New Functionalized Macroparticles for Environmentally Sustainable Biofilm Control in Water Systems
Abstract
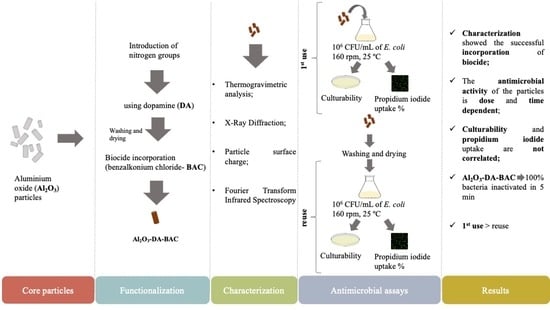
:1. Introduction
2. Results and Discussion
2.1. Functionalization Characteristics
2.1.1. Thermal Stability of Functionalized Metal Oxides
2.1.2. Adsorption/Desorption Isotherms
2.1.3. X-ray Diffraction (XRD) Analysis
2.1.4. Point of Zero Charge—pHPZC
2.1.5. Fourier-Transform Infrared Spectroscopy (FTIR) Analysis
2.2. Particles’ Antimicrobial Activity
2.3. Effect of Immobilized Biocide Concentration on the Particles’ Antimicrobial Activity
2.3.1. Culturability
2.3.2. Membrane Integrity
2.4. Effect of Particle Reuse on Their Antimicrobial Activity
2.5. Hypothesis for the Underlying Mechanism of Action of Immobilized Biocide
3. Materials and Methods
3.1. Reagents
3.2. Preparation and Functionalization of Particles
3.2.1. Initial Functionalization of Alumina Oxide Particles with DA
3.2.2. Immobilization of Biocide on the Surface of Alumina Oxide Particles
3.3. Particles Characterization
3.3.1. Thermal Stability of the Particles
3.3.2. Textural Characterization of the Particles
3.3.3. Structural Properties of the Particles
3.3.4. Determination of Particle Surface Charge
3.3.5. Fourier-Transform Infrared Spectroscopy (FTIR)
3.4. Particles Antimicrobial Activity
3.4.1. Microorganism
3.4.2. Antimicrobial Activity of the Particles against E. coli
3.4.3. Effect of Particle Functionalization and Biocide Concentration on Antimicrobial Activity
3.4.4. Effect of Reuse on Particles’ Antimicrobial Activity
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boretti, A.; Rosa, L. Reassessing the projections of the World Water Development Report. npj Clean Water 2019, 2, 1–6. [Google Scholar] [CrossRef]
- Vrouwenvelder, J.; Van der Kooij, D. Diagnosis, prediction and prevention of biofouling of NF and RO membranes. Desalination 2001, 139, 65–71. [Google Scholar] [CrossRef]
- Majamaa, K.; Johnson, J.E.; Bertheas, U. Three steps to control biofouling in reverse osmosis systems. Desalination Water Treat. 2012, 42, 107–116. [Google Scholar] [CrossRef]
- Vrouwenvelder, J.; Beyer, F.; Dahmani, K.; Hasan, N.; Galjaard, G.; Kruithof, J.; Van Loosdrecht, M. Phosphate limitation to control biofouling. Water Res. 2010, 44, 3454–3466. [Google Scholar] [CrossRef] [PubMed]
- Araújo, P.A.; Mergulhão, F.; Melo, L.; Simões, M. The ability of an antimicrobial agent to penetrate a biofilm is not correlated with its killing or removal efficiency. Biofouling 2014, 30, 675–683. [Google Scholar] [CrossRef] [Green Version]
- Xiong, Y.; Liu, Y. Biological control of microbial attachment: A promising alternative for mitigating membrane biofouling. Appl Microbiol Biotechnol 2010, 86, 825–837. [Google Scholar] [CrossRef]
- Singer, A.C.; Shaw, H.; Rhodes, V.; Hart, A. Review of antimicrobial resistance in the environment and its relevance to environmental regulators. Front. Microbiol. 2016, 7, 1728. [Google Scholar] [CrossRef] [Green Version]
- Khairnar, S.; Shinde, S.; Shrivastava, V. A Short Review on the Improvement of Antimicrobial Activity by Metal and Nonmetal Doping in Nanoscale Antimicrobial Materials. J. Nanomedicine Biotherapeutic Discov. 2019, 9, 163. [Google Scholar]
- Qu, X.; Alvarez, P.J.; Li, Q. Applications of nanotechnology in water and wastewater treatment. Water Res. 2013, 47, 3931–3946. [Google Scholar] [CrossRef]
- Akbar, A.; Sadiq, M.B.; Ali, I.; Muhammad, N.; Rehman, Z.; Khan, M.N.; Muhammad, J.; Khan, S.A.; Rehman, F.U.; Anal, A.K. Synthesis and antimicrobial activity of zinc oxide nanoparticles against foodborne pathogens Salmonella typhimurium and Staphylococcus aureus. Biocatal. Agric. Biotechnol. 2019, 17, 36–42. [Google Scholar] [CrossRef]
- Azizi-Lalabadi, M.; Ehsani, A.; Divband, B.; Alizadeh-Sani, M. Antimicrobial activity of Titanium dioxide and Zinc oxide nanoparticles supported in 4A zeolite and evaluation the morphological characteristic. Scientific reports 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benyahia, F.; O’Neill, K. Enhanced voidage correlations for packed beds of various particle shapes and sizes. Part. Sci. Technol. 2005, 23, 169–177. [Google Scholar] [CrossRef]
- Emami-Karvani, Z.; Chehrazi, P. Antibacterial activity of ZnO nanoparticle on Gram-positive and Gram-negative bacteria. Afr. J. Microbiol. Res. 2012, 5, 1368–1373. [Google Scholar]
- Jaswal, V.S.; Chaudhary, A.; Thakur, P.; Sharma, D.; Arora, A.K.; Khanna, R.; Tuli, H.S. Chapter 9: ZnO nanoparticle with promising antimicrobial and antiproliferation synergistic properties. In Comprehensive Analytical Chemistry: Engineered Nanomaterials and Phytonanotechnology: Challenges for Plant Sustainability; Verma, S.K., Das, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 87, pp. 251–262. [Google Scholar]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.-H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.-Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomedicine 2007, 3, 95–101. [Google Scholar] [CrossRef]
- Le Ouay, B.; Stellacci, F. Antibacterial activity of silver nanoparticles: A surface science insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef] [Green Version]
- Tang, S.; Zheng, J. Antibacterial Activity of Silver Nanoparticles: Structural Effects. Adv. Healthc. Mater. 2018, 7, 1701503. [Google Scholar] [CrossRef]
- Yan, X.; He, B.; Liu, L.; Qu, G.; Shi, J.; Hu, L.; Jiang, G. Antibacterial mechanism of silver nanoparticles in Pseudomonas aeruginosa: Proteomics approach. Metallomics 2018, 10, 557–564. [Google Scholar] [CrossRef]
- Asri, L.A.T.W.; Crismaru, M.; Roest, S.; Chen, Y.; Ivashenko, O.; Rudolf, P.; Tiller, J.C.; van der Mei, H.C.; Loontjens, T.J.A.; Busscher, H.J. A Shape-Adaptive, Antibacterial-Coating of Immobilized Quaternary-Ammonium Compounds Tethered on Hyperbranched Polyurea and its Mechanism of Action. Adv. Funct. Mater. 2014, 24, 346–355. [Google Scholar] [CrossRef]
- Avelelas, F.; Martins, R.; Oliveira, T.; Maia, F.; Malheiro, E.; Soares, A.M.; Loureiro, S.; Tedim, J. Efficacy and ecotoxicity of novel anti-fouling nanomaterials in target and non-target marine species. Mar. Biotechnol. 2017, 19, 164–174. [Google Scholar] [CrossRef]
- Chan, A.C.; Bravo Cadena, M.; Townley, H.E.; Fricker, M.D.; Thompson, I.P. Effective delivery of volatile biocides employing mesoporous silicates for treating biofilms. J. R. Soc. Interface 2017, 14, 20160650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, Y.; Xiao, H.; Zhang, Y. Antimicrobial polymeric materials with quaternary ammonium and phosphonium salts. Int, J. Mol. Sci 2015, 16, 3626–3655. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.-L.; Alasonati, E.; Tharaud, M.; Gelabert, A.; Fisicaro, P.; Benedetti, M.F. Flow and fate of silver nanoparticles in small French catchments under different land-uses: The first one-year study. Water Res. 2020, 176, 115722. [Google Scholar] [CrossRef]
- Akhtar, K.; Khan, S.A.; Khan, S.B.; Asiri, S.M. Nanomaterials and environmental remediation: A fundamental overview. In Nanomaterials for Environmental Applications and their Fascinating Attributes; Khan, S.B., Asiri, S.M., Akhtar, K., Eds.; Bentham Science Publishers: Sharjah, UAE, 2018; Volume 2, pp. 26–27. [Google Scholar]
- Zhang, C.; Cui, F.; Zeng, G.-m.; Jiang, M.; Yang, Z.-z.; Yu, Z.-g.; Zhu, M.-y.; Shen, L.-q. Quaternary ammonium compounds (QACs): A review on occurrence, fate and toxicity in the environment. Sci Total Environ. 2015, 518, 352–362. [Google Scholar] [CrossRef]
- Gerba, C.P. Quaternary ammonium biocides: Efficacy in application. Appl. Environ. Microbiol. 2015, 81, 464–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García, M.R.; Cabo, M.L. Optimization of E. coli inactivation by benzalkonium chloride reveals the importance of quantifying the inoculum effect on chemical disinfection. Front. Microbiol. 2018, 9, 1259. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Feng, J.; Ma, J.; Lü, B.; Jia, X. Zinc oxide sol-containing diallylmethyl alkyl quaternary ammonium salt synthesized by sol–gel process: Characterization and properties. J. Text. Inst. 2015, 106, 593–600. [Google Scholar] [CrossRef]
- Muhammad, S.; Siddiq, M.; Niazi, J.H.; Qureshi, A. Role of quaternary ammonium compound immobilized metallic graphene oxide in PMMA/PEG membrane for antibacterial, antifouling and selective gas permeability properties. Polym. Bull. 2018, 75, 5695–5712. [Google Scholar] [CrossRef]
- Choi, J.; Han, Y.; Park, S.; Park, J.; Kim, H. Pore characteristics and hydrothermal stability of mesoporous silica: Role of oleic acid. J. Nanomater. 2014, 2014, 86. [Google Scholar] [CrossRef]
- She, X.; Chen, L.; Li, C.; He, C.; He, L.; Kong, L. Functionalization of hollow mesoporous silica nanoparticles for improved 5-FU loading. J. Nanomater. 2015, 16, 108. [Google Scholar] [CrossRef]
- Zhao, W.; Chen, H.; Li, Y.; Li, L.; Lang, M.; Shi, J. Uniform rattle-type hollow magnetic mesoporous spheres as drug delivery carriers and their sustained-release property. Adv. Func Mater. 2008, 18, 2780–2788. [Google Scholar] [CrossRef]
- Liu, J. Multilayered PEI-based Films for CO2 Adsorption and Diffusion. MSc diss., University of Akron. 2013. Available online: http://rave.ohiolink.edu/etdc/view?acc_num=akron1367839488 (accessed on 15 October 2020).
- Ribena, D. Dopamine modification of interfaces between polymers and metals. Ph.D. Thesis, Technische Universiteit Eindhoven, Eindhoven, The Netherlands, 2012. [Google Scholar]
- Padhye, L.; Luzinova, Y.; Cho, M.; Mizaikoff, B.; Kim, J.-H.; Huang, C.-H. PolyDADMAC and dimethylamine as precursors of N-nitrosodimethylamine during ozonation: Reaction kinetics and mechanisms. Environ. Sci. Technol. 2011, 45, 4353–4359. [Google Scholar] [CrossRef]
- Park, S.-H.; Wei, S.; Mizaikoff, B.; Taylor, A.E.; Favero, C.; Huang, C.-H. Degradation of amine-based water treatment polymers during chloramination as N-nitrosodimethylamine (NDMA) precursors. Environ. Sci. Technol. 2009, 43, 1360–1366. [Google Scholar] [CrossRef] [PubMed]
- Hassan, K.; Elbagoury, M. Antimicrobial activity of some biocides against microorganisms isolated from a shared student kitchen. Rasayan, J. Chem. 2018, 11, 238–244. [Google Scholar]
- Drugeon, H.B.; Juvin, M.-E.; Caillon, J.; Courtieu, A.-L. Assessment of formulas for calculating critical concentration by the agar diffusion method. Antimicrob. Agents Chemother. 1987, 31, 870–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cooper, K.E. Chapter 1: The Theory of Antibiotic Inhibition Zones. In Analytical Microbiology; Kavanagh, F., Ed.; Academic Press: London, UK, 1972; Volume 2, pp. 13–30. [Google Scholar]
- Lee, D.; Cohen, R.E.; Rubner, M.F. Antibacterial properties of Ag nanoparticle loaded multilayers and formation of magnetically directed antibacterial microparticles. Langmuir 2005, 21, 9651–9659. [Google Scholar] [CrossRef] [PubMed]
- Green, J.-B.D.; Fulghum, T.; Nordhaus, M.A. A review of immobilized antimicrobial agents and methods for testing. Biointerphases 2011, 6, MR13–MR28. [Google Scholar] [CrossRef]
- Murray, P.R. The clinician and the microbiology laboratory. In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases; Elsevier: Amsterdam, The Netherlands, 2015; Volume 1, pp. 191–223. [Google Scholar]
- Zhao, P.; Li, J.; Wang, Y.; Jiang, H. Broad-spectrum antimicrobial activity of the reactive compounds generated in vitro by Manduca sexta phenoloxidase. Insect Biochemi Mol. Biol 2007, 37, 952–959. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, Z.; Lai, E.P.; Avis, T.J. Antimicrobial effect of polydopamine coating on Escherichia coli. J. Mater. Chem. 2012, 22, 21608–21612. [Google Scholar] [CrossRef]
- Capita, R.; Vicente-Velasco, M.; Rodríguez-Melcón, C.; García-Fernández, C.; Carballo, J.; Alonso-Calleja, C. Effect of low doses of biocides on the antimicrobial resistance and the biofilms of Cronobacter sakazakii and Yersinia enterocolitica. Sci. rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Grant, D.; Bott, T. Biocide dosing strategies for biofilm control. Heat Transf. Eng. 2005, 26, 44–50. [Google Scholar] [CrossRef]
- Pazos-Ortiz, E.; Roque-Ruiz, J.H.; Hinojos-Márquez, E.A.; López-Esparza, J.; Donohué-Cornejo, A.; Cuevas-González, J.C.; Espinosa-Cristóbal, L.F.; Reyes-López, S.Y. Dose-dependent antimicrobial activity of silver nanoparticles on polycaprolactone fibers against gram-positive and gram-negative bacteria. J. Nanomater. 2017, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, C.; Pereira, A.; Pereira, M.; Melo, L.; Simões, M. Physiological changes induced by the quaternary ammonium compound benzyldimethyldodecylammonium chloride on Pseudomonas fluorescens. J. Antimicrob. Chemother. 2011, 66, 1036–1043. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, M.; Azevedo, N.F.; Ivask, A. Propidium iodide staining underestimates viability of adherent bacterial cells. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Davey, H.M.; Hexley, P. Red but not dead? Membranes of stressed Saccharomyces cerevisiae are permeable to propidium iodide. Environ. Microbiol. 2011, 13, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, C.; Cypionka, H. Propidium ion enters viable cells with high membrane potential during live-dead staining. J. Microbiol. Methods 2017, 142, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Günther, S.; Hübschmann, T.; Wick, L.Y.; Harms, H.; Müller, S. Limits of propidium iodide as a cell viability indicator for environmental bacteria. Cytometry Part. A 2007, 71, 592–598. [Google Scholar] [CrossRef]
- Emerson, J.B.; Adams, R.I.; Román, C.M.B.; Brooks, B.; Coil, D.A.; Dahlhausen, K.; Ganz, H.H.; Hartmann, E.M.; Hsu, T.; Justice, N.B. Schrödinger’s microbes: Tools for distinguishing the living from the dead in microbial ecosystems. Microbiome 2017, 5, 86. [Google Scholar] [CrossRef]
- Lu, G.; Wu, D.; Fu, R. Studies on the synthesis and antibacterial activities of polymeric quaternary ammonium salts from dimethylaminoethyl methacrylate. React. Funct. Polym. 2007, 67, 355–366. [Google Scholar] [CrossRef]
- Owusu-Adom, K.; Guymon, C.A. Photopolymerization kinetics of poly (acrylate)–clay composites using polymerizable surfactants. Polymer 2008, 49, 2636–2643. [Google Scholar] [CrossRef]
- He, W.; Zhang, Y.; Li, J.; Gao, Y.; Luo, F.; Tan, H.; Wang, K.; Fu, Q. A novel surface structure consisting of contact-active antibacterial upper-layer and antifouling sub-layer derived from gemini quaternary ammonium salt polyurethanes. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ho, C.H.; Tobis, J.; Sprich, C.; Thomann, R.; Tiller, J.C. Nanoseparated polymeric networks with multiple antimicrobial properties. Adv. Mater. 2004, 16, 957–961. [Google Scholar] [CrossRef]
- Sui, Y.; Gao, X.; Wang, Z.; Gao, C. Antifouling and antibacterial improvement of surface-functionalized poly (vinylidene fluoride) membrane prepared via dihydroxyphenylalanine-initiated atom transfer radical graft polymerizations. J. Membr. Sci. 2012, 394, 107–119. [Google Scholar] [CrossRef]
- Andrade del Olmo, J.; Ruiz Rubio, L.; Saez Martinez, L.; Perez-Alvarez, V.; Vilas Vilela, J.L. Antibacterial coatings for improving the performance of biomaterials. Coatings 2020, 10, 139. [Google Scholar] [CrossRef] [Green Version]
- Bieser, A.M.; Tiller, J.C. Mechanistic considerations on contact-active antimicrobial surfaces with controlled functional group densities. Macromol. Biosci. 2011, 11, 526–534. [Google Scholar] [CrossRef]
- Hoque, J.; Akkapeddi, P.; Yadav, V.; Manjunath, G.B.; Uppu, D.S.; Konai, M.M.; Yarlagadda, V.; Sanyal, K.; Haldar, J. Broad spectrum antibacterial and antifungal polymeric paint materials: Synthesis, structure–activity relationship, and membrane-active mode of action. ACS Appl. Mater. Interfaces 2015, 7, 1804–1815. [Google Scholar] [CrossRef]
- Ricardo, S.I.d.C. Antimicrobial strategies to prevent catheters-associated medical infections. Ph.D. Thesis, Universidade de Lisboa, Lisbon, Portugal, 2017. [Google Scholar]
- Amgoth, C.; Phan, C.; Banavoth, M.; Rompivalasa, S.; Tang, G. Polymer Properties: Functionalization and Surface Modified Nanoparticles. In Role of Novel Drug Delivery Vehicles in Nanobiomedicine; IntechOpen: London, UK, 2019. [Google Scholar]
- Tuson, H.H.; Weibel, D.B. Bacteria–surface interactions. Soft. matter. 2013, 9, 4368–4380. [Google Scholar] [CrossRef] [Green Version]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Reed, R.; Reed, G. “Drop plate” method of counting viable bacteria. Can. J. Res. 1948, 26, 317–326. [Google Scholar] [CrossRef]
- Simoes, M.; Pereira, M.O.; Vieira, M. Validation of respirometry as a short-term method to assess the efficacy of biocides. Biofouling 2005, 21, 9–17. [Google Scholar] [CrossRef] [Green Version]









| Sample | SBET (m2/g) | dp (nm) | Vp0:0.95 (cm3/g) |
|---|---|---|---|
| Al2O3 | 258 | 8.2 | 0.77 |
| Al2O3-DA | 280 | 9.0 | 0.77 |
| Al2O3-DA-BAC | 240 | 8.1 | 0.60 |
| Zone of Inhibition (mm) | |
|---|---|
| Al2O3 | 0 ± 0 |
| Al2O3-DA | 0 ± 0 |
| Al2O3-DA-BAC | 18 ± 2 |
| Particles | Immobilized BAC w/w (%): Mass of BAC (g) per 100 g of Particles | Ratio Mass of Particles (g)/Volume of Solution (L) | Overall Concentration under Test (mg of Immobilized Biocide/L Solution) |
|---|---|---|---|
| Al2O3-DA-BAC | 3 | 16.8 | 500 |
| 33.5 | 1000 | ||
| 100.3 | 3000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barros, A.C.; Pereira, A.; Melo, L.F.; Sousa, J.P.S. New Functionalized Macroparticles for Environmentally Sustainable Biofilm Control in Water Systems. Antibiotics 2021, 10, 399. https://doi.org/10.3390/antibiotics10040399
Barros AC, Pereira A, Melo LF, Sousa JPS. New Functionalized Macroparticles for Environmentally Sustainable Biofilm Control in Water Systems. Antibiotics. 2021; 10(4):399. https://doi.org/10.3390/antibiotics10040399
Chicago/Turabian StyleBarros, Ana C., Ana Pereira, Luis F. Melo, and Juliana P. S. Sousa. 2021. "New Functionalized Macroparticles for Environmentally Sustainable Biofilm Control in Water Systems" Antibiotics 10, no. 4: 399. https://doi.org/10.3390/antibiotics10040399
APA StyleBarros, A. C., Pereira, A., Melo, L. F., & Sousa, J. P. S. (2021). New Functionalized Macroparticles for Environmentally Sustainable Biofilm Control in Water Systems. Antibiotics, 10(4), 399. https://doi.org/10.3390/antibiotics10040399