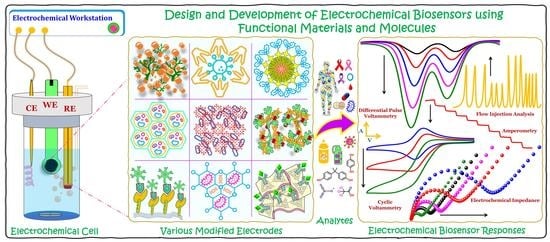
Recent Developments in the Design and Fabrication of Electrochemical Biosensors Using Functional Materials and Molecules
Abstract
:1. Introduction
2. Various Nanomaterial-Modified Electrodes
2.1. Carbon-Based Nanomaterials
2.1.1. Carbon Black
2.1.2. Graphene and Its Derivatives
2.1.3. Carbon Nanotubes
2.2. Metal Nanomaterials
2.2.1. Metal Nanoparticles
2.2.2. Metal Oxide and Sulfide Nanoparticles
2.2.3. MXenes
2.3. Quantum Dots
2.4. Organic Frameworks
2.4.1. Metal Organic Frameworks
2.4.2. Covalent Organic Frameworks
2.5. Ionic Liquids
2.5.1. Functionalized ILs
2.5.2. ILs with Nanomaterials
3. Summary and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations
| 4-NP | 4-Nitrophenol |
| ACL | Acetylcholine |
| ACLE | Acetylcholine esterase |
| AdSCV | Adsorptive-stripping cyclic voltammetry |
| AgNPs | Silver nanoparticles |
| AMIM TFSI- | 1-Allyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide |
| AMQDs | Antimonene quantum dots |
| ASA | Aspartic acid |
| ATP | Adenosine triphosphate |
| AuE | Gold electrode |
| BHb | Bovine hemoglobin |
| BMIM HFP | 1-Butyl-3-methylimidazolium hexafluorophosphate |
| BMIM TFB | 1-Butyl-3-methylimidazolium tetrafluoroborate |
| BP HFP | N-Butylpyridinium hexafluorophosphate |
| BPA | Bisphenol A |
| BSA | Bovine serum albumin |
| CA | Chronoamperometry |
| Catl | Catalase |
| CB | Carbon black |
| CBSK | Cronobacter sakazakii |
| CC | Carbon cloth |
| CE | Counter electrode |
| CFU | Colony forming unit |
| CGO | Carboxyl graphene oxide |
| CHA | Catalytic hairpin assembly |
| ChEs | Cholesterol esterase |
| ChOx | Cholesterol oxidase |
| CILE | Carbon ionic liquid electrode |
| CL | Choline |
| CLO | Choline oxidase |
| C-MWCNT | Carboxylic acid functionalized multiwalled carbon nanotube |
| CNF | Carbon nanofiber |
| CNSp | Carbon nanosphere |
| CNTs | Carbon nanotubes |
| COFs | Covalent organic frameworks |
| CONPs | Cobalt oxide nanoparticles |
| CPE | Carbon paste electrode |
| CRE | Creasome |
| CRGO | Chemically reduced graphene oxide |
| CS | Chitosan |
| CSNPs | Cobalt sulfide nanoparticles |
| CuF | Copper ferrite |
| CV | Cyclic voltammetry |
| CVD | Chemical vapor deposition |
| cyc | Cysteamine |
| CYF | Chlorpyrifos |
| Cyt-c | Cytochrome c |
| DA | Dopamine |
| DAOx | Diamine oxidase |
| DPV | Differential pulse voltammetry |
| DET | Direct electron transfer |
| DHDP | Dihexadecylphosphate |
| DMT | Dimethoate |
| DPX | Dipterex |
| E. coli | Escherichia coli |
| EDC | 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide |
| EIS | Electrochemical impedance spectroscopy |
| ERGO | Electrochemically reduced graphene oxide |
| FA | Folic acid |
| FAD | Flavin adenine dinucleotide |
| Fc | Ferrocene |
| FIA | Flow injection analysis |
| FLGR | Few-layered graphene |
| fM | Femtomolar |
| FR | Folate receptors |
| GA | Gallic acid |
| GCE | Glassy carbon electrode |
| GDY | Graphdiyne |
| Gld | Glutaraldehyde |
| GNFs | Gold nanoflowers |
| GNPs | Gold nanoparticles |
| GNSp | Gold nanosphere |
| GO | Graphene oxide |
| GOD | Glucose oxidase |
| GOQDs | Graphene oxide quantum dots |
| GR | Graphene |
| GRE | Graphite electrode |
| GRNRs | Graphene nanoribbons |
| GRNSs | Graphene nanosheets |
| GRQDs | Graphene quantum dots |
| H2O2 | Hydrogen peroxide |
| HAp | Hydroxyapatite |
| Hb | Hemoglobin |
| HCR | Hybridization chain reaction |
| HER | Human epidermal growth factor receptor |
| HQ | Hydroquinone |
| HRP | Horseradish peroxidase |
| IL | Ionic liquid |
| ITO | Indium tin oxide |
| Lac | Laccase |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| LOx | Lactate oxidase |
| MAOx | Monoamine oxidase |
| Mb | Myoglobin |
| MC | Microcuboids |
| MCH | Mercaptohexanol |
| MDA | 11-Mercaptoundecanoic acid |
| MIP | Molecularly imprinted polymer |
| miR | microRNA |
| mM | Millimolar |
| MNMs | Metal nanomaterials |
| MOFs | Metal organic frameworks |
| MPA | Mercaptopropionic acid |
| MPPy TFSI | 3-Methyl-1-propylpyridinium bis(trifluoromethyl sulfonyl)imide |
| MPTMS | (3-Mercaptopropyl)trimethoxy silane |
| MTB | Mycobacterium tuberculosis |
| MWCNT | Multiwalled carbon nanotube |
| NA | Nucleic acid |
| Nf | Nafion |
| NHS | N-hydroxysuccinimide |
| nM | Nanomolar |
| NMs | Nanomaterials |
| NS | Nanosheets |
| OFWs | Organic frameworks |
| OME | Omethoate |
| PANI | Polyaniline |
| PB | Prussian blue |
| PCR | Polymerase chain reaction |
| PDA | Phenylenediamine |
| PEDOT | Poly(3,4-ethylenedioxythiophene) |
| PEI | Polyethyleneimine |
| PGE | Pencil graphite electrode |
| pM | Picomolar |
| PPI | Poly(propylene imine) |
| PSA | Prostate specific antigen |
| PtE | Platinum electrode |
| PVFc | Polyvinyl ferrocene |
| QDs | Quantum dots |
| RE | Reference electrode |
| RGO | Reduced graphene oxide |
| RhNPs | Rhodium nanoparticles |
| SCE | Saturated calomel electrode |
| SnDS | Tin disulfide |
| SPCE | Screen printed carbon electrode |
| SPE | Screen printed electrode |
| SPF | Soy protein film |
| SPGE | Screen printed gold electrode |
| SWCNTs | Single walled carbon nanotubes |
| TCA | Trichloroacetic acid |
| TCL | Thiocholine |
| TDS | Titanium disulfide |
| TMX | Ti3C2 MXenes |
| TPC | Terephthaloyl chloride |
| Tpn | Topotecan |
| TRGO | Thermally reduced graphene oxide |
| TYR | Tyrosinase |
| WE | Working electrode |
| ZnONPs | Zinc oxide nanoparticles |
References
- Han, Q.; Pang, J.; Li, Y.; Sun, B.; Ibarlucea, B.; Liu, X.; Gemming, T.; Cheng, Q.; Zhang, S.; Liu, H.; et al. Graphene Biodevices for Early Disease Diagnosis Based on Biomarker Detection. ACS Sens. 2021, 6, 3841–3881. [Google Scholar] [CrossRef] [PubMed]
- Parsa, S.F.; Vafajoo, A.; Rostami, A.; Salarian, R.; Rabiee, M.; Rabiee, N.; Rabiee, G.; Tahriri, M.; Yadegari, A.; Vashaee, D.; et al. Early Diagnosis of Disease Using Microbead Array Technology: A Review. Anal. Chim. Acta 2018, 1032, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.; Lim, S.; Ko, H. Wearable and Flexible Sensors for User-Interactive Health-Monitoring Devices. J. Mater. Chem. B 2018, 6, 4043–4064. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, P.; Błochowiak, K. The Role of Salivary Biomarkers in the Early Diagnosis of Alzheimer’s Disease and Parkinson’s Disease. Diagnostics 2021, 11, 371. [Google Scholar] [CrossRef]
- Wang, L. Early Diagnosis of Breast Cancer. Sensors 2017, 17, 1572. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.H.; Kweon, O.Y.; Kim, H.; Yoo, J.H.; Han, S.G.; Oh, J.H. Recent Advances in Organic Sensors for Health Self-Monitoring Systems. J. Mater. Chem. C 2018, 6, 8569–8612. [Google Scholar] [CrossRef]
- Porsteinsson, A.P.; Isaacson, R.S.; Knox, S.; Sabbagh, M.N.; Rubino, I. Diagnosis of Early Alzheimer’s Disease: Clinical Practice in 2021. J. Prev. Alzheimers Dis. 2021, 8, 371–386. [Google Scholar] [CrossRef]
- Ma, S.; Lin, M.; Tang, J.; Liu, R.; Yang, Y.; Yu, Y.; Li, G.; An, T. Occurrence and Fate of Polycyclic Aromatic Hydrocarbons from Electronic Waste Dismantling Activities: A Critical Review from Environmental Pollution to Human Health. J. Hazard. Mater. 2022, 424, 127683. [Google Scholar] [CrossRef]
- Chormare, R.; Kumar, M.A. Environmental Health and Risk Assessment Metrics with Special Mention to Biotransfer, Bioaccumulation and Biomagnification of Environmental Pollutants. Chemosphere 2022, 302, 134836. [Google Scholar] [CrossRef]
- Jarin, M.; Dou, Z.; Gao, H.; Chen, Y.; Xie, X. Salinity Exchange between Seawater/Brackish Water and Domestic Wastewater through Electrodialysis for Potable Water. Front. Environ. Sci. Eng. 2023, 17, 16. [Google Scholar] [CrossRef]
- Stefan, D.S.; Bosomoiu, M.; Stefan, M. Methods for Natural and Synthetic Polymers Recovery from Textile Waste. Polymers 2022, 14, 3939. [Google Scholar] [CrossRef]
- Kanwal, Q.; Li, J.; Zeng, X. Mapping Recyclability of Industrial Waste for Anthropogenic Circularity: A Circular Economy Approach. ACS Sustain. Chem. Eng. 2021, 9, 11927–11936. [Google Scholar] [CrossRef]
- Siddiqua, A.; Hahladakis, J.N.; Al-Attiya, W.A.K.A. An Overview of the Environmental Pollution and Health Effects Associated with Waste Landfilling and Open Dumping. Environ. Sci. Pollut. Res. 2022, 29, 58514–58536. [Google Scholar] [CrossRef]
- Cai, Y.; Ling, Q.; Yi, Y.; Chen, Z.; Yang, H.; Hu, B.; Liang, L.; Wang, X. Application of Covalent Organic Frameworks in Environmental Pollution Management. Appl. Catal. A 2022, 643, 118733. [Google Scholar] [CrossRef]
- Mahamood, M.; Khan, F.R.; Zahir, F.; Javed, M.; Alhewairini, S.S. Bagarius Bagarius, and Eichhornia Crassipes Are Suitable Bioindicators of Heavy Metal Pollution, Toxicity, and Risk Assessment. Sci. Rep. 2023, 13, 1824. [Google Scholar] [CrossRef]
- Verma, N.; Velmurugan, G.V.; Winford, E.; Coburn, H.; Kotiya, D.; Leibold, N.; Radulescu, L.; Despa, S.; Chen, K.C.; van Eldik, L.J.; et al. Aβ Efflux Impairment and Inflammation Linked to Cerebrovascular Accumulation of Amyloid-Forming Amylin Secreted from Pancreas. Commun. Biol. 2023, 6, 2. [Google Scholar] [CrossRef]
- Yeganeh-Zare, S.; Farhadi, K.; Amiri, S. Rapid Detection of Apple Juice Concentrate Adulteration with Date Concentrate, Fructose and Glucose Syrup Using HPLC-RID Incorporated with Chemometric Tools. Food Chem. 2022, 370, 131015. [Google Scholar] [CrossRef]
- Wang, X.Y.; Zhu, G.B.; Cao, W.D.; Liu, Z.J.; Pan, C.G.; Hu, W.J.; Zhao, W.Y.; Sun, J.F. A Novel Ratiometric Fluorescent Probe for the Detection of Uric Acid in Human Blood Based on H2O2-Mediated Fluorescence Quenching of Gold/Silver Nanoclusters. Talanta 2019, 191, 46–53. [Google Scholar] [CrossRef]
- Kim, T.; Lee, J.; Lee, J.P.; Kim, B.N.; Kim, Y.H.; Lee, Y.S.; Min, J. Screening of Novel Peptides That Specifically Interact with Vitamin D Bound Biocomplex Proteins. Sci. Rep. 2023, 13, 2116. [Google Scholar] [CrossRef]
- Celik, C.; Demir, N.Y.; Duman, M.; Ildiz, N.; Ocsoy, I. Red Cabbage Extract-Mediated Colorimetric Sensor for Swift, Sensitive and Economic Detection of Urease-Positive Bacteria by Naked Eye and Smartphone Platform. Sci. Rep. 2023, 13, 2056. [Google Scholar] [CrossRef]
- Nukazuka, A.; Asai, S.; Hayakawa, K.; Nakagawa, K.; Kanazashi, M.; Kakizoe, H.; Hayashi, K.; Kawahara, T.; Sawada, K.; Kuno, H.; et al. Electrical Biosensing System Utilizing Ion-Producing Enzymes Conjugated with Aptamers for the Sensing of Severe Acute Respiratory Syndrome Coronavirus 2. Sens. Bio-Sens. Res. 2023, 39, 100549. [Google Scholar] [CrossRef] [PubMed]
- Cahuantzi-Muñoz, S.L.; González-Fuentes, M.A.; Ortiz-Frade, L.A.; Torres, E.; Ţălu, Ş.; Trejo, G.; Méndez-Albores, A. Electrochemical Biosensor for Sensitive Quantification of Glyphosate in Maize Kernels. Electroanalysis 2019, 31, 927–935. [Google Scholar] [CrossRef] [Green Version]
- Pascual-Lucas, M.; Allué, J.A.; Sarasa, L.; Fandos, N.; Castillo, S.; Terencio, J.; Sarasa, M.; Tartari, J.P.; Sanabria, Á.; Tárraga, L.; et al. Clinical Performance of an Antibody-Free Assay for Plasma Aβ42/Aβ40 to Detect Early Alterations of Alzheimer’s Disease in Individuals with Subjective Cognitive Decline. Alzheimers Res. Ther. 2023, 15, 2. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, Y.; Zhu, A.; Tian, Y. In Vivo Electrochemical Biosensors: Recent Advances in Molecular Design, Electrode Materials, and Electrochemical Devices. Anal. Chem. 2023, 95, 388–406. [Google Scholar] [CrossRef]
- Meloni, F.; Spychalska, K.; Zając, D.; Pilo, M.I.; Zucca, A.; Cabaj, J. Application of a Thiadiazole-Derivative in a Tyrosinase-Based Amperometric Biosensor for Epinephrine Detection. Electroanalysis 2021, 33, 1639–1645. [Google Scholar] [CrossRef]
- Hartati, Y.W.; Irkham, I.; Zulqaidah, S.; Syafira, R.S.; Kurnia, I.; Noviyanti, A.R.; Topkaya, S.N. Recent Advances in Hydroxyapatite-Based Electrochemical Biosensors: Applications and Future Perspectives. Sens. Bio-Sens. Res. 2022, 38, 100542. [Google Scholar] [CrossRef]
- Laraib, U.; Sargazi, S.; Rahdar, A.; Khatami, M.; Pandey, S. Nanotechnology-Based Approaches for Effective Detection of Tumor Markers: A Comprehensive State-of-the-Art Review. Int. J. Biol. Macromol. 2022, 195, 356–383. [Google Scholar] [CrossRef]
- Koo, K.M.; Kim, C.D.; Ju, F.N.; Kim, H.; Kim, C.H.; Kim, T.H. Recent Advances in Electrochemical Biosensors for Monitoring Animal Cell Function and Viability. Biosensors 2022, 12, 1162. [Google Scholar] [CrossRef]
- Li, H.; Qi, H.; Chang, J.; Gai, P.; Li, F. Recent Progress in Homogeneous Electrochemical Sensors and Their Designs and Applications. TrAC Trends Anal. Chem. 2022, 156, 116712. [Google Scholar] [CrossRef]
- Falina, S.; Anuar, K.; Shafiee, S.A.; Juan, J.C.; Manaf, A.A.; Kawarada, H.; Syamsul, M. Two-Dimensional Non-Carbon Materials-Based Electrochemical Printed Sensors: An Updated Review. Sensors 2022, 22, 9358. [Google Scholar] [CrossRef]
- Beluomini, M.A.; da Silva, J.L.; de Sá, A.C.; Buffon, E.; Pereira, T.C.; Stradiotto, N.R. Electrochemical Sensors Based on Molecularly Imprinted Polymer on Nanostructured Carbon Materials: A Review. J. Electroanal. Chem. 2019, 840, 343–366. [Google Scholar] [CrossRef]
- Kalambate, P.K.; Rao, Z.; Dhanjai; Wu, J.; Shen, Y.; Boddula, R.; Huang, Y. Electrochemical (Bio) Sensors Go Green. Biosens. Bioelectron. 2020, 163, 112270. [Google Scholar] [CrossRef]
- Zhang, F.T.; Cai, L.Y.; Zhou, Y.L.; Zhang, X.X. Immobilization-Free DNA-Based Homogeneous Electrochemical Biosensors. TrAC Trends Anal. Chem. 2016, 85, 17–32. [Google Scholar] [CrossRef]
- Sheikholeslam, M.; Nanda, P.; Sanati, A.; Pritzker, M.; Chen, P. Direct Electrochemistry of Hemoglobin/Peptide-Carbon Nanotube Modified Electrode for Hydrogen Peroxide Biosensing. Mater. Lett. 2023, 335, 133799. [Google Scholar] [CrossRef]
- Lawal, A.T. Progress in Utilisation of Graphene for Electrochemical Biosensors. Biosens. Bioelectron. 2018, 106, 149–178. [Google Scholar] [CrossRef]
- Liu, H.; Duan, C.; Yang, C.; Shen, W.; Wang, F.; Zhu, Z. A Novel Nitrite Biosensor Based on the Direct Electrochemistry of Hemoglobin Immobilized on MXene-Ti3C2. Sens. Actuators B 2015, 218, 60–66. [Google Scholar] [CrossRef]
- Sha, H. Direct Electrochemistry of Hemoglobin on Electrodeposited Three-Dimensional Interconnected Graphene-Silver Nanocomposite Modified Electrode. Int. J. Electrochem. Sci. 2016, 11, 9656–9665. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, N.; Xu, Y.; Liu, X.; Ma, Y.; Huang, Z.; Luo, H.; Hou, C.; Huo, D. A Novel Electrochemical Biosensor Based on AMNFs@ZIF-67 Nano Composite Material for Ultrasensitive Detection of HER2. Bioelectrochemistry 2023, 150, 108362. [Google Scholar] [CrossRef]
- Negahdary, M.; Akira Ameku, W.; Gomes Santos, B.; dos Santos Lima, I.; Gomes de Oliveira, T.; Carvalho França, M.; Angnes, L. Recent Electrochemical Sensors and Biosensors for Toxic Agents Based on Screen-Printed Electrodes Equipped with Nanomaterials. Microchem. J. 2023, 185, 108281. [Google Scholar] [CrossRef]
- Tseng, W.T.; Chou, Y.Y.; Wu, J.G.; Wang, Y.C.; Tseng, T.N.; Pan, S.W.; Luo, S.C.; Ho, M.L. An Electrochemical Conducting Polymer-Based Biosensor for Leukocyte Esterase and Nitrite Detection for Diagnosing Urinary Tract Infections: A Pilot Study. Microchem. J. 2023, 188, 108493. [Google Scholar] [CrossRef]
- Ferreira, L.M.C.; Silva, P.S.; Augusto, K.K.L.; Gomes-Júnior, P.C.; Farra, S.O.D.; Silva, T.A.; Fatibello-Filho, O.; Vicentini, F.C. Using Nanostructured Carbon Black-Based Electrochemical (Bio)Sensors for Pharmaceutical and Biomedical Analyses: A Comprehensive Review. J. Pharm. Biomed. Anal. 2022, 221, 115032. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez-Redín, G.; Silva, T.A.; Vicentini, F.C.; Fatibello-Filho, O. Effect of Carbon Black Functionalization on the Analytical Performance of a Tyrosinase Biosensor Based on Glassy Carbon Electrode Modified with Dihexadecylphosphate Film. Enzyme Microb. Technol. 2018, 116, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, Z.; Huang, J.; Wu, Y.; Mao, X.; Tan, Y.; Liu, H.; Ma, D.; Li, X.; Wang, X. Development of a Phage-Based Electrochemical Biosensor for Detection of Escherichia coli O157: H7 GXEC-N07. Bioelectrochemistry 2023, 150, 108345. [Google Scholar] [CrossRef]
- Melios, N.; Tsouti, V.; Chatzandroulis, S.; Tsekenis, G. Development of an All-Carbon Electrochemical Biosensor on a Flexible Substrate for the Sensitive Detection of Glucose. Eng. Proc. 2022, 16, 17. [Google Scholar] [CrossRef]
- Jafari, S.; Burr, L.; Migliorelli, D.; Galve, R.; Marco, M.P.; Campbell, K.; Elliott, C.; Suman, M.; Sturla, S.J.; Generelli, S. Smartphone-Based Magneto-Immunosensor on Carbon Black Modified Screen-Printed Electrodes for Point-of-Need Detection of Aflatoxin B1 in Cereals. Anal. Chim. Acta 2022, 1221, 340118. [Google Scholar] [CrossRef]
- Bollella, P.; Fusco, G.; Tortolini, C.; Sanzò, G.; Favero, G.; Gorton, L.; Antiochia, R. Beyond Graphene: Electrochemical Sensors and Biosensors for Biomarkers Detection. Biosens. Bioelectron. 2017, 89, 152–166. [Google Scholar] [CrossRef]
- Singh, S.; Hasan, M.R.; Sharma, P.; Narang, J. Graphene Nanomaterials: The Wondering Material from Synthesis to Applications. Sens. Int. 2022, 3, 100190. [Google Scholar] [CrossRef]
- Li, G.; Xia, Y.; Tian, Y.; Wu, Y.; Liu, J.; He, Q.; Chen, D. Review—Recent Developments on Graphene-Based Electrochemical Sensors toward Nitrite. J. Electrochem. Soc. 2019, 166, B881–B895. [Google Scholar] [CrossRef]
- Vermisoglou, E.; Panáček, D.; Jayaramulu, K.; Pykal, M.; Frébort, I.; Kolář, M.; Hajdúch, M.; Zbořil, R.; Otyepka, M. Human Virus Detection with Graphene-Based Materials. Biosens. Bioelectron. 2020, 166, 112436. [Google Scholar] [CrossRef]
- Justino, C.I.L.; Gomes, A.R.; Freitas, A.C.; Duarte, A.C.; Rocha-Santos, T.A.P. Graphene Based Sensors and Biosensors. TrAC Trends Anal. Chem. 2017, 91, 53–66. [Google Scholar] [CrossRef]
- Parlak, O.; Tiwari, A.; Turner, A.P.F.; Tiwari, A. Template-Directed Hierarchical Self-Assembly of Graphene Based Hybrid Structure for Electrochemical Biosensing. Biosens. Bioelectron. 2013, 49, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Gu, Y.; Lan, H.; Sun, Y.; Gao, H. Functional Graphene-Gold Nano-Composite Fabricated Electrochemical Biosensor for Direct and Rapid Detection of Bisphenol A. Anal. Chim. Acta 2015, 853, 297–302. [Google Scholar] [CrossRef]
- Kong, F.Y.; Li, W.W.; Wang, J.Y.; Fang, H.L.; Fan, D.H.; Wang, W. Direct Electrolytic Exfoliation of Graphite with Hemin and Single-Walled Carbon Nanotube: Creating Functional Hybrid Nanomaterial for Hydrogen Peroxide Detection. Anal. Chim. Acta 2015, 884, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y. SnO2 Quantum Dots Functionalized 3D Graphene Composite for Enhanced Performance of Electrochemical Myoglobin Biosensor. Int. J. Electrochem. Sci. 2020, 15, 10412–10422. [Google Scholar] [CrossRef]
- Poletti, F.; Zanfrognini, B.; Favaretto, L.; Quintano, V.; Sun, J.; Treossi, E.; Melucci, M.; Palermo, V.; Zanardi, C. Continuous Capillary-Flow Sensing of Glucose and Lactate in Sweat with an Electrochemical Sensor Based on Functionalized Graphene Oxide. Sens. Actuators B 2021, 344, 130253. [Google Scholar] [CrossRef]
- Elshafey, R.; Abo-Sobehy, G.F.; Radi, A.E. Graphene Oxide/Graphene Quantum Dots: A Platform for Probing Ds-DNA-Dimethoate Interaction and Dimethoate Sensing. J. Electroanal. Chem. 2021, 899, 115678. [Google Scholar] [CrossRef]
- Saleem, S.J.; Guler, M. Electroanalytical Determination of Paracetamol Using Pd Nanoparticles Deposited on Carboxylated Graphene Oxide Modified Glassy Carbon Electrode. Electroanalysis 2019, 31, 2187–2198. [Google Scholar] [CrossRef]
- Erden, P.E.; Erdoğan, Z.Ö.; Öztürk, F.; Koçoğlu, İ.O.; Kılıç, E. Amperometric Biosensors for Tyramine Determination Based on Graphene Oxide and Polyvinylferrocene Modified Screen-Printed Electrodes. Electroanalysis 2019, 31, 2368–2378. [Google Scholar] [CrossRef]
- Gupta, A.; Bhardwaj, S.K.; Sharma, A.L.; Deep, A. A Graphene Electrode Functionalized with Aminoterephthalic Acid for Impedimetric Immunosensing of Escherichia coli. Microchim. Acta 2019, 186, 800. [Google Scholar] [CrossRef]
- Peng, H.; Hui, Y.; Ren, R.; Wang, B.; Song, S.; He, Y.; Zhang, F. A Sensitive Electrochemical Aptasensor Based on MB-Anchored GO for the Rapid Detection of Cronobacter Sakazakii. J. Solid State Electrochem. 2019, 23, 3391–3398. [Google Scholar] [CrossRef]
- Povedano, E.; Cincotto, F.H.; Parrado, C.; Díez, P.; Sánchez, A.; Canevari, T.C.; Machado, S.A.S.; Pingarrón, J.M.; Villalonga, R. Decoration of Reduced Graphene Oxide with Rhodium Nanoparticles for the Design of a Sensitive Electrochemical Enzyme Biosensor for 17β-Estradiol. Biosens. Bioelectron. 2017, 89, 343–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebrahimi, N.; Raoof, J.B.; Ojani, R.; Ebrahimi, M. Designing a Novel DNA-Based Electrochemical Biosensor to Determine of Ba2+ Ions Both Selectively and Sensitively. Anal. Biochem. 2022, 642, 114563. [Google Scholar] [CrossRef] [PubMed]
- Halder, A.; Zhang, M.; Chi, Q. Electroactive and Biocompatible Functionalization of Graphene for the Development of Biosensing Platforms. Biosens. Bioelectron. 2017, 87, 764–771. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Liu, M.; Li, H.; Zhang, Y.; Yao, S. Synergetic Signal Amplification Based on Electrochemical Reduced Graphene Oxide-Ferrocene Derivative Hybrid and Gold Nanoparticles as an Ultra-Sensitive Detection Platform for Bisphenol A. Anal. Chim. Acta 2015, 853, 249–257. [Google Scholar] [CrossRef]
- Yang, M.H.; Kim, D.S.; Lee, T.J.; Lee, S.J.; Lee, K.G.; Choi, B.G. Polyoxometalate-Grafted Graphene Nanohybrid for Electrochemical Detection of Hydrogen Peroxide and Glucose. J. Colloid Interface Sci. 2016, 468, 51–56. [Google Scholar] [CrossRef]
- Ferrier, D.C.; Honeychurch, K.C. Carbon Nanotube (CNT)-Based Biosensors. Biosensors 2021, 11, 486. [Google Scholar] [CrossRef]
- Mujica, M.L.; Tamborelli, A.; Vaschetti, V.M.; Espinoza, L.C.; Bollo, S.; Dalmasso, P.R.; Rivas, G.A. Two Birds with One Stone: Integrating Exfoliation and Immunoaffinity Properties in Multi-Walled Carbon Nanotubes by Non-Covalent Functionalization with Human Immunoglobulin G. Microchim. Acta 2023, 190, 73. [Google Scholar] [CrossRef]
- González-Hernández, J.; Moya-Alvarado, G.; Alvarado-Gámez, A.L.; Urcuyo, R.; Barquero-Quirós, M.; Arcos-Martínez, M.J. Electrochemical Biosensor for Quantitative Determination of Fentanyl Based on Immobilized Cytochrome c on Multi-Walled Carbon Nanotubes Modified Screen-Printed Carbon Electrodes. Microchim. Acta 2022, 189, 483. [Google Scholar] [CrossRef]
- Singh, A.K.; Jaiswal, N.; Tiwari, I.; Ahmad, M.; Silva, S.R.P. Electrochemical Biosensors Based on in Situ Grown Carbon Nanotubes on Gold Microelectrode Array Fabricated on Glass Substrate for Glucose Determination. Microchim. Acta 2023, 190, 55. [Google Scholar] [CrossRef]
- Alvarez-Paguay, J.; Fernández, L.; Bolaños-Méndez, D.; González, G.; Espinoza-Montero, P.J. Evaluation of an Electrochemical Biosensor Based on Carbon Nanotubes, Hydroxyapatite and Horseradish Peroxidase for the Detection of Hydrogen Peroxide. Sens. Bio-Sens. Res. 2022, 37, 100514. [Google Scholar] [CrossRef]
- Bravo, I.; Prata, M.; Torrinha, Á.; Delerue-Matos, C.; Lorenzo, E.; Morais, S. Laccase Bioconjugate and Multi-Walled Carbon Nanotubes-Based Biosensor for Bisphenol A Analysis. Bioelectrochemistry 2022, 144, 108033. [Google Scholar] [CrossRef]
- Yang, M.; Wang, H.; Liu, P.; Cheng, J. A 3D Electrochemical Biosensor Based on Super-Aligned Carbon NanoTube Array for Point-of-Care Uric Acid Monitoring. Biosens. Bioelectron. 2021, 179, 113082. [Google Scholar] [CrossRef]
- Anshori, I.; Nuraviana Rizalputri, L.; Rona Althof, R.; Sean Surjadi, S.; Harimurti, S.; Gumilar, G.; Yuliarto, B.; Handayani, M. Functionalized Multi-Walled Carbon Nanotube/Silver Nanoparticle (f-MWCNT/AgNP) Nanocomposites as Non-Enzymatic Electrochemical Biosensors for Dopamine Detection. Nanocomposites 2021, 7, 97–108. [Google Scholar] [CrossRef]
- Pal, C.; Majumder, S. Label-Free Electrochemical Biosensor for Ultra-Low Level Detection of Ag(I) Using SsDNA Functionalized Multi-Walled Carbon Nanotube. Colloids Surf. A 2022, 645, 128927. [Google Scholar] [CrossRef]
- Lu, C.; Zhou, S.; Gao, F.; Lin, J.; Liu, J.; Zheng, J. DNA-Mediated Growth of Noble Metal Nanomaterials for Biosensing Applications. TrAC Trends Anal. Chem. 2022, 148, 116533. [Google Scholar] [CrossRef]
- Liu, P.; Qin, R.; Fu, G.; Zheng, N. Surface Coordination Chemistry of Metal Nanomaterials. J. Am. Chem. Soc. 2017, 139, 2122–2131. [Google Scholar] [CrossRef]
- Thao, D.V.P.; Thuy Ngan, D.T.; Tuan, D.V.; Lan, H.; Thi Nguyet, N.; Thu, V.V.; Hung, V.P.; Dinh Tam, P. Facile Preparation of Copper Nanoparticles in Environmentally Friendly Solvent for DNA Sensor Application. Mater. Today Commun. 2022, 33, 104161. [Google Scholar] [CrossRef]
- Castro, A.C.H.; Bezerra, Í.R.S.; Pascon, A.M.; da Silva, G.H.; Philot, E.A.; de Oliveira, V.L.; Mancini, R.S.N.; Schleder, G.R.; Castro, C.E.; de Carvalho, L.R.S.; et al. Modular Label-Free Electrochemical Biosensor Loading Nature-Inspired Peptide toward the Widespread Use of COVID-19 Antibody Tests. ACS Nano 2022, 16, 14239–14253. [Google Scholar] [CrossRef]
- Medrades, J.d.P.; Maciel, C.C.; Moraes, A.d.S.; Leite, F.d.L.; Ferreira, M. Flavin Adenine Dinucleotide Functionalized Gold Nanoparticles for the Electrochemical Detection of Dopamine. Sens. Actuators Rep. 2022, 4, 100085. [Google Scholar] [CrossRef]
- Freeman, M.H.; Hall, J.R.; Leopold, M.C. Monolayer-Protected Nanoparticle Doped Xerogels as Functional Components of Amperometric Glucose Biosensors. Anal. Chem. 2013, 85, 4057–4065. [Google Scholar] [CrossRef]
- Sanko, V.; Şenocak, A.; Tümay, S.O.; Demirbas, E. A Novel Comparative Study for Electrochemical Urea Biosensor Design: Effect of Different Ferrite Nanoparticles (MFe2O4, M: Cu, Co, Ni, Zn) in Urease Immobilized Composite System. Bioelectrochemistry 2023, 149, 108324. [Google Scholar] [CrossRef] [PubMed]
- Rawat, C.S.; Bagaria, A. Fabrication and Characterization of an Electrochemical Glucose Biosensor Using Zinc Oxide Nanoparticles and Soy Protein Isolate Film Support Matrix. MRS Commun. 2022, 12, 930–936. [Google Scholar] [CrossRef]
- Yin, Z.; Ji, Z.; Bloom, B.P.; Jayapalan, A.; Liu, M.; Zeng, X.; Waldeck, D.H.; Wei, J. Manipulating Cobalt Oxide on N-Doped Aligned Electrospun Carbon Nanofibers towards Instant Electrochemical Detection of Dopamine Secreted by Living Cells. Appl. Surf. Sci. 2022, 577, 151912. [Google Scholar] [CrossRef]
- Hilal, M.; Xie, W.; Yang, W. Straw-Sheaf–like Co3O4 for Preparation of an Electrochemical Non-Enzymatic Glucose Sensor. Microchim. Acta 2022, 189, 364. [Google Scholar] [CrossRef] [PubMed]
- Centane, S.; Mgidlana, S.; Openda, Y.; Nyokong, T. Electrochemical Detection of Human Epidermal Growth Factor Receptor 2 Using an Aptamer on Cobalt Phthalocyanines—Cerium Oxide Nanoparticle Conjugate. Bioelectrochemistry 2022, 146, 108146. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Chen, J.; Lu, X.; Yang, Y.; Song, D.; Gao, F. Porous Hierarchical Peony-like Cobalt-Based Bimetallic Oxides Structured by Ultrathin Nanosheets for Highly Sensitive Electrochemical Pesticides Detection. Sens. Actuators B 2022, 352, 131072. [Google Scholar] [CrossRef]
- Tri Murti, B.; Huang, Y.J.; Darumas Putri, A.; Lee, C.P.; Hsieh, C.M.; Wei, S.M.; Tsai, M.L.; Peng, C.W.; Yang, P.K. Free-Standing Vertically Aligned Tin Disulfide Nanosheets for Ultrasensitive Aptasensor Design toward Alzheimer’s Diagnosis Applications. Chem. Eng. J. 2023, 452, 139394. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Tang, X.; Xu, L.; Min, L.; Xue, Y.; Hu, X.; Yang, Z. Multiwalled Carbon Nanotubes Coated with Cobalt(II) Sulfide Nanoparticles for Electrochemical Sensing of Glucose via Direct Electron Transfer to Glucose Oxidase. Microchim. Acta 2020, 187, 80. [Google Scholar] [CrossRef]
- Rohaizad, N.; Mayorga-Martinez, C.C.; Sofer, Z.; Webster, R.D.; Pumera, M. Niobium-Doped TiS2: Formation of TiS3 Nanobelts and Their Effects in Enzymatic Biosensors. Biosens. Bioelectron. 2020, 155, 112114. [Google Scholar] [CrossRef]
- Yan, R.; Lu, N.; Han, S.; Lu, Z.; Xiao, Y.; Zhao, Z.; Zhang, M. Simultaneous Detection of Dual Biomarkers Using Hierarchical MoS2 Nanostructuring and Nano-Signal Amplification-Based Electrochemical Aptasensor toward Accurate Diagnosis of Prostate Cancer. Biosens. Bioelectron. 2022, 197, 113797. [Google Scholar] [CrossRef]
- Li, W.; Zhao, Z.; Yang, W.; Su, Q.; Na, C.; Zhang, X.; Zhao, R.; Song, H. Immobilization of Bovine Hemoglobin on Au Nanoparticles/MoS2 Nanosheets—Chitosan Modified Screen-Printed Electrode as Chlorpyrifos Biosensor. Enzyme Microb. Technol. 2022, 154, 109959. [Google Scholar] [CrossRef]
- Dinh Van, T.; Dang Thi Thuy, N.; Dao Vu Phuong, T.; Nguyen Thi, N.; Nguyen Thi, T.; Nguyen Phuong, T.; Vu Van, T.; Vuong-Pham, H.; Phuong Dinh, T. High-Performance Nonenzymatic Electrochemical Glucose Biosensor Based on AgNP-Decorated MoS2 Microflowers. Curr. Appl. Phys. 2022, 43, 116–123. [Google Scholar] [CrossRef]
- Jeong, J.M.; Yang, M.H.; Kim, D.S.; Lee, T.J.; Choi, B.G.; Kim, D.H. High Performance Electrochemical Glucose Sensor Based on Three-Dimensional MoS2/Graphene Aerogel. J. Colloid Interface Sci. 2017, 506, 379–385. [Google Scholar] [CrossRef]
- Yoon, J.; Shin, M.; Lim, J.; Lee, J.Y.; Choi, J.W. Recent Advances in MXene Nanocomposite-Based Biosensors. Biosensors 2020, 10, 185. [Google Scholar] [CrossRef]
- Wu, X.; Ma, P.; Sun, Y.; Du, F.; Song, D.; Xu, G. Application of MXene in Electrochemical Sensors: A Review. Electroanalysis 2021, 33, 1827–1851. [Google Scholar] [CrossRef]
- Mathew, M.; Rout, C.S. Electrochemical Biosensors based on Ti3C2Tx MXene: Future perspectives for on-site analysis. Curr. Opin. Electrochem. 2021, 30, 100782. [Google Scholar] [CrossRef]
- Wang, F.; Yang, C.; Duan, C.; Xiao, D.; Tang, Y.; Zhu, J. An Organ-Like Titanium Carbide Material (MXene) with Multilayer Structure Encapsulating Hemoglobin for a Mediator-Free Biosensor. J. Electrochem. Soc. 2015, 162, B16–B21. [Google Scholar] [CrossRef]
- Wu, L.; Lu, X.; Dhanjai; Wu, Z.S.; Dong, Y.; Wang, X.; Zheng, S.; Chen, J. 2D Transition Metal Carbide MXene as a Robust Biosensing Platform for Enzyme Immobilization and Ultrasensitive Detection of Phenol. Biosens. Bioelectron. 2018, 107, 69–75. [Google Scholar] [CrossRef]
- Wang, H.; Li, H.; Huang, Y.; Xiong, M.; Wang, F.; Li, C. A Label-Free Electrochemical Biosensor for Highly Sensitive Detection of Gliotoxin Based on DNA Nanostructure/MXene Nanocomplexes. Biosens. Bioelectron. 2019, 142, 111531. [Google Scholar] [CrossRef]
- Zhao, F.; Yao, Y.; Jiang, C.; Shao, Y.; Barceló, D.; Ying, Y.; Ping, J. Self-Reduction Bimetallic Nanoparticles on Ultrathin MXene Nanosheets as Functional Platform for Pesticide Sensing. J. Hazard. Mater. 2020, 384, 121358. [Google Scholar] [CrossRef]
- Zhao, J.; He, C.; Wu, W.; Yang, H.; Dong, J.; Wen, L.; Hu, Z.; Yang, M.; Hou, C.; Huo, D. MXene-MoS2 Heterostructure Collaborated with Catalyzed Hairpin Assembly for Label-Free Electrochemical Detection of MicroRNA-21. Talanta 2022, 237, 122927. [Google Scholar] [CrossRef]
- Song, D.; Jiang, X.; Li, Y.; Lu, X.; Luan, S.; Wang, Y.; Li, Y.; Gao, F. Metal−Organic Frameworks-Derived MnO2/Mn3O4 Microcuboids with Hierarchically Ordered Nanosheets and Ti3C2 MXene/Au NPs Composites for Electrochemical Pesticide Detection. J. Hazard. Mater. 2019, 373, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Murugan, P.; Annamalai, J.; Atchudan, R.; Govindasamy, M.; Nallaswamy, D.; Ganapathy, D.; Reshetilov, A.; Sundramoorthy, A.K. Electrochemical Sensing of Glucose Using Glucose Oxidase/PEDOT:4-Sulfocalix [4]Arene/MXene Composite Modified Electrode. Micromachines 2022, 13, 304. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Xing, Y.; Xiong, P.; Tang, H.; Li, C.; Chen, S.; Zeng, R.; Han, K.; Shi, G. Three-Dimensional Porous Ti3C2Tx MXene–Graphene Hybrid Films for Glucose Biosensing. ACS Appl. Nano Mater. 2019, 2, 6537–6545. [Google Scholar] [CrossRef]
- Farzin, M.A.; Abdoos, H. A Critical Review on Quantum Dots: From Synthesis toward Applications in Electrochemical Biosensors for Determination of Disease-Related Biomolecules. Talanta 2021, 224, 121828. [Google Scholar] [CrossRef]
- Theyagarajan, K.; Aayushi, P.S.; Devadoss, A.; Thenmozhi, K.; Senthilkumar, S. Chapter 11. Quantum Dots-Based Disposable Sensors. In Disposable Electrochemical Sensors for Healthcare Monitoring: Material Properties and Design; Pandikumar, A., Devi, K.S.S., Eds.; Royal Society of Chemistry: London, UK, 2021; pp. 314–352. [Google Scholar]
- Taghavi, F.; Moeinpour, F.; Khojastehnezhad, A.; Abnous, K.; Taghdisi, S.M. Recent Applications of Quantum Dots in Optical and Electrochemical Aptasensing Detection of Lysozyme. Anal. Biochem. 2021, 630, 114334. [Google Scholar] [CrossRef]
- Baluta, S.; Lesiak, A.; Cabaj, J. Graphene Quantum Dots-Based Electrochemical Biosensor for Catecholamine Neurotransmitters Detection. Electroanalysis 2018, 30, 1781–1790. [Google Scholar] [CrossRef]
- Wu, S.; Jiang, M.; Mao, H.; Zhao, N.; He, D.; Chen, Q.; Liu, D.; Zhang, W.; Song, X.-M. A Sensitive Cholesterol Electrochemical Biosensor Based on Biomimetic Cerasome and Graphene Quantum Dots. Anal. Bioanal. Chem. 2022, 414, 3593–3603. [Google Scholar] [CrossRef]
- Ye, W.; Guo, J.; Bao, X.; Chen, T.; Weng, W.; Chen, S.; Yang, M. Rapid and Sensitive Detection of Bacteria Response to Antibiotics Using Nanoporous Membrane and Graphene Quantum Dot (GQDs)-Based Electrochemical Biosensors. Materials 2017, 10, 603. [Google Scholar] [CrossRef] [Green Version]
- Ganganboina, A.B.; Dega, N.K.; Tran, H.L.; Darmonto, W.; Doong, R.A. Application of Sulfur-Doped Graphene Quantum Dots@gold-Carbon Nanosphere for Electrical Pulse-Induced Impedimetric Detection of Glioma Cells. Biosens. Bioelectron. 2021, 181, 113151. [Google Scholar] [CrossRef]
- Nashruddin, S.N.A.; Abdullah, J.; Mohammad Haniff, M.A.S.; Mat Zaid, M.H.; Choon, O.P.; Mohd Razip Wee, M.F. Label Free Glucose Electrochemical Biosensor Based on Poly(3,4-Ethylenedioxy Thiophene):Polystyrene Sulfonate/Titanium Carbide/Graphene Quantum Dots. Biosensors 2021, 11, 267. [Google Scholar] [CrossRef]
- Akin, M.; Bekmezci, M.; Bayat, R.; Coguplugil, Z.K.; Sen, F.; Karimi, F.; Karimi-Maleh, H. Mobile Device Integrated Graphene Oxide Quantum Dots Based Electrochemical Biosensor Design for Detection of MiR-141 as a Pancreatic Cancer Biomarker. Electrochim. Acta 2022, 435, 141390. [Google Scholar] [CrossRef]
- Fatima, B.; Hussain, D.; Bashir, S.; Hussain, H.T.; Aslam, R.; Nawaz, R.; Rashid, H.N.; Bashir, N.; Majeed, S.; Ashiq, M.N.; et al. Catalase Immobilized Antimonene Quantum Dots Used as an Electrochemical Biosensor for Quantitative Determination of H2O2 from CA-125 Diagnosed Ovarian Cancer Samples. Mater. Sci. Eng. C 2020, 117, 111296. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, J.; Huang, Q.; Tao, Y.; Gu, R.; Li, H.-Y.; Liu, H. Electrochemical Biosensor Employing PbS Colloidal Quantum Dots/Au Nanospheres-Modified Electrode for Ultrasensitive Glucose Detection. Nano Res. 2022. [Google Scholar] [CrossRef]
- Safitri, E.; Heng, L.Y.; Ahmad, M.; Tan, L.L.; Nazaruddin, N.; Suhud, K.; Chiang, C.P.; Iqhrammullah, M. Electrochemical DNA Biosensor Based on Mercaptopropionic Acid-Capped ZnS Quantum Dots for Determination of the Gender of Arowana Fish. Biosensors 2022, 12, 650. [Google Scholar] [CrossRef]
- Moazampour, M.; Zare, H.R.; Shekari, Z. Femtomolar Determination of an Ovarian Cancer Biomarker (MiR-200a) in Blood Plasma Using a Label Free Electrochemical Biosensor Based on L-Cysteine Functionalized ZnS Quantum Dots. Anal. Methods 2021, 13, 2021–2029. [Google Scholar] [CrossRef]
- Richard, Y.A.; Lincy, S.A.; Piraman, S.; Dharuman, V. Label-Free Electrochemical Detection of Cancer Biomarkers DNA and Anti-P53 at Tin Oxide Quantum Dot-Gold-DNA Nanoparticle Modified Electrode. Bioelectrochemistry 2023, 150, 108371. [Google Scholar] [CrossRef]
- Mokwebo, K.V.; Oluwafemi, O.S.; Arotiba, O.A. An Electrochemical Cholesterol Biosensor Based on a CdTe/CdSe/Znse Quantum Dots—Poly (Propylene Imine) Dendrimer Nanocomposite Immobilisation Layer. Sensors 2018, 18, 3368. [Google Scholar] [CrossRef] [Green Version]
- Majumdar, S.; Moosavi, S.M.; Jablonka, K.M.; Ongari, D.; Smit, B. Diversifying Databases of Metal Organic Frameworks for High-Throughput Computational Screening. ACS Appl. Mater. Interfaces 2021, 13, 61004–61014. [Google Scholar] [CrossRef]
- Yan, L.; Gopal, A.; Kashif, S.; Hazelton, P.; Lan, M.; Zhang, W.; Chen, X. Metal Organic Frameworks for Antibacterial Applications. Chem. Eng. J. 2022, 435, 134975. [Google Scholar] [CrossRef]
- Mehtab, T.; Yasin, G.; Arif, M.; Shakeel, M.; Korai, R.M.; Nadeem, M.; Muhammad, N.; Lu, X. Metal-Organic Frameworks for Energy Storage Devices: Batteries and Supercapacitors. J. Energy Storage 2019, 21, 632–646. [Google Scholar] [CrossRef]
- Luo, G. Electrochemical Myoglobin Biosensor Based on Magnesium Metal-Organic Frameworks and Gold Nanoparticles Composite Modified Electrode. Int. J. Electrochem. Sci. 2019, 14, 2405–2413. [Google Scholar] [CrossRef]
- Li, S.; Hu, C.; Chen, C.; Zhang, J.; Bai, Y.; Tan, C.S.; Ni, G.; He, F.; Li, W.; Ming, D. Molybdenum Disulfide Supported on Metal-Organic Frameworks as an Ultrasensitive Layer for the Electrochemical Detection of the Ovarian Cancer Biomarker CA125. ACS Appl. Bio Mater. 2021, 4, 5494–5502. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Wang, Y.; Feng, S.; Yan, M.; Huang, J.; Yang, X. A Dual-Amplification Mode and Cu-Based Metal-Organic Frameworks Mediated Electrochemical Biosensor for Sensitive Detection of MicroRNA. Biosens. Bioelectron. 2022, 202, 113992. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Hu, Y.; Wang, P.; Liu, P.; Chen, Z.; Sun, D. Electrochemical Biosensor Based on Gold Nanoflowers-Encapsulated Magnetic Metal-Organic Framework Nanozymes for Drug Evaluation with in-Situ Monitoring of H2O2 Released from H9C2 Cardiac Cells. Sens. Actuators B 2020, 311, 127909. [Google Scholar] [CrossRef]
- Du, L.; Chen, W.; Wang, J.; Cai, W.; Kong, S.; Wu, C. Folic Acid-Functionalized Zirconium Metal-Organic Frameworks Based Electrochemical Impedance Biosensor for the Cancer Cell Detection. Sens. Actuators B 2019, 301, 127073. [Google Scholar] [CrossRef]
- Chang, J.; Wang, X.; Wang, J.; Li, H.; Li, F. Nucleic Acid-Functionalized Metal-Organic Framework-Based Homogeneous Electrochemical Biosensor for Simultaneous Detection of Multiple Tumor Biomarkers. Anal. Chem. 2019, 91, 3604–3610. [Google Scholar] [CrossRef]
- Wang, L.; Meng, T.; Liang, L.; Sun, J.; Wu, S.; Wang, H.; Yang, X.; Zhang, Y. Fabrication of Amine-Functionalized Metal-Organic Frameworks with Embedded Palladium Nanoparticles for Highly Sensitive Electrochemical Detection of Telomerase Activity. Sens. Actuators B 2019, 278, 133–139. [Google Scholar] [CrossRef]
- Dong, P.; Zhu, L.; Huang, J.; Ren, J.; Lei, J. Electrocatalysis of Cerium Metal-Organic Frameworks for Ratiometric Electrochemical Detection of Telomerase Activity. Biosens. Bioelectron. 2019, 138, 111313. [Google Scholar] [CrossRef]
- Huang, S.; Lu, M.; Wang, L. Cytochrome C-Multiwalled Carbon Nanotube and Cobalt Metal Organic Framework/Gold Nanoparticle Immobilized Electrochemical Biosensor for Nitrite Detection. RSC Adv. 2020, 11, 501–509. [Google Scholar] [CrossRef]
- Gupta, A.; Bhardwaj, S.K.; Sharma, A.L.; Kim, K.H.; Deep, A. Development of an Advanced Electrochemical Biosensing Platform for E. coli Using Hybrid Metal-Organic Framework/Polyaniline Composite. Environ. Res. 2019, 171, 395–402. [Google Scholar] [CrossRef]
- Jia, Q.; Lou, Y.; Rong, F.; Zhang, S.; Wang, M.; He, L.; Zhang, Z.; Du, M. Silver Nanoparticle Embedded Polymer–Zirconium-Based Metal–Organic Framework (PolyUiO-66) for Electrochemical Biosensors of Respiratory Viruses. J. Mater. Chem. C 2021, 9, 14190–14200. [Google Scholar] [CrossRef]
- Martínez-Periñán, E.; Martínez-Fernández, M.; Segura, J.L.; Lorenzo, E. Electrochemical (Bio)Sensors Based on Covalent Organic Frameworks (COFs). Sensors 2022, 22, 4758. [Google Scholar] [CrossRef]
- Lohse, M.S.; Bein, T. Covalent Organic Frameworks: Structures, Synthesis, and Applications. Adv. Funct. Mater. 2018, 28, 1705553. [Google Scholar] [CrossRef] [Green Version]
- Geng, K.; He, T.; Liu, R.; Dalapati, S.; Tan, K.T.; Li, Z.; Tao, S.; Gong, Y.; Jiang, Q.; Jiang, D. Covalent Organic Frameworks: Design, Synthesis, and Functions. Chem. Rev. 2020, 120, 8814–8933. [Google Scholar] [CrossRef]
- Zhao, F.; Liu, H.; Mathe, S.D.R.; Dong, A.; Zhang, J. Covalent Organic Frameworks: From Materials Design to Biomedical Application. Nanomaterials 2018, 8, 15. [Google Scholar] [CrossRef] [Green Version]
- Yildirim, O.; Derkus, B. Triazine-Based 2D Covalent Organic Frameworks Improve the Electrochemical Performance of Enzymatic Biosensors. J. Mater. Sci. 2020, 55, 3034–3044. [Google Scholar] [CrossRef]
- Xiao, Y.; Wu, N.; Wang, L.; Chen, L. A Novel Paper-Based Electrochemical Biosensor Based on N,O-Rich Covalent Organic Frameworks for Carbaryl Detection. Biosensors 2022, 12, 899. [Google Scholar] [CrossRef]
- Sun, X.; Xie, Y.; Chu, H.; Long, M.; Zhang, M.; Wang, Y.; Hu, X. A Highly Sensitive Electrochemical Biosensor for the Detection of Hydroquinone Based on a Magnetic Covalent Organic Framework and Enzyme for Signal Amplification. New J. Chem. 2022, 46, 11902–11909. [Google Scholar] [CrossRef]
- Li, H.; Kou, B.; Yuan, Y.; Chai, Y.; Yuan, R. Porous Fe3O4@COF-Immobilized Gold Nanoparticles with Excellent Catalytic Performance for Sensitive Electrochemical Detection of ATP. Biosens. Bioelectron. 2022, 197, 113758. [Google Scholar] [CrossRef]
- Han, Y.; Lu, J.; Wang, M.; Sun, C.; Yang, J.; Li, G. An Electrochemical Biosensor for Exosome Detection Based on Covalent Organic Frameworks Conjugated with DNA and Horseradish Peroxidase. J. Electroanal. Chem. 2022, 920, 116576. [Google Scholar] [CrossRef]
- Zheng, J.; Zhao, H.; Ning, G.; Sun, W.; Wang, L.; Liang, H.; Xu, H.; He, C.; Li, C.P. A Novel Affinity Peptide–Antibody Sandwich Electrochemical Biosensor for PSA Based on the Signal Amplification of MnO2-Functionalized Covalent Organic Framework. Talanta 2021, 233, 122520. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Wang, L.; Yang, Y.; Song, Y.; Wang, L. A Novel Biosensor Based on Multienzyme Microcapsules Constructed from Covalent-Organic Framework. Biosens. Bioelectron. 2021, 193, 113553. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, P.; Yadav, S.; Sadique, M.A.; Khan, R.; Chaurasia, J.P.; Kumar Srivastava, A. Functional Ionic Liquids Decorated Carbon Hybrid Nanomaterials for the Electrochemical Biosensors. Biosensors 2021, 11, 414. [Google Scholar] [CrossRef]
- Cetinkaya, A.; Kaya, S.I.; Yence, M.; Budak, F.; Ozkan, S.A. Ionic Liquid-Based Materials for Electrochemical Sensor Applications in Environmental Samples. Trends Environ. Anal. Chem. 2023, 37, e00188. [Google Scholar] [CrossRef]
- Upasham, S.; Banga, I.K.; Jagannath, B.; Paul, A.; Lin, K.-C.; Muthukumar, S.; Prasad, S. Electrochemical Impedimetric Biosensors, Featuring the Use of Room Temperature Ionic Liquids (RTILs): Special Focus on Non-Faradaic Sensing. Biosens. Bioelectron. 2021, 177, 112940. [Google Scholar] [CrossRef]
- Kumar, A.; Bisht, M.; Venkatesu, P. Biocompatibility of Ionic Liquids towards Protein Stability: A Comprehensive Overview on the Current Understanding and Their Implications. Int. J. Biol. Macromol. 2017, 96, 611–651. [Google Scholar] [CrossRef]
- Tiago, G.A.O.; Matias, I.A.S.; Ribeiro, A.P.C.; Martins, L.M.D.R.S. Application of Ionic Liquids in Electrochemistry—Recent Advances. Molecules 2020, 25, 5812. [Google Scholar] [CrossRef]
- Wang, X.; Hao, J. Recent Advances in Ionic Liquid-Based Electrochemical Biosensors. Sci. Bull. 2016, 61, 1281–1295. [Google Scholar] [CrossRef] [Green Version]
- Theyagarajan, K.; Saravanakumar, D.; Senthilkumar, S.; Thenmozhi, K. Rationally Designed Naphthyl Substituted Amine Functionalized Ionic Liquid Platform for Covalent Immobilization and Direct Electrochemistry of Hemoglobin. Sci. Rep. 2019, 9, 10428. [Google Scholar] [CrossRef] [Green Version]
- Manoj, D.; Theyagarajan, K.; Saravanakumar, D.; Senthilkumar, S.; Thenmozhi, K. Aldehyde Functionalized Ionic Liquid on Electrochemically Reduced Graphene Oxide as a Versatile Platform for Covalent Immobilization of Biomolecules and Biosensing. Biosens. Bioelectron. 2018, 103, 104–112. [Google Scholar] [CrossRef]
- Theyagarajan, K.; Yadav, S.; Satija, J.; Thenmozhi, K.; Senthilkumar, S. Gold Nanoparticle-Redox Ionic Liquid Based Nanoconjugated Matrix as a Novel Multifunctional Biosensing Interface. ACS Biomater. Sci. Eng. 2020, 6, 6076–6085. [Google Scholar] [CrossRef]
- Zappi, D.; Masci, G.; Sadun, C.; Tortolini, C.; Antonelli, M.L.; Bollella, P. Evaluation of New Cholinium-Amino Acids Based Room Temperature Ionic Liquids (RTILs) as Immobilization Matrix for Electrochemical Biosensor Development: Proof-of-Concept with Trametes Versicolor Laccase. Microchem. J. 2018, 141, 346–352. [Google Scholar] [CrossRef]
- Yao, P.; Li, S.; Lambert, A.; Cheng, Q.; Yang, Z.; Zhu, X. Amino Acid-Based Imidazole Ionic Liquid: A Novel Soft Matrix for Electrochemical Biosensing Applications. ACS Sustain. Chem. Eng. 2021, 9, 4157–4166. [Google Scholar] [CrossRef]
- Xia, J.; Cao, X.; Wang, Z.; Yang, M.; Zhang, F.; Lu, B.; Li, F.; Xia, L.; Li, Y.; Xia, Y. Molecularly Imprinted Electrochemical Biosensor Based on Chitosan/Ionic Liquid-Graphene Composites Modified Electrode for Determination of Bovine Serum Albumin. Sens. Actuators B 2016, 225, 305–311. [Google Scholar] [CrossRef]
- Niamsi, W.; Larpant, N.; Kalambate, P.K.; Primpray, V.; Karuwan, C.; Rodthongkum, N.; Laiwattanapaisal, W. Paper-Based Screen-Printed Ionic-Liquid/Graphene Electrode Integrated with Prussian Blue/MXene Nanocomposites Enabled Electrochemical Detection for Glucose Sensing. Biosensors 2022, 12, 852. [Google Scholar] [CrossRef]
- Albishri, H.M.; Abd El-Hady, D. Hyphenation of Enzyme/Graphene Oxide-Ionic Liquid/Glassy Carbon Biosensors with Anodic Differential Pulse Stripping Voltammetry for Reliable Determination of Choline and Acetylcholine in Human Serum. Talanta 2019, 200, 107–114. [Google Scholar] [CrossRef]
- Işın, D.; Eksin, E.; Erdem, A. Graphene-Oxide and Ionic Liquid Modified Electrodes for Electrochemical Sensing of Breast Cancer 1 Gene. Biosensors 2022, 12, 95. [Google Scholar] [CrossRef]
- Fan, Y.; Hu, G.; Zhang, T.; Dong, X.; Zhong, Y.; Li, X.; Miao, J.; Hua, S. Determination of Glucose in Food by the Ionic Liquid and Carbon Nanotubes Modified Dual-Enzymatic Sensors. Food Anal. Methods 2016, 9, 2491–2500. [Google Scholar] [CrossRef]
- Mahmoudi-Moghaddam, H.; Tajik, S.; Beitollahi, H. A New Electrochemical DNA Biosensor Based on Modified Carbon Paste Electrode Using Graphene Quantum Dots and Ionic Liquid for Determination of Topotecan. Microchem. J. 2019, 150, 104085. [Google Scholar] [CrossRef]
- Zhang, P.F.; Wu, Z.Y.; Zhang, W.B.; He, Y.Q.; Chen, K.; Wang, T.M.; Li, H.; Zheng, H.; Li, D.H.; Yang, D.W.; et al. Establishment and Validation of a Plasma Oncofetal Chondroitin Sulfated Proteoglycan for Pan-Cancer Detection. Nat. Commun. 2023, 14, 645. [Google Scholar] [CrossRef] [PubMed]
- López-Cuenca, I.; Nebreda, A.; García-Colomo, A.; Salobrar-García, E.; de Frutos-Lucas, J.; Bruña, R.; Ramírez, A.I.; Ramirez-Toraño, F.; Salazar, J.J.; Barabash, A.; et al. Early Visual Alterations in Individuals At-Risk of Alzheimer’s Disease: A Multidisciplinary Approach. Alzheimers Res. Ther. 2023, 15, 19. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhao, D.; Han, X.; Liu, J.; Sun, Y.; Li, Y.; Duan, Y. Deposition of Hydrophilic Ti3C2Tx on a Superhydrophobic ZnO Nanorod Array for Improved Surface-Enhanced Raman Scattering Performance. J. Nanobiotechnol. 2023, 21, 17. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.-L.; Xu, Q.-Q.; Ru, J.; Li, H.; Wang, X.; Zhang, Y.-X. Co-CoO-Co3O4 Realizes Ultra Highly Sensitive Detection of 4-Nitrochlorobenzene Based on Synergistic Effect of Adsorption and Catalysis. Sens. Actuators B 2023, 381, 133426. [Google Scholar] [CrossRef]
- Al-Mhyawi, S.R.; Abdel-Hamied Abdel-Tawab, M.; el Nashar, R.M. A Novel Electrochemical Hybrid Platform for Sensitive Determination of the Aminoglycoside Antibiotic Kasugamycin Residues in Vegetables. Food Chem. 2023, 411, 135506. [Google Scholar] [CrossRef]
- Yin, F.; Mo, Y.; Liu, X.; Yang, H.; Zhou, D.; Cao, H.; Ye, T.; Xu, F. An Ultra-Sensitive and Selective Electrochemical Sensor Based on GOCS Composite and Ion Imprinted Polymer for the Rapid Detection of Cd2+ in Food Samples. Food Chem. 2023, 410, 135293. [Google Scholar] [CrossRef]
- Wang, L.; Ou, W.; Liu, H.; Wang, S.; Xia, Z.; Wang, X.; Yu, K. Electronic Structure Optimization of Titanium-Based Layered Oxide to Boost Flexible Sensing Performance. Appl. Surf. Sci. 2023, 618, 156702. [Google Scholar] [CrossRef]
- Didier, C.M.; Orrico, J.F.; Cepeda Torres, O.S.; Castro, J.M.; Baksh, A.; Rajaraman, S. Microfabricated polymer-metal biosensors for multifarious data collection from electrogenic cellular models. Microsyst. Nanoeng. 2023, 9, 22. [Google Scholar] [CrossRef]
- Ebrahimi, G.; Pakchin, P.S.; Mota, A.; Omidian, H.; Omidi, Y. Electrochemical microfluidic paper-based analytical devices for cancer biomarker detection: From 2D to 3D sensing systems. Talanta 2023, 257, 124370. [Google Scholar] [CrossRef]
- Macdonald, A.R.; Charlton, F.; Corrigan, D.K. Accelerating the development of implantable neurochemical biosensors by using existing clinically applied depth electrodes. Anal. Bioanal. Chem. 2023, 415, 1137–1147. [Google Scholar] [CrossRef]
- Ramasauskas, L.; Meskys, R.; Ratautas, D. Real-Time glucose monitoring system containing enzymatic sensor and enzymatic reference electrodes. Biosens. Bioelectron. 2020, 164, 112338. [Google Scholar] [CrossRef]
- Shi, Z.; Dai, C.; Deng, P.; Li, X.; Wu, Y.; Lv, J.; Xiong, C.; Shuai, Y.; Zhang, F.; Wang, D.; et al. Wearable battery-free smart bandage with peptide functionalized biosensors based on MXene for bacterial wound infection detection. Sens. Actuators B Chem. 2023, 383, 133598. [Google Scholar] [CrossRef]
- Shoute, L.C.T.; Abdelrasoul, G.N.; Ma, Y.; Duarte, P.A.; Edwards, C.; Zhuo, R.; Zeng, J.; Feng, Y.; Charlton, C.L.; Kanji, J.N.; et al. Label-free impedimetric immunosensor for point-of-care detection of COVID-19 antibodies. Microsyst. Nanoeng. 2023, 9, 3. [Google Scholar] [CrossRef]
- Nemiwal, M.; Zhang, T.C.; Kumar, D. Enzyme Immobilized Nanomaterials as Electrochemical Biosensors for Detection of Biomolecules. Enzyme Microb. Technol. 2022, 156, 110006. [Google Scholar] [CrossRef]
- Bisht, N.; Dwivedi, N.; Khosla, A.; Mondal, D.P.; Srivastava, A.K.; Dhand, C. Review—Recent Advances in Polydopamine-Based Electrochemical Biosensors. J. Electrochem. Soc. 2022, 169, 107505. [Google Scholar] [CrossRef]
- Chen, J.; Nilghaz, A.; Chen, X.; Liu, S.; Tian, J. Direct Electrochemical Detection of Glucose on PEDOT Functionalized Screen-Printed Electrodes. J. Electrochem. Soc. 2022, 169, 087502. [Google Scholar] [CrossRef]
- Shen, Y.; Qian, X.; Mi, X.; Tu, Y. An Ultrasensitive Electrochemiluminescent Sensing Platform for Oxygen Metabolism Based on Bioactive Magnetic Beads. Bioelectrochemistry 2022, 145, 108086. [Google Scholar] [CrossRef]








| Electrode | Analyte | Technique | Linear Range | LOD | Sensitivity | Ref. |
|---|---|---|---|---|---|---|
| GCE/fCB/DHDP/TYR | Catechol | CA | 1–39 µM | 87 nM | 539 mA/M | [42] |
| GCE/CB/CGO/Phage EP01 | E. coli | EIS | 102–107 CFU/mL | 11.8 CFU/mL | - | [43] |
| CB/Conductive C/GOD/Nf | Glucose | CA | 50 µM–2 mM | - | - | [44] |
| GCE/GR/GNPs/ChOx | H2O2 | CA | 10 µM–14 mM | 25 nM | 124.57 µA/µM/cm2 | [51] |
| GCE/GR/GNPs/ChEs | Cholesterol | CA | 25 µM–0.35 mM | 50 nM | 3.14 µA/µM/cm2 | |
| GCE/GR/GNPs/TYR/CS | BPA | DPV | 2.5 nM–3 µM | 1 nM | - | [52] |
| GCE/GR/Hemin/SWCNT | H2O2 | CA | 0.2 µM–0.4 mM | 50 nM | - | [53] |
| CILE/3D-GR-SnO2/Mb | TCA | CV | 5–94 mM | 0.35 mM | - | [54] |
| SPCE/PB/CS-GO/GOD/Nf | Glucose | FIA | 0.02–3.8 mM | 6.7 nM | 8.2 µA/mM/cm2 | [55] |
| SPCE/PB/CS-GO/Lox/Nf | Lactate | FIA | 1–50 mM | 28 nM | 0.39 µA/mM/cm2 | |
| GCE/GO/GRQDs/dsDNA | DMT | DPV | 1 fM–0.1 nM | 1 fM | [56] | |
| GCE/CGO/PdNPs/Nf | Paracetamol | CA | 40 nM–0.8 mM | 12 nM | 232.89 µA/mM/cm2 | [57] |
| SPCE/GO/PVFc/DAOx | Tyramine | CA | 0.99 µM–0.12 mM | 0.41 µM | 7.99 µA/mM | [58] |
| SPCE/GO/PVFc/MAOx | 0.99 µM–0.11 mM | 0.61 µM | 11.98 µA/mM | |||
| AuE/apt/MCH/MGO | CBSK | DPV | 20 to 2 × 106 CFU/mL | 7 CFU/mL | - | [60] |
| RGO-RhNPs/Lac | 17β-estradiol | DPV | 0.9–11 pM | 0.54 pM | 25.7 A/µM/cm2 | [61] |
| CPE/RGO/AgNPs/ssDNA | Ba2+ ions | DPV | 60 pM–0.80 nM & 1 nM–80 nM | 45 pM | - | [62] |
| GCE/PEI/RGO-Fc/ChOx | Cholesterol | CA | 2.5–25 µM | 0.5 µM | 380 mA/M/cm2 | [63] |
| GCE/PEI/RGO-Fc/GOD | Glucose | CA | 0.1–15.5 mM | 5 µM | 3.45 mA/M/cm2 | |
| GCE/GNPs/RGO-Fc | BPA | DPV | 5 nM–10 µM | 2 nM | - | [64] |
| p-IL-RGO-POM/GOD | Glucose | FIA | 2–20 mM | 67.9 µM | 14.3 µA/mM/cm2 | [65] |
| p-IL-RGO-POM | H2O2 | FIA | 0.1–20 mM | 10.2 µM | 95.5 µA/mM/cm2 | |
| SPCE/MWCNTs/Cyt-C | Fentanyl | ADSCV | 0.5–5 µg/mL | 86 ng/mL | - | [68] |
| AuE/CNT/p-PDA/GOD | Glucose | EIS | 0.2–27.5 µM | 0.2 µM | 168.03 k/Ω/M | [69] |
| GCE/HAp5-fCNT/HRP | H2O2 | CA | 10–347 µM | 1.91 µM | 63 µA/mM | [70] |
| GCE/HAp20-fCNT/HRP | 10–234 µM | 4.4 µM | 28 µA/mM | |||
| MWCNT/IL-CS/Lac | BPA | DPV | 500 nM–12 µM | 8.4 nM | 6.59 µA/µM | [71] |
| SACNTs/Urease | Uric acid | CA | 100 µM–1 mM | 1 µM | 518.8 µA/mM/cm2 | [72] |
| AuE/MWCNTs/ssDNA | Ag+ | DPV | 1 pM–10 nM | 1 pM | - | [74] |
| PtE/CuNPs/ssDNA | MTb | DPV | 1–10 nM | 1 nM | 42.5 µA/nM/cm2 | [77] |
| AuE/FAD-GNPs | DA | DPV | 0.8–8 µM | 525 nM | 1.25 µA/µM/cm2 | [79] |
| PtE/APGNC/GOD | Glucose | CA | Up to 14 mM | 23.2 µM | 0.184 µA/mM | [80] |
| GCE/PANI/CuF/Urease | Urea | DPV | 0.5–45 µM | 0.17 µM | - | [81] |
| GCE/PANI/CoF/Urease | 0.23 µM | |||||
| GCE/PANI/NiF/Urease | 0.37 µM | |||||
| GCE/PANI/ZnF/Urease | 0.42 µM | |||||
| GCE/ZnONPs/SPF/GOD | Glucose | CV | 0.1–0.3 mM | - | 52.04 µA/mM/cm2 | [82] |
| CONPs/N-CNF | DA | DPV | 10 nM–100 µM | 9 nM | - | [83] |
| GCE/CeONP/HB5 | HER2 | EIS | 1–10 ng/mL | 8 pg/mL | - | [85] |
| GCE/BMONS/ACLE | OME | DPV | 0.46 pM–0.46 µM | 0.033 pM | - | [86] |
| DPX | 3.88 pM–3.88 µM | 0.36 pM | - | |||
| GCE/MWCNT/CSNPs/GOD | Glucose | CA | 8 µM–1.5 mM | 5 µM | 15 mA/M/cm2 | [88] |
| Nb-TDS/GOD | Glucose | CA | 74.6–272.9 µM; 0.767–12.6 mM; 17.5–27.3 mM | 25.7 µM | 17.9 µA/mM/cm2 | [89] |
| SPE/MoS2NSs/GNPs/BHb | CYF | DPV | 4 nM–28.52 µM | 5.6 nM | - | [91] |
| GCE/3D MGA/GOD | Glucose | FIA | 2–20 mM | 0.29 mM | 3.36 µA/mM | [93] |
| SPCE/TMX-Au–PdNPs/ACLE | Paraoxon | CA | 0.1–1000 µg/L | 1.75 ng/L | - | [100] |
| GCE/MC/GNPs/TMX/ACLE | Methamidophos | DPV | 1 pM–1 µM | 0.134 pM | - | [102] |
| GCE/GRQDs/Lac | EP | CV | 1–120 µM | 83 nM | 2.9 µA/mM/cm2 | [108] |
| GCE/CRE/GRQDs/ChOx | Cholesterol | CV | 16 µM–6.186 mM | 5 µM | - | [109] |
| S-GRQDs/GNPs-CNSp/Angiopep-2 | Glioma cells | EIS | 100–100,000 cells/mL | 40 cells/mL | - | [111] |
| GRQDs/TMX/PEDOT/GOD | Glucose | DPV | 0–500 µM | 65 µM | 21.64 µA/mM/cm2 | [112] |
| SPE/GOQDs/ssDNA | MiR-141 | DPV | 2.3–6.1 nM | 91 fM | - | [113] |
| PBSQDs/GNSp/GOD | Glucose | DPV | 0.1 µM–10 mM | 1.432 nM | - | [115] |
| ZnSQDs/DNA | miR-200a | EIS | 10 fM–1 µM | 8.4 fM | 374.54 Ω/M | [117] |
| Electrode | Analyte | Technique | Linear Range | LOD | Sensitivity | Ref. |
|---|---|---|---|---|---|---|
| Mg-MOF/GNPs/Mb | TCA | CV | 1–200 mM | 0.33 mM | - | [123] |
| Nitrite | CV | 0.8–18 mM | 0.26 mM | |||
| SPE/MoS2-Cu-MOF/GNPs/Ab | CA125 | DPV | 0.5 mU/mL –500 U/mL | 0.5 mU | - | [124] |
| Cu-MOF/GNPs/CHA-HCR | MiR-21 | DPV | 0.1 fM–100 pM | 0.02 fM | - | [125] |
| GCE/MMOF/GNFs | H2O2 | CA | 5 µM–15 mM; 15–120 mM | 0.9 µM | - | [126] |
| GCE/FA-Zr-MOF | HeLa cells | EIS | 1 × 102–1 ×106 cells/mL | 90 cells/mL | - | [127] |
| GCE/NA-Zr-MOF/dsDNA | MiR-21 | DPV | 0.02–10 pM | 8.2 fM | - | [128] |
| Let-7a | 0.01–10 pM | 3.6 fM | ||||
| Cr-MOF/PdNPs/c-DNA | HeLa cells | DPV | 5 × 102–1.62 ×107 cells/mL | 11.25 cells/mL | - | [129] |
| Co-MOF/GNPs/MWCNT/Cyt-c | nitrite | DPV | 5 nM–1 mM | 4.4 nM | - | [131] |
| ITO/PANI/Cu-MOF/Ab | E. coli | EIS | 2–2 × 108 cfu/mL | 2 cfu/mL | - | [132] |
| SPE/TCOF/SOD | Superoxide | CA | 10 nM–100 µM | 0.5 nM | - | [138] |
| GCE/COF/ACLE | Carbaryl | EIS | 0.48–35 µM | 0.16 µM | - | [139] |
| GCE/Fe3O4COF/HRP | HQ | DPV | 0.5–300 µM | 120 nM | - | [140] |
| GCE/Fe3O4COF/GNPs/DNA | ATP | CV | 5 pM–50 µM | 1.6 pM | - | [141] |
| COF/DNA/HRP | exosomes | CA | 104–107 particles/µL | 7668 particles/µL | - | [142] |
| GCE/AMIL/Hb | Bromate | CA | 12–228 µM | 3 µM | 430.7 µA/mM/cm2 | [151] |
| 228 µM–4.42 mM | 148.4 µA/mM/cm2 | |||||
| SPCE/ERGO/AFIL/GOD | Glucose | CA | 0.05–2.4 mM | 17 µM | 17.7 µA/mM/cm2 | [152] |
| GCE/RTAFIL/GNPs/HRP | H2O2 | CA | 20 µM–0.72 mM | 3.7 µM | 63.4 µA/mM/cm2 | [153] |
| 0.72–2.77 mM | 51.1 µA/mM/cm2 | |||||
| CAA-IL/MWCNT/Lac | GA | FIA | 6 µM–0.3 mM | 3 µM | 91.9 µA/mM/cm2 | [154] |
| GCE/ASAIM-IL/GOD | Glucose | CA | 1 µM–12 mM | 0.572 µM | 38.35 µA/M/cm2 | [155] |
| SILGE/PB/TMX/GOD | Glucose | CA | 0–15 mM | 24.5 µM | - | [157] |
| GCE/AMIM-GO/CLO | CL | ADPSV | 5–1000 nM | 0.885 nM | - | [158] |
| GCE/AMIM-GO/ACLE | ACL | 1.352 nM | - | |||
| PGE/BMIM/BRC | BRCA1 | DPV | 2–10 µg/mL | 1.48 µg/mL | 1.49 µA mL/µg/cm2 | [159] |
| GCE/IL-MWCNT/Catl/GOD/CS | Glucose | DPV | 0.5–100 µM | 0.2 µM | - | [160] |
| CPE/IL-GQDs/ds-DNA | Tpn | DPV | 0.35–100 µM | 0.1 µM | - | [161] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theyagarajan, K.; Kim, Y.-J. Recent Developments in the Design and Fabrication of Electrochemical Biosensors Using Functional Materials and Molecules. Biosensors 2023, 13, 424. https://doi.org/10.3390/bios13040424
Theyagarajan K, Kim Y-J. Recent Developments in the Design and Fabrication of Electrochemical Biosensors Using Functional Materials and Molecules. Biosensors. 2023; 13(4):424. https://doi.org/10.3390/bios13040424
Chicago/Turabian StyleTheyagarajan, K., and Young-Joon Kim. 2023. "Recent Developments in the Design and Fabrication of Electrochemical Biosensors Using Functional Materials and Molecules" Biosensors 13, no. 4: 424. https://doi.org/10.3390/bios13040424
APA StyleTheyagarajan, K., & Kim, Y.-J. (2023). Recent Developments in the Design and Fabrication of Electrochemical Biosensors Using Functional Materials and Molecules. Biosensors, 13(4), 424. https://doi.org/10.3390/bios13040424