Determination of the Electrochemical Area of Screen-Printed Electrochemical Sensing Platforms
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
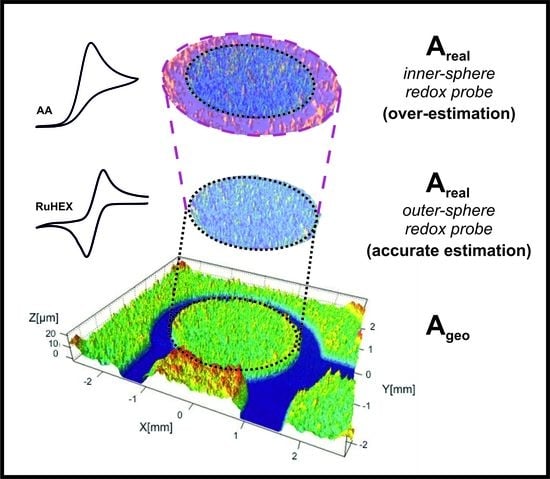
3.1. Determining the Electroactive Area Using Cyclic Voltammetry
- (1)
- Which equation should be used for each redox probe utilised? i.e., which equation from (1)–(3) is the most suitable to use? Analysis of the peak-to-peak separation (ΔEp) of the recorded voltammogram is useful, where in the reversible limit the ΔEp is ~57mV and is independent of scan rate. In the case of quasi- and irreversible conditions, the ΔEp is larger and is dependent upon the voltage scan rate. The wave-shape of the forward peak allows one to determine between reversible and irreversible conditions; a full analysis is given in reference [32].
- (2)
- The R–S equations should only be used for the forward scan [32], this is due to the fact that on the forward wave, the product is electrochemically produced and diffusion occurs, giving, as a result, a concentration of zero product within the bulk solution compared to that at the electrode surface. Consequently, on the return scan, returning the electrochemically formed product back to its starting material, a decrease in the concentration of the product has occurred, resulting in a less intense backward peak than the forward one. The Randles–Ševćik equations are only an approximation, and therefore do not represent an exact value, unlike, as for example, the case of chronocoulometry.
- (3)
- The Randles–Ševćik equations are more suitable for macroelectrodes, therefore, which size of electrode can be utilized to satisfy the Randles–Ševćik equation? i.e., how big does the electrode need to be in order to give rise to the mass transport dominated by planar diffusion? Compton has undertaken experiments inferring that working electrodes of no less than 4 mm radius should be employed for investigations in aqueous solutions [33]. Their work demonstrates that for a simple electron transfer process, the ΔEp is reduced from 60.6 mV using a radius of 0.5 mm to 57.5 mV in the case of a radius of 4 mm and larger; the quantitative change is due to the geometric electrode size increasing such radial diffusion [33].
- (4)
- One must consider, is the electrode relatively flat and non-porous? In order for Equations (1)–(3) to hold, this should be the case. In the case of a SPE, the electrode is heterogeneous, comprising a range of different carbons (graphite, carbon black) and binder(s). It should be noted that the surface roughness of a SPE is typically 0.078 µm (see Figure S1). Over the timescale of the voltammetric experiment, as determined by Compton [32,34], the diffusion layer is larger than the SPE micro-features such that the electrode kinetics are heterogeneous and dominated by the faster electrode material, i.e., the edge plane features of the graphite(s)/carbon black(s). In this case, Equations (1)–(3) hold; see references [32,34] for the categorisation of electrochemically heterogeneous surfaces that may be encountered.
- (5)
- The potential window is not reversed too early, and the analysis of the forward peak is used on the first scan [32].
- (6)
- The scan rate is not too fast to make the cyclic voltammetric response become non-reversible. This is since the Randles–Ševćik equations are derived from assuming the concentration of the electroactive species in the bulk is the same as that at the surface of the electrode, which, as highlighted above, is due to a diffusion layer developing [32].
- (7)
- In the case of determining the electrode area, a reliable diffusion coefficient (D) value needs to be utilized. A useful approach is the Wilke–Chang [35] equation to determine the diffusion coefficient:where x is the association parameter to define the effective molecular weight of the solvent with respect to the diffusion process (where x = 2.6 for water and x = 1 for non-associated solvents [35]), Ms is the molecular weight of the solvent (g mol−1), η is the viscosity of the solution (g cm−1 s−1), and V is the molar volume of solute at normal boiling point (cm3 g−1 mol−1). This equation predicts the D value with an exponential error of ±13%. As highlighted by Sitaraman et al. [36], finding the association parameter (x) becomes an issue for unknown systems, therefore the following correction has been proposed:where Ls is the latent heat of vaporization of solute at normal boiling point (cal g−1). This methodology still has an error of ±13%, but is simpler when used by experimentalists. Clearly, temperature is critical in determining the electrochemical area. Changes and fluctuations in temperature will affect the information obtained from Equations (1)–(5). Consequently, the temperature at the time of measuring the electrochemical area should be measured and factored into these equations.
- (8)
- The Randles–Ševćik equations are useful for single electron transfer processes that feature a 1:1 reaction stoichiometry, inversely however, for example, the reduction of protons to hydrogen (hydrogen evolution reaction, HER) has a 2:1 stoichiometry and experimental results deviate from theory [37]. The diffusion coefficients used here are either from the academic literature or deduced using Equation (5).
3.2. Determining the Electroactive Area Using Chronocoulometry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Metters, J.P.; Kadara, R.O.; Banks, C.E. New directions in screen printed electroanalytical sensors: An overview of recent developments. Analyst 2011, 136, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, Y.T.; Li, D.W.; Long, Y.T. Recent developments and applications of screen-printed electrodes in environmental assays—A review. Anal. Chim. Acta 2012, 734, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Kadara, R.O.; Jenkinson, N.; Banks, C.E. Disposable bismuth oxide screen printed electrodes for the high throughput screening of heavy metals. Electroanalysis 2009, 21, 2410–2414. [Google Scholar] [CrossRef]
- Foster, C.W.; de Souza, A.P.; Metters, J.P.; Bertotti, M.; Banks, C.E. Metallic modified (bismuth, antimony, tin and combinations thereof) film carbon electrodes. Analyst 2015, 140, 7598–7612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-Ibáñez, N.; García-Cruz, L.; Montiel, V.; Foster, C.W.; Banks, C.E.; Iniesta, J. Electrochemical lactate biosensor based upon chitosan/carbon nanotubes modified screen-printed graphite electrodes for the determination of lactate in embryonic cell cultures. Biosens. Bioelectron. 2016, 77, 1168–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-Ibáñez, N.; Sanjuán, I.; Montiel, M.Á.; Foster, C.W.; Banks, C.E.; Iniesta, J. L-cysteine determination in embryo cell culture media using Co (II)-phthalocyanine modified disposable screen-printed electrodes. J. Electroanal. Chem. 2016, 780, 303–310. [Google Scholar] [CrossRef]
- Lee, J.; Hussain, G.; Banks, C.E.; Silvester, D.S. Screen-printed graphite electrodes as low-cost devices for oxygen gas detection in room-temperature ionic liquids. Sensors 2017, 17, 2734. [Google Scholar] [CrossRef] [PubMed]
- Morata, A.; Viricelle, J.P.; Tarancón, A.; Dezanneau, G.; Pijolat, C.; Peiro, F.; Morante, J.R. Development and characterisation of a screen-printed mixed potential gas sensor. Sens. Actuators B Chem. 2008, 130, 561–566. [Google Scholar] [CrossRef]
- Maczuga, M.; Economou, A.; Bobrowski, A.; Prodromidis, M. Novel screen-printed antimony and tin voltammetric sensors for anodic stripping detection of Pb(II) and Cd(II). Electrochim. Acta 2013, 114, 758. [Google Scholar] [CrossRef]
- Randviir, E.P.; Metters, J.P.; Stainton, J.; Banks, C.E. Electrochemical impedance spectroscopy versus cyclic voltammetry for the electroanalytical sensing of capsaicin utilising screen printed carbon nanotube electrodes. Analyst 2013, 138, 2970–2981. [Google Scholar] [CrossRef] [PubMed]
- Ngamchuea, K.; Hurst, P.; Batchelor-McAuley, C.; Compton, R.G. Handheld electrochemical device for the determination of the strength of garlic. Sens. Actuators B Chem. 2016, 232, 138–142. [Google Scholar] [CrossRef]
- Jarzabek, G.; Borkowska, Z. On the real surface area of smooth solid electrodes. Electrochim. Acta 1997, 42, 2915–2918. [Google Scholar] [CrossRef]
- Łukaszewski, M.; Soszko, M.; Czerwiński, A. Electrochemical methods of real surface area determination of noble metal electrodes—An overview. Int. J. Electrochem. Sci. 2016, 11, 4442–4469. [Google Scholar] [CrossRef]
- Fragkou, V.; Ge, Y.; Steiner, G.; Freeman, D.; Bartetzko, N.; Turner, A.P.F. Determination of the real surface area of a screen-printed electrode by chronocoulometry. Int. J. Electrochem. Sci. 2012, 7, 6214–6220. [Google Scholar]
- Gowthaman, N.S.K.; Raj, M.A.; John, S.A. Nitrogen-doped graphene as a robust scaffold for the homogeneous deposition of copper nanostructures: A nonenzymatic disposable glucose sensor. ACS Sustain. Chem. Eng. 2017, 5, 1648–1658. [Google Scholar] [CrossRef]
- Purwidyantri, A.; Chen, C.-H.; Chen, L.-Y.; Chen, C.-C.; Luo, J.-D.; Chiou, C.-C.; Tian, Y.-C.; Lin, C.-Y.; Yang, C.-M.; Lai, H.-C.; et al. Speckled zno nanograss electrochemical sensor for staphylococcus epidermidis detection. J. Electrochem. Soc. 2017, 164, B205–B211. [Google Scholar] [CrossRef]
- Ali, M.A.; Solanki, P.R.; Srivastava, S.; Singh, S.; Agrawal, V.V.; John, R.; Malhotra, B.D. Protein functionalized carbon nanotubes-based smart lab-on-a-chip. ACS Appl. Mater. Interfaces 2015, 7, 5837–5846. [Google Scholar] [CrossRef] [PubMed]
- Jeena, S.E.; Selvaraju, T. Facile growth of Ag@Pt bimetallic nanorods on electrochemically reduced graphene oxide for an enhanced electrooxidation of hydrazine. J. Chem. Sci. 2016, 128, 357–363. [Google Scholar] [CrossRef]
- Sagarica, T.; Saha, H. Electrochemical characterization of some commercial screen-printed electrodes in different redox substrates. Curr. Sci. 2015, 109, 1427. [Google Scholar]
- Tamburri, E.; Cassani, M.C.; Ballarin, B.; Tomellini, M.; Femoni, C.; Mignani, A.; Terranova, M.L.; Orlanducci, S. Hydrogen adsorption properties of carbon nanotubes and platinum nanoparticles from a new ammonium-ethylimidazolium chloroplatinate salt. ChemSusChem 2016, 9, 1153–1165. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Tiwari, I.; Foster, C.W.; Banks, C.E. Highly sensitive and selective determination of dopamine using screen-printed electrodes modified with nanocomposite of N′-phenyl-p-phenylenediamine/multiwalled carbon nanotubes/nafion. Mater. Res. Bull. 2018, 101, 253–263. [Google Scholar] [CrossRef]
- Hikichi, A.; Muguruma, H.; Inoue, H.; Ohsawa, T. Selective determination of nicotinamide adenine dinucleotide in the presence of ascorbic acid and uric acid at a long-length carbon nanotube electrode. Electrochemistry 2017, 85, 13–16. [Google Scholar] [CrossRef]
- Hallam, P.M.; Banks, C.E. A facile approach for quantifying the density of defects (edge plane sites) of carbon nanomaterials and related structures. Phys. Chem. Chem. Phys. 2011, 13, 1210–1213. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.R.; Hong, Q.; Coles, B.A.; Compton, R.G. Variable-temperature microelectrode voltammetry: Application to diffusion coefficients and electrode reaction mechanisms. J. Phys. Chem. B 1999, 103, 2963–2969. [Google Scholar] [CrossRef]
- Kim, Y.R.; Bong, S.; Kang, Y.J.; Yang, Y.; Mahajan, R.K.; Kim, J.S.; Kim, H. Electrochemical detection of dopamine in the presence of ascorbic acid using graphene modified electrodes. Biosens. Bioelectron. 2010, 25, 2366–2369. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Foster, C.W.; Cumba, L.R.; do Carmo, D.R.; Banks, C.E. Can solvent induced surface modifications applied to screen-printed platforms enhance their electroanalytical performance? Analyst 2016, 141, 2783–2790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cumba, L.R.; Foster, C.W.; Brownson, D.A.C.; Smith, J.P.; Iniesta, J.; Thakur, B.; do Carmo, D.R.; Banks, C.E. Can the mechanical activation (polishing) of screen-printed electrodes enhance their electroanalytical response? Analyst 2016, 141, 2791–2799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Randles, J.E.B. A cathode ray polarograph. Part II.—The current-voltage curves. Trans. Faraday Soc. 1948, 44, 327–338. [Google Scholar] [CrossRef]
- Ševčík, A. Oscillographic polarography with periodical triangular voltage. Collect. Czech. Chem. Commun. 1948, 13, 349–377. [Google Scholar] [CrossRef]
- Bard, A.; Faulkner, L. Electrochemical Methods: Fundamentals and Applications; John Wiley & Sons, Inc.: New York, NY, USA, 2001. [Google Scholar]
- Brownson, D.A.C.; Banks, C.E. The Handbook of Graphene Electrochemistry; Springer: London, UK, 2014. [Google Scholar]
- Compton, R.G.; Banks, C.E. Understanding Voltammetry, 2nd ed.; Imperial College Press: London, UK, 2010. [Google Scholar]
- Ngamchuea, K.; Eloul, S.; Tschulik, K.; Compton, R.G. Planar diffusion to macro disc electrodes—What electrode size is required for the cottrell and randles-sevcik equations to apply quantitatively? J. Solid State Electrochem. 2014, 18, 3251–3257. [Google Scholar] [CrossRef]
- Davies, T.J.; Moore, R.R.; Banks, C.E.; Compton, R.G. The cyclic voltammetric response of electrochemically heterogeneous surfaces. J. Electroanal. Chem. 2004, 574, 123–152. [Google Scholar] [CrossRef]
- Wilke, C.R.; Chang, P. Correlation of diffusion coefficients in dilute solutions. AIChE J. 1955, 1, 264–270. [Google Scholar] [CrossRef] [Green Version]
- Sitaraman, R.; Ibrahim, S.H.; Kuloor, N.R. A generalized equation for diffusion in liquids. J. Chem. Eng. Data 1963, 8, 198–201. [Google Scholar] [CrossRef]
- Jiao, X.; Batchelor-McAuley, C.; Kätelhön, E.; Ellison, J.; Tschulik, K.; Compton, R.G. The subtleties of the reversible hydrogen evolution reaction arising from the nonunity stoichiometry. J. Phys. Chem. C 2015, 119, 9402–9410. [Google Scholar] [CrossRef]
- Alkire, R.C.; Barlett, P.N.; Lipkowski, J. Electrochemistry of Carbon Electrodes; John Wiley & Sons, Inc.: Weinheim, Germany, 2016; Volume 1, p. 450. [Google Scholar]
- McCreery, R.L. Advanced carbon electrode materials for molecular electrochemistry. Chem. Rev. 2008, 108, 2646–2687. [Google Scholar] [CrossRef] [PubMed]
- Anson, F.C. Innovations in the study of adsorbed reactants by chronocoulometry. Anal. Chem. 1966, 38, 54–57. [Google Scholar] [CrossRef]




| Electroactive Probe | Electrode Area Randles–Ševćik/cm2 | D/cm2 s−1 | %Real |
|---|---|---|---|
| RuHex | 0.062 | 9.10 × 10−6 | 83.25 |
| NADH | 0.049 | 7.40 × 10−6 | 65.47 |
| Dopamine | 0.090 | 6.74 × 10−6 | 120.18 |
| Capsaicin | 0.093 | 7.03 × 10−6 | 123.74 |
| TMPD | 0.057 | 6.32 × 10−6 | 75.64 |
| Ascorbic acid | 0.109 | 1.42 × 10−6 | 145.65 |
| Electroactive Probe | Electrode Area Anson/cm2 | D/cm2 s−1 | %Real |
|---|---|---|---|
| RuHex | 0.055 | 9.40 × 10−6 | 73.34 |
| NADH | 0.077 | 7.40 × 10−6 | 103.27 |
| Dopamine | 0.077 | 6.74 × 10−6 | 102.51 |
| Capsaicin | 0.057 | 7.03 × 10−6 | 75.91 |
| TMPD | 0.053 | 6.32 × 10−6 | 70.53 |
| Ascorbic acid | 0.121 | 1.42 × 10−6 | 161.21 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Miranda Ferrari, A.; Foster, C.W.; Kelly, P.J.; Brownson, D.A.C.; Banks, C.E. Determination of the Electrochemical Area of Screen-Printed Electrochemical Sensing Platforms. Biosensors 2018, 8, 53. https://doi.org/10.3390/bios8020053
García-Miranda Ferrari A, Foster CW, Kelly PJ, Brownson DAC, Banks CE. Determination of the Electrochemical Area of Screen-Printed Electrochemical Sensing Platforms. Biosensors. 2018; 8(2):53. https://doi.org/10.3390/bios8020053
Chicago/Turabian StyleGarcía-Miranda Ferrari, Alejandro, Christopher W. Foster, Peter J. Kelly, Dale A. C. Brownson, and Craig E. Banks. 2018. "Determination of the Electrochemical Area of Screen-Printed Electrochemical Sensing Platforms" Biosensors 8, no. 2: 53. https://doi.org/10.3390/bios8020053
APA StyleGarcía-Miranda Ferrari, A., Foster, C. W., Kelly, P. J., Brownson, D. A. C., & Banks, C. E. (2018). Determination of the Electrochemical Area of Screen-Printed Electrochemical Sensing Platforms. Biosensors, 8(2), 53. https://doi.org/10.3390/bios8020053