Facile Preparation of Bilayer Titanium Silicate (TS-1) Zeolite Membranes by Periodical Secondary Growth
Abstract
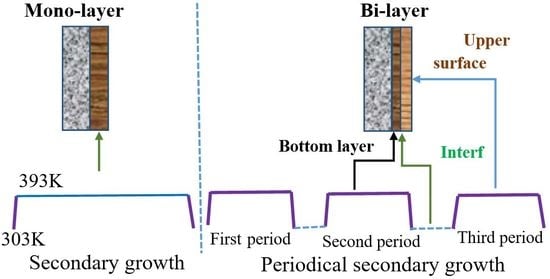
:1. Introduction
2. Experimental
2.1. Preparation
2.2. Characterization
2.3. Membrane Desalination
3. Results and Discussion
3.1. Crystal Structure and Morphology
3.2. Pore Distribution of the TS-1 Membrane
3.3. Elemental Composition and Hydrophobicity
3.4. Membrane Desalination Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lew, C.M.; Cai, R.; Yan, Y. Zeolite Thin Films: From Computer Chips to Space Stations. Acc. Chem. Res. 2010, 43, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Varoon, K.; Zhang, X.; Elyassi, B.; Brewer, D.D.; Gettel, M.; Kumar, S.; Lee, J.A.; Maheshwari, S.; Mittal, A.; Sung, C.Y.; et al. Dispersible Exfoliated Zeolite Nanosheets and Their Application as a Selective Membrane. Science 2011, 334, 72–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsapatsis, M. Toward High-Throughput Zeolite Membranes. Science 2011, 334, 767–768. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.C.T.; Kim, H.S.; Yoon, K.B. Growth of Uniformly Oriented Silica MFI and BEA Zeolite Films on Substrates. Science 2011, 334, 1533–1538. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.; Bonilla, G.; Diaz, I.; Nery, J.G.; Sujaoti, K.; Amat, M.A.; Kokkoli, E.; Terasaki, O.; Thompson, R.W.; Tsapatsis, M.; et al. Microstructural Optimization of A Zeolite Membrane for Organic Vapor Separation. Science 2003, 300, 456–460. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Jeong, H.K.; Snyder, M.A.; Stoeger, J.A.; Masel, R.I.; Tsapatsis, M. Grain Boundary Defect Elimination in a Zeolite Membrane by Rapid Thermal Processing. Science 2009, 325, 590–593. [Google Scholar] [CrossRef]
- Zhou, M.; Korelskiy, D.; Ye, P.; Grahn, M.; Hedlund, J. A Uniformly Oriented MFI Membrane for Improved CO2 Separation. Angew. Chem. Int. Ed. 2014, 53, 3492–3495. [Google Scholar] [CrossRef]
- Wang, X.D.; Zhang, B.Q.; Liu, X.F.; Lin, J.Y.S. Synthesis of B-Oriented TS-1 Films on Chitosan-Modified α-Al2O3 Substrates. Adv. Mater. 2006, 18, 3261–3265. [Google Scholar] [CrossRef]
- Chen, X.; Chen, P.; Kita, H. Pervaporation through TS-1 Membrane. Microporous Mesoporous Mater. 2008, 115, 164–169. [Google Scholar] [CrossRef]
- Chen, P.; Chen, X.; Chen, X.; Kita, H. Preparation and Catalytic Activity of Titanium Silicalite-1 Zeolite Membrane with TPABr as Template. J. Membr. Sci. 2009, 330, 369–378. [Google Scholar] [CrossRef]
- Motuzas, J.; Mikutaviciute, R.; Gerardin, E.; Julbe, A. Controlled Growth of Thin and Uniform TS-1 Membranes by MW-Assisted Heating. Microporous Mesoporous Mater. 2010, 128, 136–143. [Google Scholar] [CrossRef]
- Wang, H.; Dong, X.; Lin, Y.S. Highly Stable Bilayer MFI Zeolite Membranes for High Temperature Hydrogen Separation. J. Membr. Sci. 2014, 450, 425–432. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, C.; Lang, L.; Cui, R.; Liu, X. Selective Defect-Patching of Zeolite Membranes Using Chemical Liquid Deposition at Organic/Aqueous Interfaces. Adv. Funct. Mater. 2008, 18, 3434–3443. [Google Scholar] [CrossRef]
- Carreon, M.A.; Li, S.; Falconer, J.L.; Noble, R.D. Alumina-Supported SAPO-34 Membranes for CO2/CH4 Separation. J. Am. Chem. Soc. 2008, 130, 5412–5413. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.H.; Oh, K.Y.; Kim, S.K.; Yeo, J.G.; Lee, Y.M. Improvement in Thermal Stability of NaA Zeolite Composite Membrane by Control of Intermediate Layer Structure. J. Membr. Sci. 2011, 366, 229–236. [Google Scholar] [CrossRef]
- Jeong, H.K.; Lai, Z.; Tsapatsis, M.; Hanson, J.C. Strain of MFI Crystals in Membranes: An In Situ Synchrotron X-ray Study. Microporous Mesoporous Mater. 2005, 84, 332–337. [Google Scholar] [CrossRef]
- Kim, E.; Choi, J.; Tsapatsis, M. On Defects in Highly a-Oriented MFI Membranes. Microporous Mesoporous Mater. 2013, 170, 1–8. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Xu, L.; Zhang, B.; Ma, L. Spreading-Wetting Method for Highly Reproducible Tertiary Growth of Perfective Bilayer TS-1 Membranes. Appl. Surf. Sci. 2015, 343, 77–87. [Google Scholar] [CrossRef]
- Karanikolos, G.N.; Wydra, J.W.; Stoeger, J.A.; García, H.; Corma, A.; Tsapatsis, M. Continuous c-Oriented AlPO4-5 Films by Tertiary Growth. Chem. Mater. 2007, 19, 792–797. [Google Scholar] [CrossRef]
- Huang, A.; Liang, F.; Steinbach, F.; Gesing, T.M.; Caro, J. Neutral and Cation-Free LTA-Type Aluminophosphate (AlPO4) Molecular Sieve Membrane with High Hydrogen Permselectivity. J. Am. Chem. Soc. 2010, 132, 2140–2141. [Google Scholar] [CrossRef]
- Ng, E.-P.; Chateigner, D.; Bein, T.; Valtchev, V.; Mintova, S. Capturing Ultrasmall EMT Zeolite from Template-Free Systems. Science 2012, 335, 70–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lupulescu, A.I.; Rimer, J.D. In Situ Imaging of Silicalite-1 Surface Growth Reveals the Mechanism of Crystallization. Science 2014, 344, 729–732. [Google Scholar] [CrossRef]
- Lang, L.; Liu, X.; Jiang, H.; Lin, J.Y.S.; Zhang, B. Direct Evidence of the Evolutionary Mechanism of Zeolite Monolayers on the Substrate Surface in a Hydrothermal Reaction. Langmuir 2010, 26, 5895–5900. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, Z.; Bozhilov, K.N.; Chen, Z.; Yan, Y. TEM Investigation of Formation Mechanism of Monocrystal-Thick b-Oriented Pure Silica Zeolite MFI Film. J. Am. Chem. Soc. 2004, 126, 10732–10737. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pera-Titus, M.; Xiong, G.; Yang, W.; Landrivon, E.; Miachon, S.; Dalmon, J.A. Nanocomposite MFI-Alumina Membranes via Pore-Plugging Synthesis: Genesis of the Zeolite Material. J. Membr. Sci. 2008, 325, 973–981. [Google Scholar] [CrossRef]
- Yan, Y.; Chaudhuri, S.R.; Sarkar, A. Synthesis of Oriented Zeolite Molecular Sieve Films with Controlled Morphologies. Chem. Mater. 1996, 8, 473–479. [Google Scholar] [CrossRef]
- Zhang, X.L.; Qiu, L.F.; Ding, M.Z.; Hu, N.; Zhang, F.; Zhou, R.F.; Chen, X.S.; Kita, H. Preparation of Zeolite T Membranes by a Two-Step Temperature Process for CO2 Separation. Ind. Eng. Chem. Res. 2013, 52, 16364–16374. [Google Scholar] [CrossRef]
- Fane, A.G.; Wang, R.; Hu, M.X. Synthetic Membranes for Water Purification: Status and Future. Angew. Chem. Int. Ed. 2015, 54, 3368–3386. [Google Scholar] [CrossRef]
- Zhu, B.; Hong, Z.; Milne, N.; Doherty, C.M.; Zou, L.; Lin, Y.S.; Hill, A.J.; Gu, X.; Duke, M. Desalination of Seawater Ion Complexes by MFI-Type Zeolite Membranes: Temperature and Long Term Stability. J. Membr. Sci. 2014, 453, 126–135. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Liu, X.; Zhang, B. Preparation and Properties of Porous α-Al2O3 Based Ceramic Disk Substrates. J. Inorg. Mater. 2013, 28, 599–604. [Google Scholar] [CrossRef]
- Liu, X.; Ma, X.; Liu, Y.; Zhang, B. Preparation of Perfective TAPO-5 Membrane through Tertiary Growth with Amorphous Seed. J. Inorg. Mater. 2015, 30, 555–560. [Google Scholar] [CrossRef]
- Deng, X.; Wang, Y.; Shen, L.; Wu, H.; Liu, Y.; He, M. Low-Cost Synthesis of Titanium Silicalite-1 (TS-1) with Highly Catalytic Oxidation Performance through a Controlled Hydrolysis Process. Ind. Eng. Chem. Res. 2013, 52, 1190–1196. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, P.; Liu, X.; Zhang, B. Fabrication and Characterization of TS-1 Films on α-Al2O3 Substrates Using TiCl3 as Titanium Source. Appl. Surf. Sci. 2007, 254, 544–547. [Google Scholar] [CrossRef]
- Liu, X.; Xu, L.; Zhang, B.; Liu, X. Template Removal from AFI Aluminophosphate Molecular Sieve by Pd/SiO2 Catalytic Hydrocracking at Mild Temperature. Microporous Mesoporous Mater. 2014, 193, 127–133. [Google Scholar] [CrossRef]
- Kanezashi, M.; O’Brien, J.; Lin, Y.S. Template-Free Synthesis of MFI-Type Zeolite Membranes: Permeation Characteristics and Thermal Stability Improvement of Membrane Structure. J. Membr. Sci. 2006, 286, 213–222. [Google Scholar] [CrossRef]
- Xiao, W.; Chen, Z.; Zhou, L.; Yang, J.; Lu, J.; Wang, J. A simple Seeding Method for MFI Zeolite Membrane Synthesis on Macroporous Support by Microwave Heating. Microporous Mesoporous Mater. 2011, 142, 154–160. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Cui, R.; Zhang, B. In Situ Evaluation of Defect Size distribution for Supported Zeolite Membranes. J. Membr. Sci. 2009, 330, 259–266. [Google Scholar] [CrossRef]
- Duke, M.C.; O’Brien-Abraham, J.; Milne, N.; Zhu, B.; Lin, J.Y.S.; Diniz da Costa, J.C. Seawater Desalination Performance of MFI Type Membranes Made by Secondary Growth. Sep. Purif. Technol. 2009, 68, 343–350. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Cui, R.; Zhang, B. Formation and Reparation of Defects in Zeolite Membranes. Prog. Chem. 2008, 20, 1860–1867. [Google Scholar]
- Lang, L.; Liu, X.; Zhang, B. Synthesis and Characterization of (h0h)-Oriented Silicalite-1 Films on α-Al2O3 Substrates. Appl. Surf. Sci. 2008, 254, 2353–2358. [Google Scholar] [CrossRef]
- Weyd, M.; Richter, H.; Puhlfürß, P.; Voigt, I.; Hamel, C.; Seidel-Morgenstern, A. Transport of Binary Water–Ethanol Mixtures through a Multilayer Hydrophobic Zeolite Membrane. J. Membr. Sci. 2008, 307, 239–248. [Google Scholar] [CrossRef]
- Das, S.K. General Dusty Gas Model for Porous Media with a Specified Pore Size Distribution. Chem. Eng. Sci. 2019, 203, 293–301. [Google Scholar] [CrossRef]
- Ma, X.; Wang, H.; Wang, H.; Brien-Abraham, J.O.; Lin, Y.S. Pore Structure Characterization of Supported Polycrystalline Zeolite Membranes by Positron Annihilation Spectroscopy. J. Membr. Sci. 2015, 477, 41–48. [Google Scholar] [CrossRef] [Green Version]












| Methods | Route in Figure 1 | Crystallization Pattern | Synthesis Gel |
|---|---|---|---|
| Secondary growth | A | Continuous | original |
| Periodical secondary growth | B | Periodical crystallization | original |
| Tertiary growth | C | Periodical crystallization | refreshed |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Liu, Y.; Liu, X.; Ma, L. Facile Preparation of Bilayer Titanium Silicate (TS-1) Zeolite Membranes by Periodical Secondary Growth. Coatings 2019, 9, 850. https://doi.org/10.3390/coatings9120850
Zhang Q, Liu Y, Liu X, Ma L. Facile Preparation of Bilayer Titanium Silicate (TS-1) Zeolite Membranes by Periodical Secondary Growth. Coatings. 2019; 9(12):850. https://doi.org/10.3390/coatings9120850
Chicago/Turabian StyleZhang, Qi, Yong Liu, Xuguang Liu, and Laibo Ma. 2019. "Facile Preparation of Bilayer Titanium Silicate (TS-1) Zeolite Membranes by Periodical Secondary Growth" Coatings 9, no. 12: 850. https://doi.org/10.3390/coatings9120850
APA StyleZhang, Q., Liu, Y., Liu, X., & Ma, L. (2019). Facile Preparation of Bilayer Titanium Silicate (TS-1) Zeolite Membranes by Periodical Secondary Growth. Coatings, 9(12), 850. https://doi.org/10.3390/coatings9120850