sensor Based on Surface Plasmon Coupled Emission: In Situ Monitoring the Growth of Metal-Organic Frameworks. Anal. Chem. 2022, 94, 6430–6435. [Google Scholar] [CrossRef]
- Bhaskar, S.; Ramamurthy, S.S. Mobile Phone-Based Picomolar Detection of Tannic Acid on Nd2O3 Nanorod-Metal Thin-Film Interfaces. ACS Appl. Nano Mater. 2019, 2, 4613–4625. [Google Scholar] [CrossRef]
- Vollmer, F.; Arnold, S.; Braun, D.; Teraoka, I.; Libchaber, A. Multiplexed DNA Quantification by Spectroscopic Shift of Two Microsphere Cavities. Biophys. J. 2003, 85, 1974–1979. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Wang, J. Optical Biosensors: An Exhaustive and Comprehensive Review. Analyst 2020, 145, 1605–1628. [Google Scholar] [CrossRef] [PubMed]
- Talebi Fard, S.; Grist, S.M.; Donzella, V.; Schmidt, S.A.; Flueckiger, J.; Wang, X.; Shi, W.; Millspaugh, A.; Webb, M.; Ratner, D.M.; et al. Label-Free Silicon Photonic Biosensors for Use in Clinical Diagnostics. Silicon Photonics VIII 2013, 8629, 862909. [Google Scholar] [CrossRef]
- Li, H.; Fan, X. Characterization of Sensing Capability of Optofluidic Ring Resonator Biosensors. Appl. Phys. Lett. 2010, 97, 11105. [Google Scholar] [CrossRef]
- Yan, H.; Huang, L.; Xu, X.; Tang, N.; Chakravarty, S.; Chen, R.T. Enhanced Surface Sensitivity in Microring Resonator Biosensor Based on Subwavelength Grating Waveguides. Front. Biol. Detect. Nanosensors Syst. IX 2017, 10081, 100810G. [Google Scholar] [CrossRef]
- Dey, S.; Dolci, M.; Zijlstra, P. Single-Molecule Optical Biosensing: Recent Advances and Future Challenges. ACS Phys. Chem. Au 2023. [Google Scholar] [CrossRef]
- Cheng, L.; Mao, S.; Li, Z.; Han, Y.; Fu, H.Y. Grating Couplers on Silicon Photonics: Design Principles, Emerging Trends and Practical Issues. Micromachines 2020, 11, 666. [Google Scholar] [CrossRef]
- Wei, X.; Weiss, S.M. Guided Mode Biosensor Based on Grating Coupled Porous Silicon Waveguide. Opt. Express 2011, 19, 11330. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Zhong, N.; Zhao, X.; Ma, S.; Fu, X.; Dong, D. Recent Advances in Fiber-Optic Evanescent Wave Sensors for Monitoring Organic and Inorganic Pollutants in Water. TrAC Trends Anal. Chem. 2020, 127, 115892. [Google Scholar] [CrossRef]
- Sadani, K.; Nag, P.; Mukherji, S. LSPR Based Optical Fiber Sensor with Chitosan Capped Gold Nanoparticles on BSA for Trace Detection of Hg (II) in Water, Soil and Food Samples. Biosens. Bioelectron. 2019, 134, 90–96. [Google Scholar] [CrossRef]
- Jeong, Y.; Kook, Y.M.; Lee, K.; Koh, W.G. Metal Enhanced Fluorescence (MEF) for Biosensors: General Approaches and a Review of Recent Developments. Biosens. Bioelectron. 2018, 111, 102–116. [Google Scholar] [CrossRef] [PubMed]
- Benito-Peña, E.; Valdés, M.G.; Glahn-Martínez, B.; Moreno-Bondi, M.C. Fluorescence Based Fiber Optic and Planar Waveguide Biosensors. A Review. Anal. Chim. Acta 2016, 943, 17–40. [Google Scholar] [CrossRef] [PubMed]
- Orellana, G. Fluorescence-Based Sensors. In Optical Chemical Sensors, 1st ed.; Springer: Dordrecht, The Netherlands, 2006; pp. 99–116. ISBN 978-1-4020-4611-7. [Google Scholar]
- Liu, L.; Zhou, X.; Lu, M.; Zhang, M.; Yang, C.; Ma, R.; Memon, A.G.; Shi, H.; Qian, Y. An Array Fluorescent Biosensor Based on Planar Waveguide for Multi-Analyte Determination in Water Samples. Sens. Actuators B Chem. 2017, 240, 107–113. [Google Scholar] [CrossRef]
- Scognamiglio, V.; Antonacci, A.; Arduini, F.; Moscone, D.; Campos, E.V.R.; Fraceto, L.F.; Palleschi, G. An Eco-Designed Paper-Based Algal Biosensor for Nanoformulated Herbicide Optical Detection. J. Hazard. Mater. 2019, 373, 483–492. [Google Scholar] [CrossRef]
- Hayes, T.B.; Anderson, L.L.; Beasley, V.R.; De Solla, S.R.; Iguchi, T.; Ingraham, H.; Kestemont, P.; Kniewald, J.; Kniewald, Z.; Langlois, V.S.; et al. Demasculinization and Feminization of Male Gonads by Atrazine: Consistent Effects across Vertebrate Classes. J. Steroid Biochem. Mol. Biol. 2011, 127, 64–73. [Google Scholar] [CrossRef] [Green Version]
- Smulders, C.J.G.M.; Bueters, T.J.H.; Vailati, S.; van Kleef, R.G.D.M.; Vijvergberg, H.P.M. Block of Neuronal Nicotinic Acetylcholine Receptors by Organophosphate Insecticides. Toxicol. Sci. 2004, 82, 545–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koukouvinos, G.; Τsialla, Z.; Petrou, P.S.; Misiakos, K.; Goustouridis, D.; Ucles Moreno, A.; Fernandez-Alba, A.R.; Raptis, I.; Kakabakos, S.E. Fast Simultaneous Detection of Three Pesticides by a White Light Reflectance Spectroscopy Sensing Platform. Sens. Actuators B Chem. 2017, 238, 1214–1223. [Google Scholar] [CrossRef]
- Stavra, E.; Petrou, P.S.; Koukouvinos, G.; Kiritsis, C.; Pirmettis, I.; Papadopoulos, M.; Goustouridis, D.; Economou, A.; Misiakos, K.; Raptis, I.; et al. Simultaneous Determination of Paraquat and Atrazine in Water Samples with a White Light Reflectance Spectroscopy Biosensor. J. Hazard. Mater. 2018, 359, 67–75. [Google Scholar] [CrossRef]
- Xiao-Hong, Z.; Bao-Dong, S.; Han-Chang, S.; Lan-Hua, L.; Hong-Li, G.; Miao, H. An Evanescent Wave Multi-Channel Immunosensor System for the Highly Sensitive Detection of Small Analytes in Water Samples. Sens. Actuators B Chem. 2014, 198, 150–156. [Google Scholar] [CrossRef]
- Liu, R.; Guan, G.; Wang, S.; Zhang, Z. Core-Shell Nanostructured Molecular Imprinting Fluorescent Chemosensor for Selective Detection of Atrazine Herbicide. Analyst 2011, 136, 184–190. [Google Scholar] [CrossRef]
- Kümmerer, K. Pharmaceuticals in the Environment. Annu. Rev. Environ. Resour. 2010, 35, 57–75. [Google Scholar] [CrossRef] [Green Version]
- Sharma, V.K.; Johnson, N.; Cizmas, L.; McDonald, T.J.; Kim, H. A Review of the Influence of Treatment Strategies on Antibiotic Resistant Bacteria and Antibiotic Resistance Genes. Chemosphere 2016, 150, 702–714. [Google Scholar] [CrossRef]
- Tomassetti, M.; Conta, G.; Campanella, L.; Favero, G.; Sanzò, G.; Mazzei, F.; Antiochia, R. A Flow SPR Immunosensor Based on a Sandwich Direct Method. Biosensors 2016, 6, 22. [Google Scholar] [CrossRef] [Green Version]
- Steinke, N.; Döring, S.; Wuchrer, R.; Kroh, C.; Gerlach, G.; Härtling, T. Plasmonic Sensor for On-Site Detection of Diclofenac Molecules. Sens. Actuators B Chem. 2019, 288, 594–600. [Google Scholar] [CrossRef]
- Altintas, Z.; France, B.; Ortiz, J.O.; Tothill, I.E. Computationally Modelled Receptors for Drug Monitoring Using an Optical Based Biomimetic SPR Sensor. Sens. Actuators B Chem. 2016, 224, 726–737. [Google Scholar] [CrossRef]
- Luo, Q.; Yu, N.; Shi, C.; Wang, X.; Wu, J. Surface Plasmon Resonance Sensor for Antibiotics Detection Based on Photo-Initiated Polymerization Molecularly Imprinted Array. Talanta 2016, 161, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Weber, P.; Vogler, J.; Gauglitz, G. Development of an Optical Biosensor for the Detection of Antibiotics in the Environment. Opt. Sensors 2017, 10231, 102312L. [Google Scholar] [CrossRef]
- Kumbhat, S.; Gehlot, R.; Sharma, K.; Singh, U.; Joshi, V. Surface Plasmon Resonance Based Indirect Immunoassay for Detection of 17β-Estradiol. J. Pharm. Biomed. Anal. 2019, 163, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Wei, T. Detection of 17β-Estradiol in Water Samples by a Novel Double-Layer Molecularly Imprinted Film-Based Biosensor. Talanta 2015, 141, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, N.; Long, F.; Gao, C.; He, M.; Shi, H.-C.; Gu, A.Z. Aptamer-Based Optical Biosensor For Rapid and Sensitive Detection of 17β-Estradiol In Water Samples. Environ. Sci. Technol. 2012, 46, 3288–3294. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.H.; Zhou, X.H.; Xu, W.Q.; Song, B.D.; Shi, H.C. Highly Sensitive Detection of Sulfadimidine in Water and Dairy Products by Means of an Evanescent Wave Optical Biosensor. RSC Adv. 2014, 4, 60227–60233. [Google Scholar] [CrossRef]
- Scott, I.M.; Kraft, L.J.; Parker, J.D.; Daniel, K.; Kustick, S.; Kennen, D.; McMurry, J.L. A Real Time Optical Biosensor Assay for Amoxicillin and Other β-Lactams in Water Samples. Georg. J. Sci. 2010, 68, 2010. [Google Scholar]
- Sciacca, B.; Secret, E.; Pace, S.; Gonzalez, P.; Geobaldo, F.; Quignard, F.; Cunin, F. Chitosan-Functionalized Porous Silicon Optical Transducer for the Detection of Carboxylic Acid-Containing Drugs in Water. J. Mater. Chem. 2011, 21, 2294–2302. [Google Scholar] [CrossRef]
- Stackelberg, P.E.; Furlong, E.T.; Meyer, M.T.; Zaugg, S.D.; Henderson, A.K.; Reissman, D.B. Persistence of Pharmaceutical Compounds and Other Organic Wastewater Contaminants in a Conventional Drinking-Water-Treatment Plant. Sci. Total Environ. 2004, 329, 99–113. [Google Scholar] [CrossRef] [Green Version]
- Hegnerová, K.; Piliarik, M.; Šteinbachová, M.; Flegelová, Z.; Černohorská, H.; Homola, J. Detection of Bisphenol A Using a Novel Surface Plasmon Resonance Biosensor. Anal. Bioanal. Chem. 2010, 398, 1963–1966. [Google Scholar] [CrossRef]
- Hegnerová, K.; Homola, J. Surface Plasmon Resonance Sensor for Detection of Bisphenol A in Drinking Water. Sens. Actuators B Chem. 2010, 151, 177–179. [Google Scholar] [CrossRef]
- Ma, K.; Ekblad, T.; Koerkamp, M.K.; Kelderman, H.; van Wijlen, M.; Duarah, A.; Yue, J.; Zhang, L.; Wong, M.V.M.; Lim, M.H. Contaminant Detection in Treated Water Using Optiqua’s MiniLabTM Biosensing System: A Case Study for Bisphenol A. Int. J. Environ. Anal. Chem. 2015, 95, 366–378. [Google Scholar] [CrossRef]
- Masdor, N.A.; Altintas, Z.; Tothill, I.E. Surface Plasmon Resonance Immunosensor for the Detection of Campylobacter Jejuni. Chemosensors 2017, 5, 16. [Google Scholar] [CrossRef]
- Foudeh, A.M.; Trigui, H.; Mendis, N.; Faucher, S.P.; Veres, T.; Tabrizian, M. Rapid and Specific SPRi Detection of L. Pneumophila in Complex Environmental Water Samples. Anal. Bioanal. Chem. 2015, 407, 5541–5545. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zou, H.; Li, M.; Sun, C.; Ren, D.; Li, Y. Fiber Optic Surface Plasmon Resonance Sensor for Detection of E. Coli O157:H7 Based on Antimicrobial Peptides and AgNPs-RGO. Biosens. Bioelectron. 2018, 117, 347–353. [Google Scholar] [CrossRef]
- Sanati, P.; Hashemi, S.S.; Bahadoran, M.; Babadi, A.A.; Akbari, E. Detection of Escherichia Coli K12 in Water Using Slot Waveguide in Cascaded Ring Resonator. Silicon 2022, 14, 851–857. [Google Scholar] [CrossRef]
- Kim, D.W.; Chun, H.J.; Kim, J.H.; Yoon, H.; Yoon, H.C. A Non-Spectroscopic Optical Biosensor for the Detection of Pathogenic Salmonella Typhimurium Based on a Stem-Loop DNA Probe and Retro-Reflective Signaling. Nano Converg. 2019, 6, 16. [Google Scholar] [CrossRef]
- Angelopoulou, M.; Petrou, P.; Misiakos, K.; Raptis, I.; Kakabakos, S. Simultaneous Detection of Salmonella Typhimurium and Escherichia Coli O157:H7 in Drinking Water with Mach–Zehnder Interferometers Monolithically Integrated on Silicon Chips †. Eng. Proc. 2022, 16, 12269. [Google Scholar] [CrossRef]
- Nabok, A.; Al-Jawdah, A.M.; Gémes, B.; Takács, E.; Székács, A. An Optical Planar Waveguide-Based Immunosensors for Determination of Fusarium Mycotoxin Zearalenone. Toxins 2021, 13, 89. [Google Scholar] [CrossRef]
- Yildirim, N.; Long, F.; Gu, A.Z. Aptamer Based E-Coli Detection in Waste Waters by Portable Optical Biosensor System. In Proceedings of the 2014 40th Annual Northeast Bioengineering Conference (NEBEC), Boston, MA, USA, 25–27 April 2014; pp. 1–3. [Google Scholar] [CrossRef]
- Herranz, S.; Marazuela, M.D.; Moreno-Bondi, M.C. Automated Portable Array Biosensor for Multisample Microcystin Analysis in Freshwater Samples. Biosens. Bioelectron. 2012, 33, 50–55. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, H.; Chen, S.; Yu, H.; Quan, X. Colloidal Graphene as a Transducer in Homogeneous Fluorescence-Based Immunosensor for Rapid and Sensitive Analysis of Microcystin-LR. Environ. Sci. Technol. 2012, 46, 12567–12574. [Google Scholar] [CrossRef]
- Shi, H.C.; Song, B.D.; Long, F.; Zhou, X.H.; He, M.; Lv, Q.; Yang, H.Y. Automated Online Optical Biosensing System for Continuous Real-Time Determination of Microcystin-LR with High Sensitivity and Specificity: Early Warning for Cyanotoxin Risk in Drinking Water Sources. Environ. Sci. Technol. 2013, 47, 4434–4441. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wu, J.; Sun, Y.; Zhang, Y.; Wen, Z.; Dai, H.; Wang, H.; Li, Z. A Graphene Oxide Based Biosensor for Microcystins Detection by Fluorescence Resonance Energy Transfer. Biosens. Bioelectron. 2012, 38, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Zhou, X.; Tang, Y.; He, M.; Zhang, X.; Shi, H.; Xiang, Y. Practical, Highly Sensitive, and Regenerable Evanescent-Wave Biosensor for Detection of Hg2+ and Pb2+ in Water. Biosens. Bioelectron. 2016, 80, 265–272. [Google Scholar] [CrossRef]
- Gupta, S.; Sarkar, S.; Katranidis, A.; Bhattacharya, J. Development of a Cell-Free Optical Biosensor for Detection of a Broad Range of Mercury Contaminants in Water: A Plasmid DNA-Based Approach. ACS Omega 2019, 4, 9480–9487. [Google Scholar] [CrossRef] [PubMed]
- Bismuth, M.; Zaltzer, E.; Muthukumar, D.; Suckeveriene, R.; Shtenberg, G. Real-Time Detection of Copper Contaminants in Environmental Water Using Porous Silicon Fabry-Pérot Interferometers. Analyst 2021, 146, 5160–5168. [Google Scholar] [CrossRef]
- Kumar, D.N.; Reingewirtz, S.; Shemesh, M.; Suckeveriene, R.; Shtenberg, G. DNAzyme-Based Biosensor for Sub Ppb Lead Ions Detection Using Porous Silicon Fabry-Pérot Interferometer. Sens. Actuators B Chem. 2022, 362, 131761. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, L.; Zhou, B.; Liu, W.; Ge, J.; Wu, J.; Wang, Y.; Wang, P. Ultrasensitive and Selective Gold Film-Based Detection of Mercury (II) in Tap Water Using a Laser Scanning Confocal Imaging-Surface Plasmon Resonance System in Real Time. Biosens. Bioelectron. 2013, 47, 391–395. [Google Scholar] [CrossRef]
- Chang, C.C.; Lin, S.; Wei, S.C.; Chen, C.Y.; Lin, C.W. An Amplified Surface Plasmon Resonance “Turn-on” Sensor for Mercury Ion Using Gold Nanoparticles. Biosens. Bioelectron. 2011, 30, 235–240. [Google Scholar] [CrossRef]
- Yildirim, N.; Long, F.; He, M.; Gao, C.; Shi, H.C.; Gu, A.Z. A Portable DNAzyme-Based Optical Biosensor for Highly Sensitive and Selective Detection of Lead (II) in Water Sample. Talanta 2014, 129, 617–622. [Google Scholar] [CrossRef]
- Long, F.; Gao, C.; Shi, H.C.; He, M.; Zhu, A.N.; Klibanov, A.M.; Gu, A.Z. Reusable Evanescent Wave DNA Biosensor for Rapid, Highly Sensitive, and Selective Detection of Mercury Ions. Biosens. Bioelectron. 2011, 26, 4018–4023. [Google Scholar] [CrossRef] [PubMed]
- Long, F.; Zhu, A.; Shi, H.; Wang, H.; Liu, J. Rapid On-Site/in-Situ Detection of Heavy Metal Ions in Environmental Water Using a Structure-Switching DNA Optical Biosensor. Sci. Rep. 2013, 3, 1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shtenberg, G.; Massad-Ivanir, N.; Segal, E. Detection of Trace Heavy Metal Ions in Water by Nanostructured Porous Si Biosensors. Analyst 2015, 140, 4507–4514. [Google Scholar] [CrossRef] [PubMed]
- Eltzov, E.; Yehuda, A.; Marks, R.S. Creation of a New Portable Biosensor for Water Toxicity Determination. Sens. Actuators B Chem. 2015, 221, 1044–1054. [Google Scholar] [CrossRef]
- Xu, T.; Close, D.M.; Sayler, G.S.; Ripp, S. Genetically Modified Whole-Cell Bioreporters for Environmental Assessment. Ecol. Indic. 2013, 28, 125–141. [Google Scholar] [CrossRef] [Green Version]
- Refaat, D.; Aggour, M.G.; Farghali, A.A.; Mahajan, R.; Wiklander, J.G.; Nicholls, I.A.; Piletsky, S.A. Strategies for Molecular Imprinting and the Evolution of MIP Nanoparticles as Plastic Antibodies—Synthesis and Applications. Int. J. Mol. Sci. 2019, 20, 6304. [Google Scholar] [CrossRef] [Green Version]
- Parisi, O.I.; Francomano, F.; Dattilo, M.; Patitucci, F.; Prete, S.; Amone, F.; Puoci, F. The Evolution of Molecular Recognition: From Antibodies to Molecularly Imprinted Polymers (MIPs) as Artificial Counterpart. J. Funct. Biomater. 2022, 13, 12. [Google Scholar] [CrossRef]
- Bhaskar, S.; Thacharakkal, D.; Ramamurthy, S.S.; Subramaniam, C. Metal-Dielectric Interfacial Engineering with Mesoporous Nano-Carbon Florets for 1000-Fold Fluorescence Enhancements: Smartphone-Enabled Visual Detection of Perindopril Erbumine at a Single-Molecular Level. ACS Sustain. Chem. Eng. 2023, 11, 78–91. [Google Scholar] [CrossRef]
- Yuk, J.S.; Guignon, E.F.; Lynes, M.A. Highly Sensitive Grating Coupler-Based Surface Plasmon-Coupled Emission (SPCE) Biosensor for Immunoassay. Analyst 2013, 138, 2576–2582. [Google Scholar] [CrossRef]
- Rai, A.; Bhaskar, S.; Ganesh, K.M.; Ramamurthy, S.S. Hottest Hotspots from the Coldest Cold: Welcome to Nano 4.0. ACS Appl. Nano Mater. 2022, 5, 12245–12264. [Google Scholar] [CrossRef]
- Bhaskar, S.; Srinivasan, V.; Ramamurthy, S.S. Nd2O3-Ag Nanostructures for Plasmonic Biosensing, Antimicrobial, and Anticancer Applications. ACS Appl. Nano Mater. 2022, 6, 1129–1145. [Google Scholar] [CrossRef]
- Gaddam, R.R.; Narayan, R.; Raju, K.V.S.N. Fluorescence Spectroscopy of Nanofillers and Their Polymer Nanocomposites. In Spectroscopy of Polymer Nanocomposites, 1st ed.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 158–180. ISBN 9780323401838. [Google Scholar]
- Akkilic, N.; Geschwindner, S.; Höök, F. Single-Molecule Biosensors: Recent Advances and Applications. Biosens. Bioelectron. 2020, 151, 111944. [Google Scholar] [CrossRef]
- Ruby, R. A Snapshot in Time: The Future in Filters for Cell Phones. IEEE Microw. Mag. 2015, 16, 46–59. [Google Scholar] [CrossRef]
- Wang, H.; Heintzmann, R.; Diederich, B. The Power in Your Pocket—Uncover Smartphones for Use as Cutting-Edge Microscopic Instruments in Science and Research. Adv. Opt. Technol. 2021, 10, 89–108. [Google Scholar] [CrossRef]
- McCracken, K.E.; Tat, T.; Paz, V.; Yoon, J.Y. Smartphone-Based Fluorescence Detection of Bisphenol A from Water Samples. RSC Adv. 2017, 7, 9237–9243. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.Y.; Liu, X.; Shen, L.; Chen, L.; Fang, W.H. Machine Learning with Multilevel Descriptors for Screening of Inorganic Nonlinear Optical Crystals. J. Phys. Chem. C 2021, 125, 25175–25188. [Google Scholar] [CrossRef]
- Fairbairn, C.E.; Kang, D.; Bosch, N. Using Machine Learning for Real-Time BAC Estimation from a New-Generation Transdermal Biosensor in the Laboratory. Drug Alcohol Depend. 2020, 216, 108205. [Google Scholar] [CrossRef]
- Arano-Martinez, J.A.; Martínez-González, C.L.; Salazar, M.I.; Torres-Torres, C. A Framework for Biosensors Assisted by Multiphoton Effects and Machine Learning. Biosensors 2022, 12, 710. [Google Scholar] [CrossRef]
- Guo, Z.; Kang, Y.; Liang, S.; Zhang, J. Detection of Hg(II) in Adsorption Experiment by a Lateral Flow Biosensor Based on Streptavidin-Biotinylated DNA Probes Modified Gold Nanoparticles and Smartphone Reader. Environ. Pollut. 2020, 266, 115389. [Google Scholar] [CrossRef]









| Biological Element | Affinity | Specificity | Sensibility (LOD, ng L−1) | Stability | Versatility | Cost |
|---|---|---|---|---|---|---|
| Enzyme | High | Medium/high | 12,000–1 × 10−4 | Medium/low | High | Low |
| Antibody | High | High | 250–0.07 × 10−6 | High | High | High |
| DNA/RNA | Very High | Very high | 4.14–4.4 × 10−3 | High | Low | Low |
| Cell | Medium | Medium | 2900–0.5 | High | Medium | Low |
| MIP | High | High | 1900–0.08 | High | High | Low |
| Target Analyte | Type of Biosensor | Limit of Detection (ppb) | Sample | Bio | Reference |
|---|---|---|---|---|---|
| 2,4-D | Fluorescence | 7.53 | Drinking water | Antibody | [89] |
| 2,4-D | Fluorescence | 2.17 | Deionized water | Antibody | [95] |
| Diuron | Fluorescence | 10 | Deionized water | Cell | [55] |
| Isoproturon | 10 | ||||
| Atrazine | 10 | ||||
| Atrazine | Fluorescence | 80 | Tap water | Cell | [90] |
| Atrazine | Fluorescence | 0.77 | Lake water | MIP | [96] |
| Atrazine | Grating couplers | 0.05 | Tap and river water | Antibody | [6] |
| Methyl-parathion | Fiber optic | 0.063 | Deionized water | Enzyme | [41] |
| Chlorpyrifos | Interferometer | 0.03 | Spiked bottled water | Antibody | [93] |
| Thiabendazole | 0.04 | ||||
| Imazalil | 0.03 | ||||
| Atrazine | Interferometer | 0.04 | Deionized water | Antibody | [94] |
| Paraquat | 0.05 | ||||
| Fenitrothion | Interferometer | 0.29 | Tap water | Antibody | [46] |
| Phenobucarb | Resonator | 1 × 10−4 | River water | Enzyme | [42] |
| Dimethoate | 1 × 10−3 |
| Target Analyte | Type of Biosensor | Limit of Detection (ppb) | Sample | Bio | Reference |
|---|---|---|---|---|---|
| Ampicilin | SPR | 0.3 × 106 | Deionized water | Antibody | [99] |
| Tetracycline | SPR/LSPR | 0.97 | Deionized water | MIP | [57] |
| Tetracycline | SPR/LSPR | <ppb | River water | Aptamer and antibody | [53] |
| Metoprolol | SPR | 1.9 | Drinking water | MIP | [101] |
| Ciprofloxacin | SPR | 0.08 | Deionized water | MIP | [102] |
| Diclofenac | SPR | 1 | Deionized water | Antibody | [100] |
| 17β-estradiol | SPR | 0.001 | Deionized water | Antibody | [104] |
| 17β-estradiol | SPR/Grating | 0.0015 | Spiked tap and pond water | ER hERα | [34] |
| 17β-estradiol | SPR | 6.8 × 10−5 | Wastewater | MIP | [105] |
| 17β-estradiol | Fluorescence | 0.14 | Wastewater | DNA | [106] |
| Sulfadimine | Fluorescence | 0.06 | Wastewater, lake and bottled water | Antibody | [107] |
| Diclofenac | Fluorescence | 2900 | Wastewater | Cell | [54] |
| Ciprofloxacin | Fluorescence | 1900 | River water | MIP | [58] |
| Estrogen | Fluorescence | 1.05 | Wastewater | Estrogen receptors ERα and Erβ | [33] |
| Ciprofloxacin | Fiber optic | 3.3 × 10−6 | Wastewater | Antibody | [47] |
| Penicillin | Interferometer | 250 | Deionized water | Antibody | [103] |
| Amoxicillin | Interferometer | >1 | Wastewater, lake and drinking water | Antibody | [108] |
| Ibuprofen | Interferometer | 1000 | Deionized water | Chitosan | [109] |
| Target Analyte | Type of Biosensor | Limit of Detection (ppb) | Sample | Bio | Reference |
|---|---|---|---|---|---|
| Dichloroethane | Fiber optic | 1000 | River, tap and bottled water | Enzyme | [44] |
| Naphthalene | SPR | 0.76 | Sea water | Antibody | [49] |
| Bisphenol A | SPR | 5.2 × 10−3 | Deionized water | Antibody | [48] |
| Bisphenol A | SPR | 0.14 | Wastewater | Antibody | [111] |
| Bisphenol A | SPR | 0.04 | Drinking water | Antibody | [112] |
| 1,2-dibromoethane | Fluorescence | 2400 | River water | Enzyme | [43] |
| 1,2,3-trichloropropane | 1400 | ||||
| 1,2-di-chloroethane | 2700 | ||||
| 3-chloro-2-(chloromethyl)-1-propene | 1400 | ||||
| γ-hexa-chlorocyclohexane | 12,100 | ||||
| Bisphenol A | Fluorescence | 0.03 | Drinking water | Antibody | [89] |
| Bisphenol A | Fluorescence | 0.001 | River, tap and bottled water | DNA | [52] |
| Bisphenol A | Fluorescence | 0.076 | Lake and tap water | Antibody | [18] |
| Bisphenol A | Fluorescence | 0.025 | Deionized water | Antibody | [95] |
| Bisphenol A | Interferometer | 0.5 | Treated water | Antibody | [113] |
| Target Analyte | Type of Biosensor | Limit of Detection | Sample | Bio | Reference |
|---|---|---|---|---|---|
| Microorganisms | |||||
| L. pneumophila | SPRi | 3 × 104 CFU/mL | Spiked water from a cooling tower | DNA | [115] |
| C. jejuni | SPR | 4 × 104 CFU/mL | Deionized water | Antibody | [114] |
| E. coli O157:H7 | SPR | 5 × 102 CFU/mL | Spiked tap water | Peptide | [116] |
| E. coli | Interferometer | 103 cells/mL | Deionized water | Lectins of Concanavalin A | [35] |
| S. aureus | 103 cells/mL | ||||
| E. coli | Interferometer | 110 CFU/mL | Drinking water | Antibody | [119] |
| S. typhimurium | 40 CFU/mL | ||||
| E. coli | Resonator | 3.33 × 10−5 RIU | Drinking water | Antibody | [117] |
| E. coli | Fluorescence | 10 CFU/mL | Wastewater | DNA | [121] |
| S. aureus | Grating | 244 CFU/mL | Deionized water | Antibody | [50] |
| S. typhimurium | Retroreflector | 2.84 pM | Deionized water | DNA | [118] |
| Toxins | |||||
| Microcystin-LR | Fluorescence | 0.67 ppb | Drinking water | Antibody | [89] |
| Microcystin-LR | Fluorescence | 0.03 ppb | Deionized water | Antibody | [95] |
| Microcystin-LR | Fluorescence | 0.016 ppb | Fresh water | Antibody | [122] |
| Microcystin-LR | Fluorescence | 0.14 ppb | Drinking water | DNA and antibody | [123] |
| Microcystin-LR | Fluorescence | 0.09 ppb | Lake water | Antibody | [124] |
| Microcystin-LR | Fluorescence | 0.5 ppb | Lake water | DNA | [125] |
| Microcystin-RR | 0.3 ppb | ||||
| Ochratoxin A | Interferometer | 1 × 10−3 ppb | Deionized water | Antibody | [51] |
| Zearalenone | Interferometer | 0.01 ppb | Deionized water | Antibody | [120] |
| Target Analyte | Type of Biosensor | Limit of Detection (ppb) | Sample | Bio | Reference |
|---|---|---|---|---|---|
| Hg | Optical fiber | 5001 | Deionized water | Enzyme | [45] |
| Hg | LSPR | 0.1 | Spiked seawater, wastewater and tap water | BSA and Chitosan | [85] |
| Hg | LSPR | 0.5 | Tap water | Cell | [56] |
| Cd | 0.5 | ||||
| Hg | SPR | 0.01 | Tap water | DNA | [130] |
| Hg | SPR | 0.2 | Tap and pond water | DNA | [131] |
| Hg | Optical fiber | 4.4 × 10−3 | Drinking water | DNA | [126] |
| Pb | 4.14 | ||||
| Pb | Optical fiber | 0.21 | Wastewater | DNA | [132] |
| Hg | Optical fiber | 0.58 | Wastewater, tap and bottled water | DNA | [133] |
| Hg | Fluorescence | 1 | Deionized water | DNA | [127] |
| Hg | Fluorescence | 0.24 | Surface water | DNA | [134] |
| Cu | Resonator | 2.5 × 10−3 | Drinking water | Stearic acid | [36] |
| Cu | Interferometer | 0.53 | Ground water, irrigation water and tap water | Polyethylenimine | [128] |
| Cu | Interferometer | 104 | Tap, irrigation and drain water | Enzyme | [135] |
| Ag | 56 | ||||
| Pb | 125 | ||||
| Pb | Interferometer | 0.49 | Ground water, irrigation water and tap water | DNA | [129] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera-Domínguez, M.; Morales-Luna, G.; Mahlknecht, J.; Cheng, Q.; Aguilar-Hernández, I.; Ornelas-Soto, N. Optical Biosensors and Their Applications for the Detection of Water Pollutants. Biosensors 2023, 13, 370. https://doi.org/10.3390/bios13030370
Herrera-Domínguez M, Morales-Luna G, Mahlknecht J, Cheng Q, Aguilar-Hernández I, Ornelas-Soto N. Optical Biosensors and Their Applications for the Detection of Water Pollutants. Biosensors. 2023; 13(3):370. https://doi.org/10.3390/bios13030370
Chicago/Turabian StyleHerrera-Domínguez, Marcela, Gesuri Morales-Luna, Jürgen Mahlknecht, Quan Cheng, Iris Aguilar-Hernández, and Nancy Ornelas-Soto. 2023. "Optical Biosensors and Their Applications for the Detection of Water Pollutants" Biosensors 13, no. 3: 370. https://doi.org/10.3390/bios13030370
APA StyleHerrera-Domínguez, M., Morales-Luna, G., Mahlknecht, J., Cheng, Q., Aguilar-Hernández, I., & Ornelas-Soto, N. (2023). Optical Biosensors and Their Applications for the Detection of Water Pollutants. Biosensors, 13(3), 370. https://doi.org/10.3390/bios13030370