Precise Dose of Folic Acid Supplementation Is Essential for Embryonic Heart Development in Zebrafish
Abstract
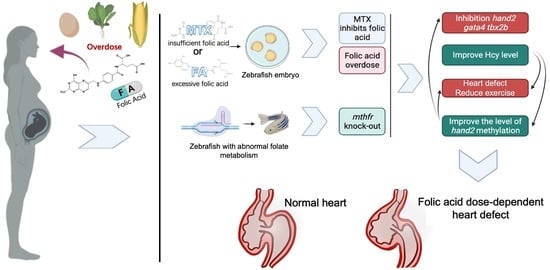
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Zebrafish Maintenance and Care
2.2. Visible Light Imaging and Fluorescence Imaging of Zebrafish Embryos
2.3. Microinjection of Zebrafish Embryos with CRISPR/Cas9 Knock-Out
2.4. Protein Structure Prediction
2.5. Cardiac Physiology Measurement
2.6. In Situ Hybridization
2.7. Quantitative Real-Time PCR
2.8. Luciferase Assays
2.9. Statistical Analysis
2.10. Behavioral Test
2.11. S-Adenosylmethionine Content Determination
2.12. Assessment of DNA Methylation Level
3. Results
3.1. Folic Acid and MTX Showed Dose-Dependent Effects on Early Cardiac Development in Zebrafish
3.2. Insufficient or Excessive Folic Acid Leads to Heart Looping Defect and Abnormal Homocysteine Metabolism
3.3. Abnormal Folate Metabolism Leads to Behavior Disorder of Zebrafish Embryos
3.4. CRISPR/Cas9 Mediated mthfr Gene Knock-Out Model in Zebrafish
3.5. Folic Acid Deficiency and Excessive Supplement Lead to the Changes of Heart Related Genes
3.6. Folate Metabolism Changes Defect the Methylation of hand2
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verhoef, P.; Stampfer, M.J. Prospective Studies of Homocysteine and Cardiovascular Disease. Nutr. Rev. 1995, 53, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Bethke, L.; Webb, E.; Murray, A.; Schoemaker, M.; Feychting, M.; Lönn, S.; Ahlbom, A.; Malmer, B.; Henriksson, R.; Auvinen, A.; et al. Functional Polymorphisms in Folate Metabolism Genes Influence the Risk of Meningioma and Glioma. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1195–1202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deloughery, T. Common mutation in methylenetetrahydrofolate reductase. Correlation with homocysteine metabolism and late-onset vascular disease. Circulation 1996, 94, 3074. [Google Scholar] [CrossRef] [PubMed]
- Morse, N.L. Benefits of Docosahexaenoic Acid, Folic Acid, Vitamin D and Iodine on Foetal and Infant Brain Development and Function Following Maternal Supplementation during Pregnancy and Lactation. Nutrients 2012, 4, 799–840. [Google Scholar] [CrossRef] [Green Version]
- Christensen, K.E.; Mikael, L.G.; Leung, K.-Y.; Lévesque, N.; Deng, L.; Wu, Q.; Malysheva, O.V.; Best, A.; Caudill, M.A.; Greene, N.D.E.; et al. High folic acid consumption leads to pseudo-MTHFR deficiency, altered lipid metabolism, and liver injury in mice. Am. J. Clin. Nutr. 2015, 101, 646–658. [Google Scholar] [CrossRef] [Green Version]
- Plumptre, L.; Masih, S.P.; Ly, A.; Aufreiter, S.; Sohn, K.-J.; Croxford, R.; Lausman, A.Y.; Berger, H.; O’Connor, D.; Kim, Y.-I. High concentrations of folate and unmetabolized folic acid in a cohort of pregnant Canadian women and umbilical cord blood. Am. J. Clin. Nutr. 2015, 102, 848–857. [Google Scholar] [CrossRef]
- Murray, L.K.; Smith, M.J.; Jadavji, N.M. Maternal oversupplementation with folic acid and its impact on neurodevelopment of offspring. Nutr. Rev. 2018, 76, 708–721. [Google Scholar] [CrossRef]
- Scher, A.I.; Terwindt, G.M.; Verschuren, W.M.M.; Kruit, M.; Blom, H.; Kowa, H.; Frants, R.R.; van den Maagdenberg, A.M.J.M.; Van Buchem, M.A.; Ferrari, M.D.; et al. Migraine and MTHFR C677T genotype in a population-based sample. Ann. Neurol. 2005, 59, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Fan, S.; Zhi, X.; Wang, D.; Li, Y.; Wang, Y.; Wang, Y.; Wei, J.; Zheng, Q.; Sun, G. Associations of MTHFR C677T and MTRR A66G Gene Polymorphisms with Metabolic Syndrome: A Case-Control Study in Northern China. Int. J. Mol. Sci. 2014, 15, 21687–21702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, J.; Ye, J.; Kamphorst, J.; Shlomi, T.; Thompson, C.B.; Rabinowitz, J.D. Quantitative flux analysis reveals folate-dependent NADPH production. Nature 2014, 510, 298–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hobbs, C.A.; Cleves, M.A.; Macleod, S.L.; Erickson, S.W.; Tang, X.; Li, J.; Li, M.; Nick, T.; Malik, S. National Birth Defects Prevention, Conotruncal heart defects and common variants in maternal and fetal genes in folate, homocysteine, and transsulfuration pathways. Birth. Defects Res. A Clin. Mol. Teratol. 2014, 100, 116–126. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Wang, F.; Shi, K.-H.; Tao, H.; Li, Y.; Zhao, R.; Lu, H.; Duan, W.; Qiao, B.; Zhao, S.-M.; et al. Lower Circulating Folate Induced by a Fidgetin Intronic Variant Is Associated with Reduced Congenital Heart Disease Susceptibility. Circulation 2017, 135, 1733–1748. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Sharma, V.; Kumar, A.; Thakur, A.; Bajwa, K.K.; Malakar, D. 170 Effect of Folic Acid Supplementation on In Vitro Maturation of Oocytes and Folate Cycle. Reprod. Fertil. Dev. 2018, 30, 224–225. [Google Scholar] [CrossRef]
- Westerfield, M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish. 2000. Available online: http://zfin.org/zf_info/zfbook/zfbk.html (accessed on 21 September 2020).
- Liu, D.; Wang, Z.; Xiao, A.; Zhang, Y.; Li, W.; Zu, Y.; Yao, S.; Lin, S.; Zhang, B. Efficient Gene Targeting in Zebrafish Mediated by a Zebrafish-Codon-Optimized Cas9 and Evaluation of Off-Targeting Effect. J. Genet. Genom. 2014, 41, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qin, W.; Lu, X.; Xu, J.; Huang, H.; Bai, H.; Li, S.; Lin, S. Programmable base editing of zebrafish genome using a modified CRISPR-Cas9 system. Nat. Commun. 2017, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Zu, Y.; Li, Z.; Li, W.; Ying, L.; Yang, J.; Wang, X.; He, S.; Liu, D.; Zhu, Z.; et al. Kctd10 regulates heart morphogenesis by repressing the transcriptional activity of Tbx5a in zebrafish. Nat. Commun. 2014, 5, 3153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aquilina-Beck, A.; Ilagan, K.; Liu, Q.; Liang, J.O. Nodal signaling is required for closure of the anterior neural tube in zebrafish. BMC Dev. Biol. 2007, 7, 126. [Google Scholar] [CrossRef] [Green Version]
- Jones, P.A. Functions of DNA methylation: Islands, start sites, gene bodies and beyond. Nat. Rev. Genet. 2012, 13, 484–492. [Google Scholar] [CrossRef]
- Lowery, L.A.; Sive, H. Strategies of vertebrate neurulation and a re-evaluation of teleost neural tube formation. Mech. Dev. 2004, 121, 1189–1197. [Google Scholar] [CrossRef]
- Polar, P.; Kairo, M.T.; Peterkin, D.; Moore, D.; Pegram, R.; John, S.-A. Assessment of Fungal Isolates for Development of a MycoAcaricide for Cattle Tick Control. Vector-Borne Zoonotic Dis. 2005, 5, 276–284. [Google Scholar] [CrossRef]
- Le Nôtre, J.; Brissieux, L.; Sémeril, D.; Bruneau, C.; Dixneuf, P.H. Tandem isomerization/Claisen transformation of allyl homoallyl and diallyl ethers into gamma, delta-unsaturated aldehydes with a new three component catalyst Ru-3(CO)(12)/imidazolinium salt/Cs2CO3. Chem. Commun. 2002, 1772–1773. [Google Scholar] [CrossRef] [PubMed]
- Sofia, H.J.; Chen, G.; Hetzler, B.G.; Reyes-Spindola, J.F.; Miller, N.E. Radical SAM, a novel protein superfamily linking unre-solved steps in familiar biosynthetic pathways with radical mechanisms: Functional characterization using new analysis and information visualization methods. Nucleic Acids Res. 2001, 29, 1097–1106. [Google Scholar] [CrossRef] [Green Version]
- Veeman, M.T.; Slusarski, D.C.; Kaykas, A.; Louie, S.H.; Moon, R.T. Zebrafish Prickle, a Modulator of Noncanonical Wnt/Fz Signaling, Regulates Gastrulation Movements. Curr. Biol. 2003, 13, 680–685. [Google Scholar] [CrossRef] [Green Version]
- Herrmann, F.; Gross, A.; Zhou, D.; Kestler, H.A.; Kuhl, M. A Boolean Model of the Cardiac Gene Regulatory Network Determining First and Second Heart Field Identity. PLoS ONE 2012, 7, e46798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, S.; Gui, Y.; Wang, Y.; Qian, L.; Liu, X.; Jiang, Q.; Song, H. Effects of methotrexate on the developments of heart and vessel in zebrafish. Acta Biochim. Biophys. Sin. 2009, 41, 86–96. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.-N.; Gui, Y.-H.; Wang, Y.-X.; Qian, L.-X.; Jiang, Q.; Liu, D.; Song, H.-Y. Effect of dihydrofolate reductase gene knock-down on the expression of heart and neural crest derivatives expressed transcript 2 in zebrafish cardiac development. Chin. Med. J. 2007, 120, 1166–1171. [Google Scholar] [CrossRef] [PubMed]
- Yelon, D.; Ticho, B.; Halpern, M.E.; Ruvinsky, I.; Ho, R.K.; Silver, L.M.; Stainier, D.Y. The bHLH transcription factor hand2 plays parallel roles in zebrafish heart and pectoral fin development. Development 2000, 127, 2573–2582. [Google Scholar] [CrossRef]
- Lievers, K.J.; Boers, G.H.; Verhoef, P.; Heijer, M.; Kluijtmans, L.A.; Put, N.M.; Trijbels, F.J.; Blom, H.J. A second common variant in the methylenetetrahydrofolate reductase (MTHFR) gene and its relationship to MTHFR enzyme activity, homocysteine, and cardiovascular disease risk. J. Mol. Med. 2001, 79, 522–528. [Google Scholar] [CrossRef]
- Maron, B.A.; Loscalzo, A. The Treatment of Hyperhomocysteinemia. Annu. Rev. Med. 2009, 60, 39–54. [Google Scholar] [CrossRef] [Green Version]
- Hoffman, J.I.; Kaplan, S. The incidence of congenital heart disease. J. Am. Coll. Cardiol. 2002, 39, 1890–1900. [Google Scholar] [CrossRef] [Green Version]
- Schober, F.A.; Moore, D.; Atanassov, I.; Moedas, M.F.; Clemente, P.; Végvári, Á.; El Fissi, N.; Filograna, R.; Bucher, A.-L.; Hinze, Y.; et al. The one-carbon pool controls mitochondrial energy metabolism via complex I and iron-sulfur clusters. Sci. Adv. 2021, 7, eabf0717. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Bonner, J.R.; Bernard, D.J.; Sanchez, E.L.; Sause, E.T.; Prentice, R.R.; Burgess, S.M.; Brody, L.C. Disruption of the folate pathway in zebrafish causes developmental defects. BMC Dev. Biol. 2012, 12, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, X.; Wang, B.; Jin, D.; Liu, K.; Wang, H.; Chen, L.; Zu, Y. Precise Dose of Folic Acid Supplementation Is Essential for Embryonic Heart Development in Zebrafish. Biology 2022, 11, 28. https://doi.org/10.3390/biology11010028
Han X, Wang B, Jin D, Liu K, Wang H, Chen L, Zu Y. Precise Dose of Folic Acid Supplementation Is Essential for Embryonic Heart Development in Zebrafish. Biology. 2022; 11(1):28. https://doi.org/10.3390/biology11010028
Chicago/Turabian StyleHan, Xuhui, Bingqi Wang, Dongxu Jin, Kuang Liu, Hongjie Wang, Liangbiao Chen, and Yao Zu. 2022. "Precise Dose of Folic Acid Supplementation Is Essential for Embryonic Heart Development in Zebrafish" Biology 11, no. 1: 28. https://doi.org/10.3390/biology11010028
APA StyleHan, X., Wang, B., Jin, D., Liu, K., Wang, H., Chen, L., & Zu, Y. (2022). Precise Dose of Folic Acid Supplementation Is Essential for Embryonic Heart Development in Zebrafish. Biology, 11(1), 28. https://doi.org/10.3390/biology11010028