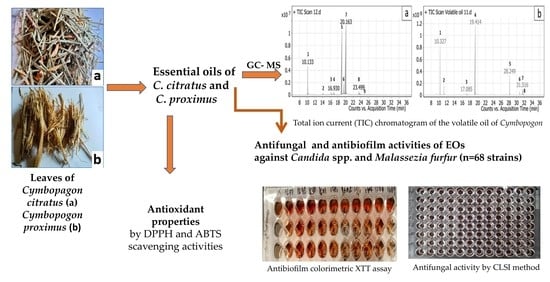
Antifungal, Antioxidant and Antibiofilm Activities of Essential Oils of Cymbopogon spp.
Abstract
:1. Introduction
2. Results
2.1. Chemical Composition of C. citratus and C. proximus Essential Oils
2.2. Antifungal Activity
2.3. Inhibitory Effects of Cymbopogon citratus and Cymbopogon proximus EOs on Candida spp. and Malassezia furfur Biofilms
2.4. In Vitro Antioxidant Activity of C. citratus and C. proximus EOs
3. Discussion
4. Materials and Methods
4.1. Plant Material and Essential Oil Isolation
4.2. Identification of the Chemical Composition of EOs by Gas Chromatography–Mass Spectrometry Analysis (GC-MS)
4.3. Antifungal Activities
4.3.1. Yeast Strains
4.3.2. Antifungal Activity
4.4. Inhibitory Effects of Cymbopogon citratus and Cymbopogon proximus EOs on Candida spp. and Malassezia furfur Biofilms
4.5. In Vitro Antioxidant Activity of C. citratus and C. proximus EOs
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Erb, M.; Kliebenstein, D.J. Plant Secondary Metabolites as Defenses, Regulators, and Primary Metabolites: The Blurred Functional Trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef]
- Iqbal, Z.; Iqbal, M.S.; Hashem, A.; Abd Allah, E.F.; Ansari, M.I. Plant defense responses to biotic stress and its interplay with fluctuating dark/light conditions. Front. Plant Sci. 2021, 12, 297. [Google Scholar] [CrossRef] [PubMed]
- Abegaz, B.M.; Kinfe, H.H. Secondary metabolites, their structural diversity, bioactivity, and ecological functions: An overview. Fundam. Concepts. 2020, 1, 7–54. [Google Scholar] [CrossRef]
- Lang, G.; Buchbauer, G. A review on recent research results (2008–2010) on essential oils as antimicrobials and antifungals. A review. Flavour. Fragr. J. 2012, 27, 13–39. [Google Scholar] [CrossRef]
- Evangelista, A.G.; Corrêa, J.A.F.; Pinto, A.C.S.M.; Luciano, F.B. The impact of essential oils on antibiotic use in animal production regarding antimicrobial resistance–a review. Crit. Rev. Food. Sci. Nutr. 2021, 8, 1–17. [Google Scholar] [CrossRef]
- Cafarchia, C.; De Laurentis, N.; Milillo, M.A.; Losacco, V.; Puccini, V. Antifungal activity of essential oils from leaves and flowers of Inula viscosa (Asteraceae) by Apulian region. Parassitologia 2002, 44, 153–156. [Google Scholar] [PubMed]
- Peixoto, L.R.; Rosalen, P.L.; Ferreira, G.L.S.; Freires, I.A.; de Carvalho, F.G.; Castellano, L.R.; De Castro, R.D. Antifungal activity, mode of action and anti-biofilm effects of Laurus nobilis Linnaeus essential oil against Candida spp. Arch. Oral. Biol. 2017, 73, 179–185. [Google Scholar] [CrossRef]
- Abd Rashed, A.; Rathi, D.N.G.; Ahmad Nasir, N.A.H.; Abd Rahman, A.Z. Antifungal properties of essential oils and their compounds for application in skin fungal infections: Conventional and nonconventional approaches. Molecules 2021, 26, 1093. [Google Scholar] [CrossRef]
- Rhimi, W.; Theelen, B.; Boekhout, T.; Aneke, C.I.; Otranto, D.; Cafarchia, C. Conventional therapy and new antifungal drugs against Malassezia infections. Med. Mycol. 2021, 59, 215–234. [Google Scholar] [CrossRef]
- Bismarck, D.; Dusold, A.; Heusinger, A.; Müller, E. Antifungal in vitro Activity of Essential Oils against Clinical Isolates of Malassezia pachydermatis from Canine Ears: A Report from a Practice Laboratory. Complement. Med. Res. 2020, 27, 143–154. [Google Scholar] [CrossRef]
- Siva Sai, C.; Mathur, N. Inhibitory Potential of Essential Oils on Malassezia strains by Various Plants. Biol. Life Sci. Forum 2020, 4, 46. [Google Scholar] [CrossRef]
- Saunte, D.; Gaitanis, G.; Hay, R.J. Malassezia-Associated Skin Diseases, the Use of Diagnostics and Treatment. Front. Cell. Infect. Microbiol. 2020, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- Eix, E.F.; Nett, J.E. How biofilm growth affects Candida-host interactions. Front Microbiol. 2020, 11, 1437. [Google Scholar] [CrossRef] [PubMed]
- Stephane, F.F.Y.; Juleshttps, B.K.J. Terpenoids as important bioactive constituents of essential oils. In Essential Oils-Bioactive Compounds, New Perspectives and Applications; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; Feo, V.D. Essential oils and antifungal activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avoseh, O.; Oyedeji, O.; Rungqu, P.; Nkeh-Chungag, B.; Oyedeji, A. Cymbopogon species; ethnopharmacology, phytochemistry and the pharmacological importance. Molecules 2015, 20, 7438. [Google Scholar] [CrossRef]
- Anggraeni, N.I.; Hidayat, I.W.; Rachman, S.D.; Ersanda. Bioactivity of essential oil from lemongrass (Cymbopogon citratus Stapf) as antioxidant agent. AIP Conf. Proc. 2018, 1927, 030007. [Google Scholar] [CrossRef]
- Al-Taweel, A.; Fawzy, G.; Perveen, S.; El Tahir, K. Gas chromatographic mass analysis and further pharmacological actions of Cymbopogon proximus essential oil. Drug. Res. 2013, 63, 484–488. [Google Scholar] [CrossRef]
- Selim, S.A. Chemical composition, antioxidant and antimicrobial activity of the essential oil and methanol extract of the Egyptian lemongrass Cymbopogon proximus Stapf. Grasas Aceites 2011, 62, 55–61. [Google Scholar] [CrossRef]
- Madi, Y.F.; Choucry, M.A.; Meselhy, M.R.; El-Kashoury, E.S.A. Essential oil of Cymbopogon citratus cultivated in Egypt: Seasonal variation in chemical composition and anticholinesterase activity. Nat. Prod. Res. 2020, 35, 4063–4067. [Google Scholar] [CrossRef]
- Mahmoud, E.A.M.; Al-Askalany, S.A.; Hanafy, E.A. Antioxidant, antibacterial and cytotoxic effect of Cymbopogon citratus, Mentha longifolia, and Artemisia absinthium essential oils. Egypt. J. Chem. 2022, 65, 287–296. [Google Scholar] [CrossRef]
- Shaikh, S.A.M.; Singh, B.G.; Barik, A.; Balaji, N.V.; Subbaraju, G.V.; Naik, D.B.; Pri-yadarsini, K.I. Unravelling the effect of β-diketo group modification on the antioxidant mech-anism of curcumin derivatives: A combined experimental and DFT approach. J. Mol. Struct. 2019, 1193, 166–176. [Google Scholar] [CrossRef]
- Menut, C.; Bessiere, J.M.; Samate, D.; Djibo, A.K.; Buchbauer, G.; Schopper, B. Aromatic plants of tropical west Africa. XI. chemical composition, antioxidant and antiradical properties of the essential oils of three Cymbopogon species from Burkina Faso. J. Essent. Oil Res. 2000, 12, 207–212. [Google Scholar] [CrossRef]
- El Tahir, K.E.H.; Abdel-Kader, M.S. Chemical and pharmacological study of Cymbopogon proximus volatile oil. Res. J. Med. Plant. 2008, 2, 53–60. [Google Scholar] [CrossRef] [Green Version]
- Boukhatem, M.N.; Ferhat, M.A.; Kameli, A.; Saidi, F.; Kebir, H.T. Lemon grass (Cymbopogon citratus) essential oil as a potent anti-inflammatory and antifungal drugs. Libyan. J. Med. 2014, 19, 25431. [Google Scholar] [CrossRef] [PubMed]
- Del Carmen Vázquez-Briones, M.; Hernández, L.R.; Guerrero-Beltrán, J.Á. Physicochemical and antioxidant properties of Cymbopogon citratus essential oil. J. Food Res. 2015, 4, 36. [Google Scholar] [CrossRef]
- Zabka, M.; Pavela, R.; Prokinova, E. Antifungal activity and chemical composition of twenty essential oils against significant indoor and outdoor toxigenic and aeroallergenic fungi. Chemosphere 2014, 112, 443–448. [Google Scholar] [CrossRef]
- Miron, D.; Battisti, F.; Silva, F.K.; Lana, A.D.; Pippi, B.; Casanova, B.; Simone, G.; Fuentefria, A.; Mayorga, P.; Schapoval, E.E. Antifungal activity and mechanism of action of monoterpenes against dermatophytes and yeasts. Rev. Bras. Farmacogn. 2014, 24, 660–667. [Google Scholar] [CrossRef]
- Sharma, Y.; Khan, L.A.; Manzoor, N. Anti-Candida activity of geraniol involves disruption of cell membrane integrity and function. J. Mycol. Med. 2016, 26, 244–254. [Google Scholar] [CrossRef]
- Iraji, A.; Yazdanpanah, S.; Alizadeh, F.; Mirza Mohammadi, S.; Ghasemi, Y.; Pakshir, K.; Yang, Y.; Zomorodian, K. Screening the antifungal activities of monoterpenes and their isomers against Candida species. J. Appl. Microbiol. 2020, 129, 1541–1551. [Google Scholar] [CrossRef]
- Pedroso, R.D.S.; Balbino, B.L.; Andrade, G.; Dias, M.C.P.S.; Alvarenga, T.A.; Pedroso, R.C.N.; Pimenta, L.P.; Lucarini, R.; Pauletti, P.M.; Januário, A.H.; et al. In vitro and in vivo anti-Candida spp. activity of plant-derived products. Plants 2019, 8, 494. [Google Scholar] [CrossRef] [Green Version]
- Silva, C.D.B.D.; Guterres, S.S.; Weisheimer, V.; Schapoval, E.E. Antifungal activity of the lemongrass oil and citral against Candida spp. Braz. J. Infect. Dis. 2008, 12, 63–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taweechaisupapong, S.; Aieamsaard, J.; Chitropas, P.; Khunkitti, W. Inhibitory effect of lemongrass oil and ts major constituents on Candida biofilm and germ tube formation. S. Afr. J. Bot. 2012, 81, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Viljoen, A.M.; Petkar, S.; Van Vuuren, S.F.; Figueiredo, A.C.; Pedro, L.G.; Barroso, J.G. The chemo-geographical variation in essential oil composition and the antimicrobial properties of “wild mint”–Mentha longifolia subsp. polyadena (Lamiaceae) in southern Africa. J. Essent. Oil. Res. 2006, 18, 60–65. [Google Scholar] [CrossRef]
- Jaradat, N.A.; Al Zabadi, H.; Rahhal, B.; Hussein, A.M.A.; Mahmoud, J.S.; Mansour, B.; Issa, A. The effect of inhalation of Citrus sinensis flowers and Mentha spicata leave essential oils on lung function and exercise performance: A quasi-experimental uncontrolled before and after study. J. Int. Soc. Sports. Nutr. 2016, 13, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chee, H.Y.; Lee, M.H. In vitro activity of celery essential oil against Malassezia furfur. Mycobiology 2009, 37, 67–68. [Google Scholar] [CrossRef] [Green Version]
- Ebani, V.V.; Mancianti, F. Use of Essential Oils in Veterinary Medicine to Combat Bacterial and Fungal Infections. Vet. Sci. 2020, 7, 193. [Google Scholar] [CrossRef]
- Guillot, J.; Bond, R. Malassezia yeasts in veterinary dermatology: An updated overview. Front. Cell. Infect. Microbiol. 2020, 10, 79. [Google Scholar] [CrossRef]
- Rhimi, W.; Theelen, B.; Boekhout, T.; Otranto, D.; Cafarchia, C. Malassezia spp. yeasts of emerging concern in fungemia. Front. Cell. Infect. Microbiol. 2020, 10, 370. [Google Scholar] [CrossRef]
- Peano, A.; Gallo, M.G. Management of Malassezia-related diseases in the dog. Parassitologia 2008, 50, 85–88. [Google Scholar] [PubMed]
- Almeida, L.D.F.; Paula, J.F.; Almeida, R.V.; Williams, D.W.; Hebling, J.; Cavalcanti, Y.W. Efficacy of citronella and cinnamon essential oils on Candida albicans biofilms. Acta Odontol. Scand. 2016, 74, 393–398. [Google Scholar] [CrossRef]
- Porfírio, E.M.; Melo, H.M.; Pereira, A.M.G.; Cavalcante, T.T.A.; Gomes, G.A.; Carvalho, M.G.D.; Júnior, F.E.A.C. In vitro antibacterial and antibiofilm activity of Lippia alba essential oil, citral, and carvone against Staphylococcus aureus. Sci. World J. 2017, 2017, 4962707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zieniuk, B.; Groborz, K.; Wołoszynowska, M.; Ratusz, K.; Białecka-Florjańczyk, E.; Fabiszewska, A. Enzymatic Synthesis of Lipophilic Esters of Phenolic Compounds, Evaluation of their Antioxidant Activity and Effect on the Oxidative Stability of Selected Oils. Biomolecules 2021, 11, 314. [Google Scholar] [CrossRef] [PubMed]
- Zielińska-Błajet, M.; Feder-Kubis, J. Monoterpenes and their derivatives—Recent development in biological and medical applications. Int. J. Mol. Sci. 2020, 21, 7078. [Google Scholar] [CrossRef] [PubMed]
- Hellali, N.; Mahammed, M.H.; Ramdane, F.; Talli, A. Antimicrobial and antioxidant activities of Cymbopogon schoenanthus (L.) spreng. essential oil, growing in Illizi-Algeria. J. Med. Plant Res. 2016, 10, 188–194. [Google Scholar] [CrossRef]
- Ong, K.S.; Mawang, C.I.; Daniel-Jambun, D.; Lim, Y.Y.; Lee, S.M. Current anti-biofilm strategies and potential of antioxidants in biofilm control. Expert. Rev. Anti. Infect. Ther. 2018, 16, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Gambino, M.; Cappitelli, F. Mini-review: Biofilm responses to oxidative stress. Biofouling 2016, 32, 167–178. [Google Scholar] [CrossRef]
- Gabrani, R.; Sharma, G.; Dang, S.; Gupta, S. Interplay among bacterial resistance, biofilm formation and oxidative stress for nosocomial infections. In Free Radicals in Human Health and Disease; Springer: New Delhi, India, 2015; pp. 369–379. [Google Scholar] [CrossRef]
- Seneviratne, C.J.; Wang, Y.; Jin, L.; Abiko, Y.; Samaranayake, L.P. Candida albicans biofilm formation is associated with increased anti-oxidative capacities. Proteomics 2008, 8, 2936–2947. [Google Scholar] [CrossRef]
- Gupta, P.; Gupta, H.; Poluri, K.M. Geraniol eradicates Candida glabrata biofilm by targeting multiple cellular pathways. Appl. Microbiol. Biotechnol. 2021, 105, 5589–5605. [Google Scholar] [CrossRef]
- Sun, L.; Liao, K.; Wang, D. Effects of magnolol and honokiol on adhesion, yeast-hyphal transition, and formation of biofilm by Candida albicans. PLoS ONE 2015, 10, e0117695. [Google Scholar] [CrossRef] [Green Version]
- Qian, W.; Liu, M.; Fu, Y.; Wang, T.; Zhang, J.; Yang, M.; Sun, Z.; Li, X.; Li, Y. Antimicrobial and Antibiofilm Activities of Citral Against Carbapenem-Resistant Enterobacter cloacae. Foodborne Pathog. Dis. 2020, 17, 459–465. [Google Scholar] [CrossRef]
- Lalko, J.; Api, A.M. Investigation of the Dermal Sensitization Potential of Various Essential oils in the Local Lymph Node Assay. Food Chem. Toxicol. 2006, 44, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Althurwi, H.N.; Abdel-Kader, M.S.; Alharthy, K.M.; Salkini, M.A.; Albaqami, F.F. Cymbopogon proximus essential oil protects rats against isoproterenol-induced cardiac hypertrophy and fibrosis. Molecules 2020, 25, 1786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhimi, W.; Aneke, C.I.; Annoscia, G.; Camarda, A.; Mosca, A.; Cantacessi, C.; Ottranto, D.; Cafarchia, C. Virulence and in vitro antifungal susceptibility of Candida albicans and Candida catenulata from laying hens. Int. Microbiol. 2021, 24, 57–63. [Google Scholar] [CrossRef]
- Iatta, R.; Figueredo, L.A.; Montagna, M.T.; Otranto, D.; Cafarchia, C. In vitro antifungal susceptibility of Malassezia furfur from bloodstream infections. J. Med. Microbiol. 2014, 63, 1467–1473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhimi, W.; Salem, I.B.; Iatta, R.; Chaabane, H.; Saidi, M.; Boulila, A.; Cafarchia, C. Dittrichia viscosa L. leaves lipid extract: An unexploited source of essential fatty acids and tocopherols with antifungal and anti-inflammatory properties. Ind. Crops Prod. 2018, 113, 196–201. [Google Scholar] [CrossRef]
- Pierce, C.G.; Uppuluri, P.; Tristan, A.R.; Wormley, F.L.; Mowat, E.; Ramage, G.; Lopez-Ribot, J.L. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat. Protoc. 2008, 3, 1494–1500. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Rhimi, W.; Hlel, R.; Ben Salem, I.; Boulila, A.; Rejeb, A.; Saidi, M. Dittrichia viscosa L. ethanolic extract based ointment with antiradical, antioxidant, and healing wound activities. Biomed. Res. Int. 2019, 2019, 4081253. [Google Scholar] [CrossRef] [Green Version]




| Peak No. | RT | Name | Formula | Classification | MS (M/e) | Area % | |||
|---|---|---|---|---|---|---|---|---|---|
| m/z | No Scans | Main Significant Fragments | Base Peak | ||||||
| 1 | 10.133 | β-Myrcene | C10H16 | monoterpenes | 136.23 | 18 | 69, 79, 93, 121 | 93 | 5.82 |
| 2 | 14.046 | Linalyl acetate | C12H20O2 | monoterpenes | 196.29 | 52 | 55, 69, 79, 93, 107, 121, 136, 150 | 93 | 0.58 |
| 3 | 16.289 | trans-Verbenol | C10H16O | monoterpenoid alcohol | 152.23 | 51 | 55, 67, 91, 109, 134 | 91 | 1.01 |
| 4 | 16.93 | Isoneral | C10H16O | monoterpenes | 152.23 | 51 | 55, 67, 81, 91, 109, 119, 134, 152 | 67, 81 | 1.26 |
| 5 | 19.082 | Z-Citral B (Neral) | C10H16O | monoterpenes | 152.24 | 45 | 69, 94, 134 | 69 | 37.49 |
| 6 | 19.568 | Nerol | C10H18O | monoterpenoid alcohol | 154.25 | 40 | 69, 79, 93, 121, 154 | 69 | 3.65 |
| 7 | 20.163 | E-Citral A (Geranial) | C10H16O | monoterpenes | 152.23 | 53 | 69, 84, 109, 152 | 69 | 48.2 |
| 8 | 23.499 | Grandlure II | C10H18O | monoterpenes | 154.25 | 75 | 55, 69, 79, 93, 121, 136, 154 | 69 | 1.91 |
| 9 | 24.993 | trans-α-Bergamotene | C15H24 | bicyclic sesquiterpenoids | 204.35 | 20 | 55, 69, 79, 93, 107, 119, 135, 161 | 93 | 0.07 |
| Total Identification | 99.99 | ||||||||
| Total monoterpenes | 99.92 | ||||||||
| Total sesquiterpenes | 0.07 | ||||||||
| Peak No. | RT | Name | Formula | Classification | MS (M/e) | Area % | |||
|---|---|---|---|---|---|---|---|---|---|
| m/z | No Scans | Main Significant Fragments | Base Peak | ||||||
| 1 | 10.327 | α-Terpinolene | C10H16 | monoterpenes | 136.23 | 18 | 93, 120 | 93 | 15.7 |
| 2 | 11.26 | cis-β-terpinyl acetate | C12H20O2 | monoterpenes | 196.28 | 22 | 68, 93 | 93 | 2.91 |
| 3 | 17.085 | α-Terpineol | C10H18O | monoterpenoid alcohol | 154.25 | 54 | 59, 93 | 93 | 1.44 |
| 4 | 19.414 | piperitone | C10H16O | monoterpenes | 152.23 | 52 | 69, 82, 109 | 82 | 66.99 |
| 5 | 28.249 | β-Elemol | C15H26O | sesquiterpenes | 222.37 | 52 | 59, 93, 161 | 59 | 5.87 |
| 6 | 30.818 | Selinenol | C15H26O | sesquiterpenes | 222.37 | 23 | 91, 133, 189 | 189 | 1.56 |
| 7 | 31.516 | β-Eudesmol | C15H26O | sesquiterpenes | 222.37 | 23 | 59, 91, 149, 204 | 59 | 2.42 |
| 8 | 31.636 | γ-Eudesmol | C15H26O | sesquiterpenes | 222.37 | 23 | 59, 91, 149, 204 | 59 | 3.11 |
| Total Identification | 100 | ||||||||
| Total monoterpenes | 87.04 | ||||||||
| Total sesquiterpenes | 12.96 | ||||||||
| Yeast spp. | MIC Values | C. citratus EO | C. proximus EO | FLZ | |||
|---|---|---|---|---|---|---|---|
| MIC µL/mL | MFC µL/mL | MIC µL/mL | MFC µL/mL | MIC µL/mL | MFC µL/mL | ||
| Candida tropicalis (n = 7) | Range | 2.5 | 2.5 | 20 | <20 | 4 | 4 |
| MIC90 | 2.5 | 2.5 | 20 | <20 | 4 | 4 | |
| Candida catenulate (n = 10) | Range | 2.5–5 | 5 | 20 | <20 | 8 | 8 |
| MIC90 | 5 | 5 | 20 | <20 | 8 | 8 | |
| Candida krusei (n = 10) | Range | 2.5 | 2.5 | 20 | <20 | >32 | >32 |
| MIC90 | 2.5 | 2.5 | 20 | <20 | >32 | >32 | |
| Candida guilliermondii (n = 10) | Range | 2.5–5 | 5 | 20 | <20 | 8 | 8 |
| MIC90 | 2.5 | 5 | 20 | <20 | 8 | 8 | |
| Candida albicans (n = 12) | Range | 2.5 | 2.5 | 20 | <20 | 4 | 4 |
| MIC90 | 2.5 | 2.5 | 20 | <20 | 4 | 4 | |
| Malassezia furfur (n = 9) | Range | 1.25 | 2.5 | 2.5 | 2.5 | >32 | >32 |
| MIC90 | 1.25 | 2.5 | 2.5 | 2.5 | >32 | >32 | |
| Candida parapsilosis (n = 8) | Range | 2.5 | 2.5 | 20 | <20 | 4 | 4 |
| MIC90 | 2.5 | 2.5 | 20 | <20 | 4 | 4 | |
| Candida parapsilosis ATCC 22019 | Range | 2.5 | 2.5 | 20 | <20 | 4 | 4 |
| MIC90 | 2.5 | 2.5 | 20 | <20 | 4 | 4 | |
| Candida krusei ATCC 6258 | Range | 2.5 | 2.5 | 20 | <20 | >32 | >32 |
| MIC90 | 2.5 | 2.5 | 20 | <20 | >32 | >32 | |
| C. tropcalis (n = 7) | C. catenulata (n = 10) | C. krusei (n = 10) | C. guillermondii (n = 10) | C. albicans (n = 12) | M. furfur (n = 9) | C. parapsilosis (n = 8) | C. krusei ATCC 6258 | C. parapsilosis ATCC 22019 | |
|---|---|---|---|---|---|---|---|---|---|
| EO C. proximus (80 µL/mL) | 71.56 ± 22.5 a | 43.14 ± 17.8 a,d | 64.86 ± 22.7 a,d | 77.36 ± 7.4 a,d | 35.94 ± 19.0 a | 64.47 ± 11.9 a | 78.77 ± 1.9 a | 94.96 ± 1.9 a | 78.77 ± 8.8 a |
| EO C. proximus (40 µL/mL) | 67.82 ± 12.8 b,c | 33.14 ± 18.5 b,c | 54.73 ± 19.3 b,c | 83.82 ± 16.5 b,c | 27.65 ± 11.7 b | 77.36 ± 8.2 b | 85.44 ± 20.7 b | 91.32 ± 3.9 b | 85.44 ± 2.0 b |
| EO C. citratus (20 µL/mL) | 90.42 ± 3.2 a,c | 83.64 ± 10.6 a,b | 96.39 ± 2.8 a,b | 93.64 ± 1.5 a,b | 94.85 ± 3.6 a,b | 74.01 ± 11.5 c | 93.68 ± 26.6 c | 95.32 ± 1.4 c | 93.68 ± 1.4 c |
| EO C. citratus (10 µL/mL) | 86.99 ± 5.4 b | 83.86 ± 10.3 c,d | 95.50 ± 2.5 d,c | 92.73 ± 2.0 c,d | 93.79 ± 4.30 c,d | 84.21 ± 5.1 a | 91.32 ± 24.7 d | 90.34 ± 0.5 d | 91.32 ± 1.9 d |
| FLZ (16 µL/mL) | 45.48 ± 3.4 a,b | 57.22 ± 5.3 a,b | 27.70 ± 15.3 a,b | 49.16 ± 7.0 a,b | 52.97 ± 5.9 a,b | 51.99 ± 15.1 a,b,c | 44.42 ± 25.6 a,b,c,d | 19.68 ± 13.1 a,b,c,d | 44.42 ± 6.8 a,b,c,d |
| Yeast Species | Collection Code | Origins |
|---|---|---|
| Candida tropicalis (n = 7) | CD1693, CD1694, CD 1695, CD1700, CD1701, CD1702, CD1703 | Lizards feces |
| Candida catenulata (n = 10) | CD 1777, CD1778, CD1568, CD1569, CD1575, CD1577, CD1578, CD1579, CD1580, CD1581 | Lizards, Laying hens feces |
| Candida krusei (n = 10) | CD 1631, CD 1635, CD1638, CD 1641, CD1642, CD 1645, CD 1650, CD 1651, CD 1659, CD 1661, CD1662 | Wild boars feces |
| Candida guilliermondii (n = 10) | CD 1606, CD 1644, CD 1653, CD 1675, CD1676, CD 1733, CD1738, CD1740, CD 1741, CD1743 | Lizards and wild boars feces |
| Candida albicans (n = 12) | CD1601, CD1613, CD1616, CD1618, CD1620, CD1637, CD 1721, CD1729, CD1730, CD1755, CD1757, CD1760 | Lizards and wild boar feces |
| Malassezia furfur (n = 9) | CD 1008, CD1009, CD1029, CD1030 CD1042, CD1043, CD1058, CD1490, CD1492 | Human skin |
| Candida parapsilosis (n = 8) | CD1679, CD1681, CD1682, CD1683, CD1684, CD1691, CD1735, CD1736 | Lizards and wild boar feces |
| Candida krusei | ATCC 6258 | American Type Culture Collection |
| Candida parapsilosis | ATCC 22019 | American Type Culture Collection |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rhimi, W.; Mohammed, M.A.; Zarea, A.A.K.; Greco, G.; Tempesta, M.; Otranto, D.; Cafarchia, C. Antifungal, Antioxidant and Antibiofilm Activities of Essential Oils of Cymbopogon spp. Antibiotics 2022, 11, 829. https://doi.org/10.3390/antibiotics11060829
Rhimi W, Mohammed MA, Zarea AAK, Greco G, Tempesta M, Otranto D, Cafarchia C. Antifungal, Antioxidant and Antibiofilm Activities of Essential Oils of Cymbopogon spp. Antibiotics. 2022; 11(6):829. https://doi.org/10.3390/antibiotics11060829
Chicago/Turabian StyleRhimi, Wafa, Mona A. Mohammed, Aya Attia Koraney Zarea, Grazia Greco, Maria Tempesta, Domenico Otranto, and Claudia Cafarchia. 2022. "Antifungal, Antioxidant and Antibiofilm Activities of Essential Oils of Cymbopogon spp." Antibiotics 11, no. 6: 829. https://doi.org/10.3390/antibiotics11060829
APA StyleRhimi, W., Mohammed, M. A., Zarea, A. A. K., Greco, G., Tempesta, M., Otranto, D., & Cafarchia, C. (2022). Antifungal, Antioxidant and Antibiofilm Activities of Essential Oils of Cymbopogon spp. Antibiotics, 11(6), 829. https://doi.org/10.3390/antibiotics11060829