Effects of the Amount and Type of Diol Ring Openers on the Properties of Oligolactide Acrylates for UV-Curable Printing Inks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
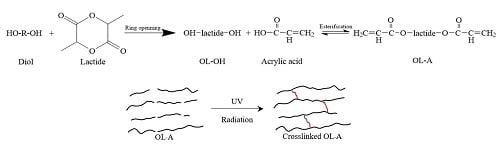
2.2. Synthesis of Oligolactide Diols (OL-OHs)
2.3. Synthesis of Oligolactide Acrylates (OL-As)
2.4. Characterization of Oligolactide Diols (OL-OHs) and Oligolactide Acrylates (OL-As)
2.5. Preparation of UV-Curable Screen Printing Inks
2.6. Characterizatin of UV-Curable Screen Printing Inks
3. Results and Discussion
3.1. Synthesis of Oligolactide Diols (OL-OHs)
3.2. Molecular Weights of Oligolactide Diols (OL-OHs)
3.3. Synthesis of Oligolactide Acrylates (OL-As)
3.4. Rheological Behavior of Oligolactide Acrylates
3.5. Thermal Properties of Lactide Oligomers
3.6. Properties of Screen Printing Inks
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wicks, Z.W., Jr.; Jones, F.N.; Pappas, S.P.; Wicks, D.A. Organic Coatings: Science and Technology, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Colak, S.; Küsefoğlu, S.H. Synthesis and interfacial properties of aminosilane derivative of acrylated epoxidized soybean oil. J. Appl. Polym. Sci. 2007, 104, 2244–2253. [Google Scholar] [CrossRef]
- Ali, M.A.; Ooi, T.L.; Salmiah, A.; Ishiaku, U.S.; Ishak, Z.A. New polyester acrylate resins from palm oil for wood coating application. J. Appl. Polym. Sci. 2001, 79, 2156–2163. [Google Scholar] [CrossRef]
- Zhang, D.; Liang, H.; Bu, J.; Xiong, L.; Huang, S.; Zhang, D.D.; Huang, S.M. UV curable soybean-oil hybrid systems based on thiol-acrylate and thiol-ene-acrylate chemistry. J. Appl. Polym. Sci. 2015, 132, 42095. [Google Scholar] [CrossRef]
- Sin, L.T.; Rahmat, A.R.; Rahman, W.A.W.A. Polylactic Acid: PLA Biopolymer Technology and Applications, 1st ed.; William Andrew Inc.: London, UK, 2012. [Google Scholar]
- Miller, K.R.; Soucek, M.D. Photopolymerization of biocompatible films containing poly (lactic acid). Eur. Polym. J. 2012, 48, 2107–2116. [Google Scholar] [CrossRef]
- Storey, R.F.; Warren, S.C.; Allison, C.J.; Puckett, A.D. Methacrylate-endcapped poly (d,l-lactide-co-trimethylene carbonate) oligomers. Network formation by thermal free-radical curing. Polymer 1997, 38, 6295–6301. [Google Scholar] [CrossRef]
- Shukla, V.; Bajpai, M.; Singh, D.K.; Singh, M.; Shukla, R. Review of basic chemistry of UV-curing technology. Pigm. Resin Technol. 2004, 33, 272–279. [Google Scholar] [CrossRef]
- Kulkarni, R.D.; Chaudhari, M.E.; Mishra, S. UV cure acrylate monomers: Synthesis, analysis and storage. Pigm. Resin Technol. 2013, 42, 53–67. [Google Scholar] [CrossRef]
- Tounthai, J.; Petchsuk, A.; Opaprakasit, P.; Opaprakasit, M. Curable polyester precursors from polylactic acid glycolyzed products. Polym. Bull. 2013, 70, 2223–2238. [Google Scholar] [CrossRef]
- Liu, J.; Peizhen, C.; Jiang, H.; Chen, L. Synthesis and chain extension of hydroxyl-terminated poly (lactic acid) oligomers and application in the blends. Polym. Compos. 2013, 34, 305–312. [Google Scholar] [CrossRef]
- Fenn, D.R.; Kulfan, J.L. Crosslinked Coatings Comprising Lactide. U.S. Patent 8,076,001, 13 December 2011. [Google Scholar]
- Izadi-Vasafi, H.; Sadeghi, M.; Mir, G.; Garmabi, H. Synthesis of hydroxyl-terminated poly (lactic acid) via polycondensation: An equation to predict molecular weight based on the reaction parameters. J. Appl. Polym. Sci. 2012, 125, E604–E605. [Google Scholar] [CrossRef]
- Kulsiriswad, S.; Srikulkit, K.; Saravari, O. An oligolactide Acrylate for UV-Curable Screen Printing Inks. J. Eng. Appl. Sci. 2016, 11, 2775–2780. [Google Scholar]
- Bishop, T.E.; Derks, F.J.; Tortorello, A. Radiation Curable Coating Composition. U.S. Patent 6,714,712, 30 March 2004. [Google Scholar]
- Fischer, W.; Margotte, D.; Meixner, J. Process for Preparing Esters of Ethylenically Unsaturated Carboxylic Acids. U.S. Patent 6,153,788, 28 November 2000. [Google Scholar]
- Reich, W.; Jager, U.; Beck, E.; Keil, E.; Erhardt, U.; Nuber, A. Preparation of Radiation-Curable Acrylates. U.S. Patent 5,516,860, 14 May 1996. [Google Scholar]
- ASTM D1639 Standard Test Method for Acid Value of Organic Coating Materials; ASTM International: West Conshohocken, PA, USA, 1996.
- Wu, J.; Zhang, R.; Ma, G.; Hou, C.; Zhang, H. Preparation and properties of fluorinated oligomer with tertiary amine structure in the UV curable coatings. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- ASTM D4752 Standard Practice for Measuring MEK Resistance of Ethyl Silicate (Inorganic) Zinc-Rich Primers by Solvent Rub; ASTM International: West Conshohocken, PA, USA, 2015.
- ASTM D3359 Standard Test Methods for Rating Adhesion by Tape Test; ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM D3363 Standard Test Method for Film Hardness by Pencil Test; ASTM International: West Conshohocken, PA, USA, 2011.
- ASTM D1647 Standard Test Methods for Resistance of Dried Films of Varnishes to Water and Alkali; ASTM International: West Conshohocken, PA, USA, 1996.
- Nikolic, L.; Ristic, I.; Adnadjevic, B.; Nikolic, V.; Jovanovic, J.; Stankovic, M. Novel microwave-assisted synthesis of poly (d,l-lactide): The influence of monomer/initiator molar ratio on the product properties. Sensors 2010, 10, 5063–5073. [Google Scholar] [CrossRef] [PubMed]
- Braun, B.; Dorgan, J.R.; Dec, S.F. Infrared spectroscopic determination of lactide concentration in polylactide: An improved methodology. Macromolecules 2006, 39, 9302–9310. [Google Scholar] [CrossRef]
- Korhonen, H.; Helminen, A.; Seppälä, J.V. Synthesis of polylactides in the presence of co-initiators with different numbers of hydroxyl groups. Polymer 2001, 42, 7541–7549. [Google Scholar] [CrossRef]
- Helminen, A.O.; Korhonen, H.; Seppälä, J.V. Structure modification and crosslinking of methacrylated polylactide oligomers. J. Appl. Polym. Sci. 2002, 86, 3616–3624. [Google Scholar] [CrossRef]
- Lokai, M.; Beck, E.; Reich, W.; Enenkel, P.; Marky, H.; Königer, R. (Meth) Acrylic Esters Containing Urethane Groups, Their Preparation, Radiation-Curable Coating Compositions and a Process for Preparing These Coating Compositions. U.S. Patent 6,319,983, 20 November 2001. [Google Scholar]
- Oprea, S.; Vlad, S.; Stanciu, A.; Macoveanu, M. Epoxy urethane acrylate. Eur. Polym. J. 2000, 36, 373–378. [Google Scholar] [CrossRef]
- Coullerez, G.R.; Lowe, C.; Pechy, P.; Kausch, H.H.; Hilborn, J.N. Synthesis of acrylate functional telechelic poly (lactic acid) oligomer by transesterification. J. Mater. Sci. Mater. M. 2000, 11, 505–510. [Google Scholar] [CrossRef]
- Schwalm, R. UV Coatings: Basics, Recent Developments and New Applications, 1st ed.; Elsevier: London, UK, 2006. [Google Scholar]












| Sample | l-Lactide (g) | Diol (g) | Stannous Octoate (g) | Toluene (g) |
|---|---|---|---|---|
| BDOH-900 | 100.00 | 11.11 | 0.28 | 11.11 |
| BDOH-1200 | 100.00 | 8.11 | 0.28 | 10.81 |
| BDOH-1500 | 100.00 | 6.38 | 0.28 | 10.64 |
| HDOH-900 | 100.00 | 15.07 | 0.28 | 11.51 |
| HDOH-1200 | 100.00 | 10.86 | 0.28 | 11.09 |
| HDOH-1500 | 100.00 | 8.58 | 0.28 | 10.86 |
| DDOH-900 | 100.00 | 24.07 | 0.29 | 12.41 |
| DDOH-1200 | 100.00 | 16.96 | 0.28 | 11.70 |
| DDOH-1500 | 100.00 | 13.12 | 0.28 | 11.31 |
| Sample | Theoretical | 1H-NMR | ||
|---|---|---|---|---|
| DP | Mn (g/mol) | DP | Mn (g/mol) | |
| BDOH-900 | 5.6 | 900 | 5.7 | 910 |
| BDOH-1200 | 7.7 | 1200 | 7.3 | 1146 |
| BDOH-1500 | 9.8 | 1500 | 8.7 | 1342 |
| HDOH-900 | 5.4 | 900 | 5.8 | 958 |
| HDOH-1200 | 7.5 | 1200 | 7.3 | 1176 |
| HDOH-1500 | 9.6 | 1500 | 9.0 | 1413 |
| DDOH-900 | 5.0 | 900 | 5.7 | 988 |
| DDOH-1200 | 7.1 | 1200 | 7.5 | 1259 |
| DDOH-1500 | 9.2 | 1500 | 9.1 | 1487 |
| Sample | 1H-NMR Integral | Degree of Functionalization (%) | |||
|---|---|---|---|---|---|
| I6.42 | I6.13 | I5.81 | I4.32 | ||
| BDA-900 | 1.00 | 0.93 | 0.99 | 0.57 | 63% |
| BDA-1200 | 1.00 | 0.94 | 1.01 | 0.83 | 54% |
| BDA-1500 | 1.00 | 0.97 | 1.02 | 1.04 | 49% |
| HAD-900 | 1.00 | 1.00 | 1.06 | 0.62 | 62% |
| HAD-1200 | 1.00 | 0.97 | 1.03 | 0.68 | 59% |
| HAD-1500 | 1.00 | 0.97 | 1.01 | 0.74 | 57% |
| DDA-900 | 1.00 | 0.96 | 1.02 | 0.46 | 69% |
| DDA-1200 | 1.00 | 0.97 | 1.02 | 0.59 | 63% |
| DDA-1500 | 1.00 | 0.91 | 0.98 | 0.75 | 56% |
| Sample | Acid Value (mg KOH/g) | Viscosity (mPa·s) | |
|---|---|---|---|
| Before Neutralization | After Neutralization | ||
| BDAN-900 | 14.6 | 4.6 | 5300 |
| BDAN-1200 | 12.3 | 4.1 | 13,000 |
| BDAN-1500 | 15.1 | 3.1 | 23,000 |
| HDAN-900 | 11.0 | 4.7 | 3700 |
| HDAN-1200 | 13.0 | 3.9 | 12,300 |
| HDAN-1500 | 14.6 | 4.5 | 18,300 |
| DDAN-900 | 10.4 | 4.3 | 2900 |
| DDAN-1200 | 10.5 | 5.1 | 8500 |
| DDAN-1500 | 13.3 | 4.4 | 13,900 |
| Original Oligolactide Diols | Tg (°C) | |||
|---|---|---|---|---|
| Diol | Acrylate | Neutralized Acrylate | Cured Ink | |
| BDOH-900 | 1.0 | −7.3 | 1.0 | 36.8 |
| BDOH-1200 | 11.2 | −2.1 | 10.3 | 30.3 |
| BDOH-1500 | 17.1 | −1.2 | 18.1 | 34.4 |
| HDOH-900 | −4.4 | −12.8 | −5.7 | 30.8 |
| HDOH-1200 | 6.2 | −1.9 | 6.6 | 35.3 |
| HDOH-1500 | 11.4 | −3.1 | 14.3 | 35.2 |
| DDOH-900 | −15.2 | −25.0 | −15.4 | 25.0 |
| DDOH-1200 | −0.8 | −8.2 | −0.9 | 33.2 |
| DDOH-1500 | 8.5 | −7.7 | 7.4 | 31.7 |
| Samples | Double Bond Conversion (%) |
|---|---|
| BDAN-900 | 95 |
| BDAN-1200 | 95 |
| BDAN-1500 | 96 |
| HDAN-900 | 94 |
| HDAN-1200 | 94 |
| HDAN-1500 | 96 |
| DCAN-900 | 94 |
| DCAN-1200 | 94 |
| DCAN-1500 | 92 |
| EB524 | 93 |
| Sample | MEK Double Rubs | Adhesion a | Flexibility (Ø/mm) | Impact Resistance (Inch-Pound) | Pencil Hardness | Water Resistance b |
|---|---|---|---|---|---|---|
| BDAN-900 | 200+ | 5B | 3 | 160 | 3H | Excellent c |
| BDAN-1200 | 200+ | 5B | 3 | 160 | 3H | Excellent c |
| BDAN-1500 | 200+ | 5B | 3 | 160 | 3H | Excellent c |
| HDAN-900 | 200+ | 5B | 3 | 160 | 3H | Excellent c |
| HDAN-1200 | 200+ | 5B | 3 | 160 | 3H | Excellent c |
| HDAN-1500 | 200+ | 5B | 3 | 160 | 3H | Excellent c |
| DDAN-900 | 200+ | 5B | 3 | 160 | 3H | Excellent c |
| DDAN-1200 | 200+ | 5B | 3 | 160 | 3H | Excellent c |
| DDAN-1500 | 200+ | 5B | 3 | 160 | 3H | Excellent c |
| EB524 | 50 | 5B | 3 | 160 | 2H | Excellent c |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulsiriswad, S.; Srikulkit, K.; Saravari, O. Effects of the Amount and Type of Diol Ring Openers on the Properties of Oligolactide Acrylates for UV-Curable Printing Inks. Coatings 2017, 7, 174. https://doi.org/10.3390/coatings7100174
Kulsiriswad S, Srikulkit K, Saravari O. Effects of the Amount and Type of Diol Ring Openers on the Properties of Oligolactide Acrylates for UV-Curable Printing Inks. Coatings. 2017; 7(10):174. https://doi.org/10.3390/coatings7100174
Chicago/Turabian StyleKulsiriswad, Santi, Kawee Srikulkit, and Onusa Saravari. 2017. "Effects of the Amount and Type of Diol Ring Openers on the Properties of Oligolactide Acrylates for UV-Curable Printing Inks" Coatings 7, no. 10: 174. https://doi.org/10.3390/coatings7100174
APA StyleKulsiriswad, S., Srikulkit, K., & Saravari, O. (2017). Effects of the Amount and Type of Diol Ring Openers on the Properties of Oligolactide Acrylates for UV-Curable Printing Inks. Coatings, 7(10), 174. https://doi.org/10.3390/coatings7100174