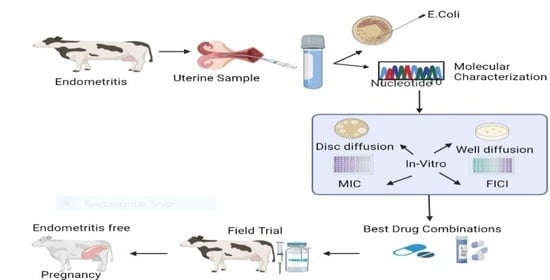
Evidence-Based Tracking of MDR E. coli from Bovine Endometritis and Its Elimination by Effective Novel Therapeutics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tracking Bovine Endometritis
2.2. Isolation and Confirmation of E. coli
2.3. Molecular Confirmation of E. coli
2.3.1. Sequencing of the Local E. coli Isolate
2.3.2. Gene Structure and Motifs Elicitation
2.4. Assessment of the Antibiotic Resistance Profile of E. coli
2.5. In Vitro Therapeutic Testing of Antibiotics
2.5.1. Well Diffusion Assay
2.5.2. Synergy Testing Using Broth Dilution Method
2.6. Field Evaluation of In Vitro Outcomes
2.7. Statistical Analysis
3. Results
3.1. Prevalence of Endometritis and E. coli
3.2. Sequencing Results of the Local E. coli Isolate
3.2.1. Nucleotide Analysis
3.2.2. Phylogenetic Analysis
3.2.3. Gene Structure and Motif Analysis
3.3. Antibiogram of Endometritis-Originated E. coli
3.4. In Vitro Therapeutics of Commonly Used Anti-Microbials
3.4.1. Wells Zones of Microbial Growth Inhibition
3.4.2. Synergy Testing of Anti-Microbials
3.5. Field Trial Outcome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gilbert, R.O. Bovine endometritis: The burden of proof. Cornell Vet. 1992, 82, 11–14. [Google Scholar] [PubMed]
- Gautam, G.; Nakao, T.; Yusuf, M.; Koike, K. Prevalence of endometritis during the postpartum period and its impact on subsequent reproductive performance in two Japanese dairy herds. Anim. Reprod. Sci. 2009, 116, 175–187. [Google Scholar] [CrossRef]
- Blood, D.C. Veterinary Medicine: A Textbook of the Diseases of Cattle, Sheep, Pigs, Goats and Horses; James, A., Radostits, O.M., Eds.; Bailliere Tindall: London, UK, 1983; ISBN 0702009873. [Google Scholar]
- Turk, R.; Samardžija, M.; Bačić, G. Oxidative stress and reproductive disorders in dairy cows. In Dairy Cows: Nutrition, Fertility and Milk Production; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2013; pp. 57–98. ISBN 9781611229585. [Google Scholar]
- Abdel-Latif, M.A.; EL-Gohary, E.S.; Gabr, A.A.; El-Hawary, A.F.; Ahmed, S.A.; Ebrahim, S.A.; Fathala, M.M. Impact of Supplementing Propylene Glycol and Calcium Propionate to Primiparous Buffalo Cows During the Late Gestation and Early Lactation Period on Reproductive Performance and Metabolic Parameters. Alexandria J. Vet. Sci. 2016, 51, 114–121. [Google Scholar]
- Földi, J.; Kulcsar, M.; Pecsi, A.; Huyghe, B.; De Sa, C.; Lohuis, J.; Cox, P.; Huszenicza, G. Bacterial complications of postpartum uterine involution in cattle. Anim. Reprod. Sci. 2006, 96, 265–281. [Google Scholar] [CrossRef]
- Bicalho, R.C.; Santos, T.M.A.; Gilbert, R.O.; Caixeta, L.S.; Teixeira, L.M.; Bicalho, M.L.S.; Machado, V.S. Susceptibility of Escherichia coli isolated from uteri of postpartum dairy cows to antibiotic and environmental bacteriophages. Part I: Isolation and lytic activity estimation of bacteriophages. J. Dairy Sci. 2010, 93, 93–104. [Google Scholar] [CrossRef]
- Gautam, G.; Nakao, T.; Koike, K.; Long, S.T.; Yusuf, M.; Ranasinghe, R.; Hayashi, A. Spontaneous recovery or persistence of postpartum endometritis and risk factors for its persistence in Holstein cows. Theriogenology 2010, 73, 168–179. [Google Scholar] [CrossRef]
- Suojala, L.; Kaartinen, L.; Pyörälä, S. Treatment for bovine Escherichia coli mastitis—an evidence-based approach. J. Vet. Pharmacol. Ther. 2013, 36, 521–531. [Google Scholar] [CrossRef]
- Ericsson Unnerstad, H.; Lindberg, A.; Persson Waller, K.; Ekman, T.; Artursson, K.; Nilsson-Öst, M.; Bengtsson, B. Microbial aetiology of acute clinical mastitis and agent-specific risk factors. Vet. Microbiol. 2009, 137, 90–97. [Google Scholar] [CrossRef] [Green Version]
- Shoaib, M.; Rahman, S.U.; Aqib, A.I.; Ashfaq, K.; Naveed, A.; Kulyar, M.F.-A.; Bhutta, Z.A.; Younas, M.S.; Sarwar, I.; Naseer, M.A. Diversified Epidemiological Pattern and Antibiogram of mecA Gene in Staphylococcus aureus Isolates of Pets, Pet Owners and Environment. Pak. Vet. J. 2020, 40, 331–336. [Google Scholar]
- Lippolis, J.D.; Holman, D.B.; Brunelle, B.W.; Thacker, T.C.; Bearson, B.L.; Reinhardt, T.A.; Sacco, R.E.; Casey, T.A. Genomic and transcriptomic analysis of Escherichia coli strains associated with persistent and transient bovine mastitis and the role of colanic acid. Infect. Immun. 2018, 86, e00566-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasman, H.; Mevius, D.; Veldman, K.; Olesen, I.; Aarestrup, F.M. β-Lactamases among extended-spectrum β-lactamase (ESBL)-resistant Salmonella from poultry, poultry products and human patients in The Netherlands. J. Antimicrob. Chemother. 2005, 56, 115–121. [Google Scholar] [CrossRef] [Green Version]
- Mackeen, A.D.; Packard, R.E.; Ota, E.; Speer, L. Antibiotic regimens for postpartum endometritis. Cochrane Database Syst. Rev. 2015, 2015, CD001067. [Google Scholar] [CrossRef] [PubMed]
- Cheesman, M.J.; Ilanko, A.; Blonk, B.; Cock, I.E. Developing New Antimicrobial Therapies: Are Synergistic Combinations of Plant Extracts/Compounds with Conventional Antibiotics the Solution? Pharmacogn. Rev. 2017, 11, 57–72. [Google Scholar] [CrossRef] [Green Version]
- Aslam, B.; Wang, W.; Arshad, M.I.; Khurshid, M.; Muzammil, S.; Rasool, M.H.; Nisar, M.A.; Alvi, R.F.; Aslam, M.A.; Qamar, M.U.; et al. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azawi, O.I.; Omran, S.N.; Hadad, J.J. Clinical, bacteriological, and histopathological study of toxic puerperal metritis in Iraqi buffalo. J. Dairy Sci. 2007, 90, 4654–4660. [Google Scholar] [CrossRef]
- Ahmadi, M.R.; Hosseini, A.; Gheisari, H.R.; Yavari, M. Preliminary trial in treatment of postpartum endometritis with intrauterine application of hyperimmune serum in dairy cows. Asian Pacific J. Trop. Dis. 2014, 4, 360–365. [Google Scholar] [CrossRef]
- Thrusfield, M. Sampling in Veterinary Epidemiology; Blackwell Science Ltd.: London, UK, 2007; pp. 214–256. [Google Scholar]
- Fissore, R.A.; Edmondson, A.J.; Pashen, R.L.; Bondurant, R.H. The use of ultrasonography for the study of the bovine reproductive tract. II. Non-pregnant, pregnant and pathological conditions of the uterus. Anim. Reprod. Sci. 1986, 12, 167–177. [Google Scholar] [CrossRef]
- Pierson, R.A.; Ginther, O.J. Ultrasonic imaging of the ovaries and uterus in cattle. Theriogenology 1988, 29, 21–37. [Google Scholar] [CrossRef]
- Ihnatsenka, B.; Boezaart, A.P. Ultrasound: Basic understanding and learning the language. Int. J. Shoulder Surg. 2010, 4, 55. [Google Scholar]
- Sarwar, I.; Ashar, A.; Mahfooz, A.; Aqib, A.I.; Saleem, M.I.; Butt, A.A.; Bhutta, Z.A.; Shoaib, M.; Kulyar, M.F.-A.; Ilyas, A. Evaluation of Antibacterial Potential of Raw Turmeric, Nano-Turmeric, and NSAIDs against Multiple Drug Resistant Staphylococcus aureus and E. coli Isolated from Animal Wounds. Pak. Vet. J. 2021, 41, 209–214. [Google Scholar]
- Holt, J.G.; Krieg, N.R.; Sneath, P.H.A.; Staley, J.T.; Williams, S.T. Bergey’s Manual Of Determinative Bacteriology, 9th ed.; William Wilkins: Baltimore, MD, USA, 1994. [Google Scholar]
- Sambrook, J.; Russell, D.W. Molecular Cloning-Sambrook & Russel-Vol. 1, 2, 3; Cold Springs Harbor Lab Press: Long Island, NY, USA, 2001. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Williams, N.; Misleh, C.; Li, W.W. MEME: Discovering and analyzing DNA and protein sequence motifs. Nucleic Acids Res. 2006, 34, W369–W373. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing of Anaerobic Bacteria: Informational Supplement; Clinical and Laboratory Standards Institute (CLSI): Annapolis, MD, USA, 2009. [Google Scholar]
- Anwar, M.A.; Aqib, A.I.; Ashfaq, K.; Deeba, F.; Khan, M.K.; Khan, S.R.; Muzammil, I.; Shoaib, M.; Naseer, M.A.; Riaz, T.; et al. Antimicrobial resistance modulation of MDR E. coli by antibiotic coated ZnO nanoparticles. Microb. Pathog. 2020, 148, 104450. [Google Scholar] [CrossRef] [PubMed]
- Aqib, A.; Saqib, M.; Khan, S.; Ahmad, T.; Raza, S.; Naseer, M.; Shoaib, M.; Sarwar, I.; Kulyar, M.F.; Bhutta, Z.; et al. Non-steroidal anti-inflammatory drugs, plant extracts, and characterized microparticles to modulate antimicrobial resistance of epidemic mecA positive S. aureus of dairy origin. Appl. Nanosci. 2021, 11, 553–563. [Google Scholar] [CrossRef]
- Shin, E.-K.; Jeong, J.-K.; Choi, I.-S.; Kang, H.-G.; Hur, T.-Y.; Jung, Y.-H.; Kim, I.-H. Relationships among ketosis, serum metabolites, body condition, and reproductive outcomes in dairy cows. Theriogenology 2015, 84, 252–260. [Google Scholar] [CrossRef]
- Thrusfield, M.V. Veterinary Epidemiology; Blackwell Science: Ames, IA, USA, 2007; ISBN 9781405156271. [Google Scholar]
- Wang, G.-Q.; Wu, C.-M.; Du, X.-D.; Shen, Z.-Q.; Song, L.-H.; Chen, X.; Shen, J.-Z. Characterization of integrons-mediated antimicrobial resistance among Escherichia coli strains isolated from bovine mastitis. Vet. Microbiol. 2008, 127, 73–78. [Google Scholar] [CrossRef]
- Hinthong, W.; Pumipuntu, N.; Santajit, S.; Kulpeanprasit, S.; Buranasinsup, S.; Sookrung, N.; Chaicumpa, W.; Aiumurai, P.; Indrawattana, N. Detection and drug resistance profile of Escherichia coli from subclinical mastitis cows and water supply in dairy farms in Saraburi Province, Thailand. PeerJ 2017, 5, e3431. [Google Scholar] [CrossRef] [Green Version]
- Sheldon, I.M.; Dobson, H. Postpartum uterine health in cattle. Anim. Reprod. Sci. 2004, 82, 295–306. [Google Scholar] [CrossRef]
- Deori, S.; Phookan, A. bovine postpartum metritis and its therapeutics: A Review. Indian J. Sci. Technol. 2015, 8, 1. [Google Scholar] [CrossRef]
- Azawi, O.I. Postpartum uterine infection in cattle. Anim. Reprod. Sci. 2008, 105, 187–208. [Google Scholar] [CrossRef]
- Palanisamy, K.; Udhayavel, S.; Malmarugan, S.; Rajeswar, J. Antibiogram pattern of bacteria causing endometritis in cows. Vet. World 2013, 6, 100–102. [Google Scholar] [CrossRef]
- Koba, I.S.; Lysenko, A.A.; Koshchaev, A.G.; Rodin, I.A.; Shantyz, A.U. Effective treatment of chronic endometritis in cows by Florinazol preparation. Indian Vet. J. 2017, 94, 15–18. [Google Scholar]
- Rehman, S.U.; Feng, T.; Wu, S.; Luo, X.; Lei, A.; Luobu, B.; Hassan, F.-U.; Liu, Q. Comparative Genomics, Evolutionary and Gene Regulatory Regions Analysis of Casein Gene Family in Bubalus bubalis. Front. Genet. 2021, 12, 662609. [Google Scholar] [CrossRef]
- Rehman, S.U.; Nadeem, A.; Javed, M.; Hassan, F.-U.; Luo, X.; Khalid, R.B.; Liu, Q. Genomic Identification, Evolution and Sequence Analysis of the Heat-Shock Protein Gene Family in Buffalo. Genes 2020, 11, 1388. [Google Scholar] [CrossRef]
- Sabat, A.J.; van Zanten, E.; Akkerboom, V.; Wisselink, G.; van Slochteren, K.; de Boer, R.F.; Hendrix, R.; Friedrich, A.W.; Rossen, J.W.A.; Kooistra-Smid, A.M.D.M. Targeted next-generation sequencing of the 16S-23S rRNA region for culture-independent bacterial identification—increased discrimination of closely related species. Sci. Rep. 2017, 7, 3434. [Google Scholar] [CrossRef]
- Patel, J.B. 16S rRNA gene sequencing for bacterial pathogen identification in the clinical laboratory. Mol. Diagn. J. Devoted Underst. Hum. Dis. Clin. Appl. Mol. Biol. 2001, 6, 313–321. [Google Scholar] [CrossRef]
- Liu, J.; Guan, G.; Li, Y.; Liu, A.; Luo, J.; Yin, H. A Molecular Survey of Babesia Species and Detection of a New Babesia Species by DNA Related to B. venatorum from White Yaks in Tianzhu, China. Front. Microbiol. 2017, 8, 419. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, D.; Sarma, P.M.; Krishnan, S.; Mishra, S.; Lal, B. Evaluation of genetic diversity among Pseudomonas citronellolis strains isolated from oily sludge-contaminated sites. Appl. Environ. Microbiol. 2003, 69, 1435–1441. [Google Scholar] [CrossRef] [Green Version]
- Becker, K.; Harmsen, D.; Mellmann, A.; Meier, C.; Schumann, P.; Peters, G.; Von Eiff, C. Development and evaluation of a quality-controlled ribosomal sequence database for 16S ribosomal DNA-based identification of Staphylococcus species. J. Clin. Microbiol. 2004, 42, 4988–4995. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Liu, D.; Cao, B.; Han, W.; Liu, Y.; Liu, F.; Guo, X.; Bastin, D.A.; Feng, L.; Wang, L. Development of a serotype-specific DNA microarray for identification of some Shigella and pathogenic Escherichia coli strains. J. Clin. Microbiol. 2006, 44, 4376–4383. [Google Scholar] [CrossRef] [Green Version]
- Oladeinde, B.H.; Omoregie, R.; Olley, M.; Anunibe, J.A. Urinary tract infection in a rural community of Nigeria. N. Am. J. Med. Sci. 2011, 3, 75. [Google Scholar] [CrossRef]
- Mohanty, N.; Das, P.; Pany, S.; Sarangi, L.N.; Ranabijuli, S.; Panda, H. Isolation and antibiogram of Staphylococcus, Streptococcus and Escherichia coli isolates from clinical and subclinical cases of bovine mastitis. Vet. World 2013, 6, 739–743. [Google Scholar] [CrossRef] [Green Version]
- Haftu, R.; Menghistu, H.T.; Gugsa, G.; Kalayou, S. Prevalence, bacterial causes, and antimicrobial susceptibility profile of mastitis isolates from cows in large-scale dairy farms of Northern Ethiopia. Trop. Anim. Health Prod. 2012, 44, 1765–1771. [Google Scholar] [CrossRef]
- Kocyigit, R.; Yilmaz, O.; Özenc, E.; Mehmet, U. Effect of some risk factors on subclinical mastitis in dairy cows. Kocatepe Vet. Derg. 2016, 9, 185–193. [Google Scholar] [CrossRef]
- Wenz, J.R.; Barrington, G.M.; Garry, F.B.; McSweeney, K.D.; Dinsmore, R.P.; Goodell, G.; Callan, R.J. Bacteremia associated with naturally occuring acute coliform mastitis in dairy cows. J. Am. Vet. Med. Assoc. 2001, 219, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Najeeb, M.; Anjum, A.; Ahmad, M.-D.; Khan, H.; Ali, M.; Sattar, M.M.K. Bacterial etiology of subclinical mastitis in dairy goats and multiple drug resistance of the isolates. J. Anim. Plant. Sci. 2013, 23, 1541–1544. [Google Scholar]
- Hemmatinezhad, B.; Khamesipour, F.; Mohammadi, M.; Safarpoor Dehkordi, F.; Mashak, Z. Microbiological Investigation of O-Serogroups, Virulence Factors and Antimicrobial Resistance Properties of Shiga Toxin-Producing Escherichia Coli Isolated from Ostrich, Turkey and Quail Meats. J. Food Saf. 2015, 35, 491–500. [Google Scholar] [CrossRef]
- Dehkordi, F.S.; Yazdani, F.; Mozafari, J.; Valizadeh, Y. Virulence factors, serogroups and antimicrobial resistance properties of Escherichia coli strains in fermented dairy products. BMC Res. Notes 2014, 7, 217. [Google Scholar] [CrossRef]
- Shahrani, M.; Dehkordi, F.S.; Momtaz, H. Characterization of Escherichia coli virulence genes, pathotypes and antibiotic resistance properties in diarrheic calves in Iran. Biol. Res. 2014, 47, 28. [Google Scholar] [CrossRef] [Green Version]
- Bessalah, S.; Fairbrother, J.M.; Salhi, I.; Vanier, G.; Khorchani, T.; Seddik, M.M.; Hammadi, M. Antimicrobial resistance and molecular characterization of virulence genes, phylogenetic groups of Escherichia coli isolated from diarrheic and healthy camel-calves in Tunisia. Comp. Immunol. Microbiol. Infect. Dis. 2016, 49, 1–7. [Google Scholar] [CrossRef]
- Su, Y.; Yu, C.-Y.; Tsai, Y.; Wang, S.-H.; Lee, C.; Chu, C. Fluoroquinolone-resistant and extended-spectrum β-lactamase-producing Escherichia coli from the milk of cows with clinical mastitis in Southern Taiwan. J. Microbiol. Immunol. Infect. 2016, 49, 892–901. [Google Scholar] [CrossRef] [Green Version]
- Salmon, S.A.; Watts, J.L. Minimum inhibitory concentration determinations for various antimicrobial agents against 1570 bacterial isolates from turkey poults. Avian Dis. 2000, 1, 85–98. [Google Scholar] [CrossRef]
- San Millan, A.; Escudero, J.A.; Gifford, D.R.; Mazel, D.; MacLean, R.C. Multicopy plasmids potentiate the evolution of antibiotic resistance in bacteria. Nat. Ecol. Evol. 2016, 1, 10. [Google Scholar] [CrossRef]
- Ibrahem, E.J.; Yasin, Y.S.; Jasim, O.K. Antibacterial Activity of Zinc Oxide Nanoparticles Against Staphylococcus Aureus and Pseudomonas Aeruginosa Isolated from Burn Wound Infections. Cihan Univ. Sci. J. 2017, 10, 24086. [Google Scholar] [CrossRef] [Green Version]
- Chan, E.W.L.; Yee, Z.Y.; Raja, I.; Yap, J.K.Y. Synergistic effect of non-steroidal anti-inflammatory drugs (NSAIDs) on antibacterial activity of cefuroxime and chloramphenicol against methicillin-resistant Staphylococcus aureus. J. Glob. Antimicrob. Resist. 2017, 10, 70–74. [Google Scholar] [CrossRef]
- Diaz-Sanchez, S.; D’Souza, D.; Biswas, D.; Hanning, I. Botanical alternatives to antibiotics for use in organic poultry production1. Poult. Sci. 2015, 94, 1419–1430. [Google Scholar] [CrossRef]
- Palombo, E.A.; Semple, S.J. Antibacterial activity of Australian plant extracts against methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci (VRE). J. Basic Microbiol. 2002, 42, 444–448. [Google Scholar] [CrossRef]
- Punjabi, K.; Mehta, S.; Chavan, R.; Chitalia, V.; Deogharkar, D.; Deshpande, S. Efficiency of biosynthesized silver and zinc nanoparticles against multi-drug resistant pathogens. Front. Microbiol. 2018, 9, 2207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, S.; Vishakha, K.; Das, S.; Dutta, M.; Mukherjee, D.; Mondal, J.; Mondal, S.; Ganguli, A. Antibacterial, anti-biofilm activity and mechanism of action of pancreatin doped zinc oxide nanoparticles against methicillin resistant Staphylococcus aureus. Colloids Surf. B Biointerfaces 2020, 190, 110921. [Google Scholar] [CrossRef] [PubMed]
- Tateda, K.; Matsumoto, T.; Miyazaki, S.; Yamaguchi, K. Efficacy of β-lactam antibiotics combined with gentamicin against penicillin-resistant pneumococcal pneumonia in CBA/J mice. J. Antimicrob. Chemother. 1999, 43, 367–371. [Google Scholar] [CrossRef] [Green Version]
- Halassy, B.; Kurtović, T.; Brgles, M.; Lang Balija, M.; Forčić, D. Factors influencing preclinical in vivo evaluation of mumps vaccine strain immunogenicity. Hum. Vaccin. Immunother. 2015, 11, 2446–2454. [Google Scholar] [CrossRef] [Green Version]
- Bohn, T.; Carriere, F.; Day, L.; Deglaire, A.; Egger, L.; Freitas, D.; Golding, M.; Le Feunteun, S.; Macierzanka, A.; Menard, O.; et al. Correlation between in vitro and in vivo data on food digestion. What can we predict with static in vitro digestion models? Crit. Rev. Food Sci. Nutr. 2018, 58, 2239–2261. [Google Scholar] [CrossRef] [Green Version]
- Carfora, V.; Caprioli, A.; Marri, N.; Sagrafoli, D.; Boselli, C.; Giacinti, G.; Giangolini, G.; Sorbara, L.; Dottarelli, S.; Battisti, A.; et al. Enterotoxin genes, enterotoxin production, and methicillin resistance in Staphylococcus aureus isolated from milk and dairy products in Central Italy. Int. Dairy J. 2015, 42, 12–15. [Google Scholar] [CrossRef] [Green Version]
- Davies, S.N.; Martin, D.; Millar, J.D.; Aram, J.A.; Church, J.; Lodge, D. Differences in results from in vivo and in vitro studies on the use-dependency of N-methylaspartate antagonism by MK-801 and other phencyclidine receptor ligands. Eur. J. Pharmacol. 1988, 145, 141–151. [Google Scholar] [CrossRef]
- Nagadurga, D.H. Bioavailability and Bioequivalence Studies. In Pharmaceutical Formulation Design-Recent Practices; IntechOpen: London, UK, 2019. [Google Scholar]
- Voehringer, P.; Nicholson, J.R. Minimum Information in In Vivo Research BT—Good Research Practice in Non-Clinical Pharmacology and Biomedicine; Bespalov, A., Michel, M.C., Steckler, T., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 197–222. ISBN 978-3-030-33656-1. [Google Scholar]
- Ocampo, P.S.; Lázár, V.; Papp, B.; Arnoldini, M.; Abel zur Wiesch, P.; Busa-Fekete, R.; Fekete, G.; Pál, C.; Ackermann, M.; Bonhoeffer, S. Antagonism between bacteriostatic and bactericidal antibiotics is prevalent. Antimicrob. Agents Chemother. 2014, 58, 4573–4582. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Farm Name | Prevalence of Endometritis | Prevalence of E. coli | |||||
|---|---|---|---|---|---|---|---|
| No. of Animals Screened (A) | Endometritis Positive (B) | Prevalence (B/A × 100) | Confidence Interval (95%) | E. coli Positive (C) | E. coli % (C/B × 100) | Confidence Interval (95%) | |
| Usama Dairies | 27 | 9 | 33.33 | 18.64–52.17 | 9 | 100 | 70.09–100 |
| Sahu Dairies | 27 | 8 | 29.63 | 15.85–48.48 | 8 | 100 | 67.56–100 |
| United Dairies | 18 | 8 | 44.44 | 24.56–66.28 | 8 | 100 | 67.56–100 |
| Rajpoot Dairies | 34 | 14 | 41.18 | 26.37–57.78 | 5 | 35.71 | 16.34–61.23 |
| Horizon Dairies | 54 | 26 | 48.15 | 35.4–61.15 | 19 | 73.08 | 53.92–86.3 |
| Abdullah Dairies | 12 | 5 | 41.67 | 19.33–68.05 | 5 | 100 | 56.55–100 |
| Masab Dairies | 28 | 13 | 46.43 | 29.53–64.19 | 7 | 53.85 | 29.15–76.8 |
| Sifari Dairies | 48 | 26 | 54.167 | 40.29–67.43 | 13 | 50 | 32.06–67.94 |
| Sial Dairies | 38 | 16 | 42.10 | 27.86–57.81 | 14 | 87.50 | 63.98–96.5 |
| Hiraj Dairies | 18 | 8 | 44.44 | 24.56–66.28 | 8 | 100 | 67.56–100 |
| Total | 304 | 133 | 43.75 | 96 | 72.18 | ||
| Nucleotide | Frequency |
|---|---|
| A | 0.227 |
| C | 0.273 |
| T | 0.227 |
| G | 0.273 |
| Antibiotics | Potency (µg) | Resistant (%) | Intermediate (%) | Sensitive (%) |
|---|---|---|---|---|
| Fusidic acid | 10 | 80 | 20 | 0 |
| Enrofloxacin | 10 | 10 | 20 | 70 |
| Ciprofloxacin | 5 | 0 | 30 | 70 |
| Trimethoprim Sulfamethoxazole | 25 | 0 | 10 | 90 |
| Amoxicillin | 5 | 50 | 20 | 30 |
| Chloramphenicol | 30 | 0 | 30 | 70 |
| Vancomycin | 30 | 70 | 30 | 0 |
| Gentamicin | 10 | 10 | 40 | 50 |
| Linezolid | 30 | 40 | 30 | 30 |
| Cefoxitin | 30 | 30 | 30 | 40 |
| Drugs/Antibiotics Used | Mean ± Std. | p-Value | |
|---|---|---|---|
| Drug’s Name | Combination of Drugs | ||
| Co-amoxiclav | Alone | 7 ± 1.414 | 0.073 |
| C + E | 5 ± 1.414 | ||
| C + M | 6 ± 2.828 | ||
| C + G | 10.5 ± 2.121 | ||
| C + O | 10.5 ± 0.707 | ||
| C + A | 7.0 ± 1.414 | ||
| C + S | 8.5 ± 0.707 | ||
| Enrofloxacin | Alone | 5 ± 0.00 | 0.162 |
| E + M | 5 ± 1.414 | ||
| E + G | 3 ± 0.00 | ||
| E + O | 7.5 ± 2.121 | ||
| E + C | 5 ± 1.414 | ||
| E + A | 10.5 ± 6.364 | ||
| E + S | 10.0 ± 1.414 | ||
| Metronidazole | Alone | 3 ± 0 | 0.246 |
| M + G | 4.5 ± 0.707 | ||
| M + O | 7 ± 1.414 | ||
| M + C | 6 ± 2.828 | ||
| M + E | 5 ± 1.414 | ||
| M + A | 5.5 ± 0.707 | ||
| M + S | 8.5 ± 3.535 | ||
| Oxytetracycline | Alone | 4 ± 1.414 | 0.016 |
| O + C | 10.5 ± 0.707 | ||
| O + M | 7 ± 1.414 | ||
| O + E | 7.5 ± 2.121 | ||
| O + G | 6.5 ± 0.707 | ||
| O + A | 8.5 ± 0.707 | ||
| O + S | 9.5 ± 0.707 | ||
| Gentamicin | Alone | 10.5 ± 0.707 | 0.001 |
| G + O | 6.5 ± 0.707 | ||
| G + C | 10.5 ± 2.121 | ||
| G + E | 3 ± 0 | ||
| G + M | 4.5 ± 0.707 | ||
| G + A | 11.0 ± 1.414 | ||
| G + S | 10.5 ± 0.707 | ||
| Amoxicillin | Alone | 6.5 ± 0.707 | 0.344 |
| A + S | 6.5 ± 0.707 | ||
| A + C | 7.0 ± 1.414 | ||
| A + M | 5.5 ± 0.707 | ||
| A + O | 8.5 ± 0.707 | ||
| A + E | 10.5 ± 6.364 | ||
| A + G | 11.0 ± 1.414 | ||
| Streptomycin | Alone | 7.5 ± 0.707 | 0.266 |
| S + A | 6.5 ± 0.707 | ||
| S + C | 8.5 ± 0.707 | ||
| S + M | 8.5 ± 3.535 | ||
| S + O | 9.5 ± 0.707 | ||
| S + E | 10.0 ± 1.414 | ||
| S + G | 10.5 ± 0.707 | ||
| Combinations | MIC AB | MIC A | FIC A | MIC BA | MIC B | FIC B | FICI | Results |
|---|---|---|---|---|---|---|---|---|
| amoxi + co-amoxiclav | 23.4375 | 15.625 | 1.5 | 5.859375 | 7.8125 | 0.75 | 2.25 | Indifferent |
| amoxi + metro | 7.8125 | 15.625 | 0.5 | 62.5 | 187.5 | 0.333333 | 0.833333 | Additive |
| amoxi + enro | 5.859375 | 15.625 | 0.375 | 0.976563 | 1.513672 | 0.645161 | 1.020161 | Indifferent |
| amoxi + strepto | 5.859375 | 15.625 | 0.375 | 7.8125 | 18.22917 | 0.428571 | 0.803571 | Additive |
| amoxi + genta | 4.557292 | 15.625 | 0.291667 | 1.953125 | 2.929688 | 0.666667 | 0.958333 | Additive |
| amoxi + oxy | 31.25 | 15.625 | 2 | 31.25 | 23.4375 | 1.333333 | 3.333333 | Indifferent |
| co-amoxiclav + metro | 5.859375 | 7.8125 | 0.75 | 125 | 187.5 | 0.666667 | 1.416667 | Indifferent |
| co-amoxiclav + enro | 3.90625 | 7.8125 | 0.5 | 0.976563 | 1.513672 | 0.645161 | 1.145161 | Indifferent |
| co-amoxiclav + strepto | 2.929688 | 7.8125 | 0.375 | 15.625 | 18.22917 | 0.857143 | 1.232143 | Indifferent |
| co-amoxiclav + genta | 3.90625 | 7.8125 | 0.5 | 0.976563 | 2.929688 | 0.333333 | 0.833333 | Additive |
| co-amoxiclav + oxy | 7.8125 | 7.8125 | 1 | 31.25 | 23.4375 | 1.333333 | 2.333333 | Indifferent |
| metro + enro | 72.91667 | 187.5 | 0.388889 | 0.488281 | 1.513672 | 0.322581 | 0.71147 | Additive |
| metro + strepto | 250 | 187.5 | 1.333333 | 15.625 | 18.22917 | 0.857143 | 2.190476 | Indifferent |
| metro + genta | 125 | 187.5 | 0.666667 | 1.953125 | 2.929688 | 0.666667 | 1.333333 | Indifferent |
| metro + oxy | 250 | 187.5 | 1.333333 | 31.25 | 23.4375 | 1.333333 | 2.666667 | Indifferent |
| enro + strepto | 3.90625 | 1.513672 | 2.580645 | 20.50781 | 18.22917 | 1.125 | 3.705645 | Indifferent |
| enro + genta | 0.488281 | 1.513672 | 0.322581 | 1.953125 | 2.929688 | 0.666667 | 0.989247 | Additive |
| enro + oxy | 4.557292 | 1.513672 | 3.010753 | 31.25 | 23.4375 | 1.333333 | 4.344086 | Antagonistic |
| strepto + genta | 15.625 | 18.22917 | 0.857143 | 3.90625 | 2.929688 | 1.333333 | 2.190476 | Indifferent |
| strepto + oxy | 20.50781 | 18.22917 | 1.125 | 15.625 | 23.4375 | 0.666667 | 1.791667 | Indifferent |
| genta + oxy | 4.557292 | 2.929688 | 1.555556 | 31.25 | 23.4375 | 1.333333 | 2.888889 | Indifferent |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shafique, L.; Wu, S.; Aqib, A.I.; Ali, M.M.; Ijaz, M.; Naseer, M.A.; Sarwar, Z.; Ahmed, R.; Saleem, A.; Qudratullah; et al. Evidence-Based Tracking of MDR E. coli from Bovine Endometritis and Its Elimination by Effective Novel Therapeutics. Antibiotics 2021, 10, 997. https://doi.org/10.3390/antibiotics10080997
Shafique L, Wu S, Aqib AI, Ali MM, Ijaz M, Naseer MA, Sarwar Z, Ahmed R, Saleem A, Qudratullah, et al. Evidence-Based Tracking of MDR E. coli from Bovine Endometritis and Its Elimination by Effective Novel Therapeutics. Antibiotics. 2021; 10(8):997. https://doi.org/10.3390/antibiotics10080997
Chicago/Turabian StyleShafique, Laiba, Siwen Wu, Amjad Islam Aqib, Muhammad Muddassir Ali, Misbah Ijaz, Muhammad Aamir Naseer, Zaeem Sarwar, Rais Ahmed, Arslan Saleem, Qudratullah, and et al. 2021. "Evidence-Based Tracking of MDR E. coli from Bovine Endometritis and Its Elimination by Effective Novel Therapeutics" Antibiotics 10, no. 8: 997. https://doi.org/10.3390/antibiotics10080997
APA StyleShafique, L., Wu, S., Aqib, A. I., Ali, M. M., Ijaz, M., Naseer, M. A., Sarwar, Z., Ahmed, R., Saleem, A., Qudratullah, Ahmad, A. S., Pan, H., & Liu, Q. (2021). Evidence-Based Tracking of MDR E. coli from Bovine Endometritis and Its Elimination by Effective Novel Therapeutics. Antibiotics, 10(8), 997. https://doi.org/10.3390/antibiotics10080997