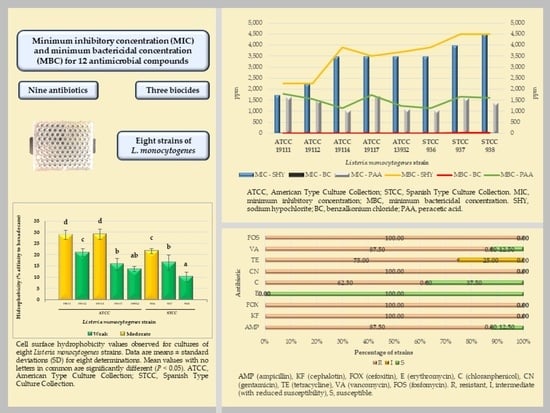
Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) for Twelve Antimicrobials (Biocides and Antibiotics) in Eight Strains of Listeria monocytogenes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Culturing Conditions
2.2. Determination of the Minimum Inhibitory Concentration (MIC)
2.3. Determination of the Minimum Bactericidal Concentration (MBC)
2.4. Determination of Cell Surface Hydrophobicity (CSH)
2.5. Statistical Analysis
3. Results
3.1. Minimum Inhibitory Concentration (MIC) and Minimum Bactricidal Concentration (MBC) for the Biocides
3.2. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) for Antibiotics
3.3. Relationship between MICs and MBCs of Biocides and Antibiotics
3.4. Cell Surface Hydrophobicity
4. Discussion
4.1. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) for Biocides
4.2. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) for Antibiotics
4.3. Relationship between MICs and MBCs of Biocides and Antibiotics
4.4. Cell Surface Hydrophobicity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Skowron, K.; Kwiecińska-Piróga, J.; Grudlewska, K.; Świeca, A.; Paluszak, Z.; Bauza-Kaszewska, B.; Wałecka-Zacharska, E.; Gospodarek-Komkowska, E. The occurrence, transmission, virulence and antibiotic resistance of Listeria monocytogenes in fish processing plant. Int. J. Food Microbiol. 2018, 282, 71–83. [Google Scholar] [CrossRef]
- Wagner, M.; McLauchin, J. Handbook of Listeria Monocytogenes; Liu, D., Ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 3–25. [Google Scholar]
- Nwaiwu, O. What are the recognized species of the genus Listeria? Access Microbiol. 2020, 2, e000153. [Google Scholar] [CrossRef] [PubMed]
- Pirie, J.H. The genus Listerella pirie. Science 1940, 91, 383. [Google Scholar] [CrossRef]
- Seeliger, H.P.R. A pathogen listerien: L. innocua sp. n. (Seeliger et Schoofs, 1977). Zent. Bakteriol. Mikrobiol. Hygiene. 1. Abt. Originale. A Med. Mikrobiol. Infekt. Parasitol. 1981, 249, 487–493. [Google Scholar]
- Rocourt, J.; Grimont, P.A.D. Listeria welshimeri sp. nov. and Listeria seeligeri sp. nov. Int. J. Syst. Bacteriol. 1983, 33, 866–869. [Google Scholar] [CrossRef] [Green Version]
- Seeliger, H.P.R.; Rocourt, J.; Schrettenbrunner, A.; Grimont, P.A.D.; Jones, D. Notes: Listeria ivanovii sp. nov. Int. J. Syst. Evol. Microbiol. 1984, 34, 336–337. [Google Scholar] [CrossRef]
- Rocourt, J.; Boerlin, P.; Grimont, F.; Jacquet, C.; Piffaretti, J.C. Assignment of Listeria grayi and Listeria murrayi to a single species, Listeria grayi, with a revised description of Listeria grayi. Int. J. Syst. Evol. Microbiol. 1992, 42, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Graves, L.M.; Helsel, L.O.; Steigerwalt, A.G.; Morey, R.E.; Daneshvar, M.I.; Roof, S.E.; Orsi, R.H.; Fortes, E.D.; Milillo, S.R.; Bakker, H.C.; et al. Listeria marthii sp. nov., isolated from the natural environment, Finger Lakes National Forest. Int. J. Syst. Evol. Microbiol. 2010, 60, 1280–1288. [Google Scholar] [CrossRef]
- Leclercq, A.; Clermont, D.; Bizet, C.; Grimont, P.A.D.; Le Flèche-Matéos, A.; Roche, S.M.; Buchrieser, C.; Cadet-Daniel, V.; Le Monier, A.; Lecuit, M.; et al. Listeria rocourtiae sp. nov. Int. J. Syst. Evol. Microbiol. 2010, 60, 2210–2214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertsch, D.; Rau, J.; Eugster, M.R.; Haug, M.C.; Lawson, P.A.; Lacroix, C.; Meile, L. Listeria fleischmannii sp. nov., isolated from cheese. Int. J. Syst. Evol. Microbiol. 2013, 63 Pt 2, 526–532. [Google Scholar] [CrossRef]
- Lang Halter, E.; Neuhaus, K.; Scherer, S. Listeria weihenstephanensis sp. nov., isolated from the water plant Lemna trisulca taken from a freshwater pond. Int. J. Syst. Evol. Microbiol. 2013, 63 Pt 2, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Den Bakker, H.C.; Warchocki, S.; Wright, E.M.; Allred, A.F.; Ahlstrom, C.; Manuel, C.S.; Stasiewicz, J.; Burrell, A.; Roof, S.; Strawn, L.K.; et al. Listeria floridensis sp. nov., Listeria aquatica sp. nov., Listeria cornellensis sp. nov., Listeria riparia sp. nov. and Listeria grandensis sp. nov., from agricultural and natural environments. Int. J. Syst. Evol. Microbiol. 2014, 64 Pt 6, 1882–1889. [Google Scholar] [CrossRef]
- Weller, D.; Andrus, A.; Wiedmann, M.; Den Bakker, H.C. Listeria booriae sp. nov. and Listeria newyorkensis sp. nov., from food processing environments in the USA. Int. J. Syst. Evol. Microbiol. 2015, 65 Pt 1, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Doijad, S.P.; Poharkar, K.V.; Kale, S.B.; Kerkar, S.; Kalorey, D.R.; Kurkure, N.V.; Rawool, D.B.; Malik, S.V.S.M.; Ahmad, R.Y.; Hudel, M.; et al. Listeria goaensis sp. nov. Int. J. Syst. Evol. Microbiol. 2018, 68, 3285–3291. [Google Scholar] [CrossRef]
- Núñez-Montero, K.; Leclercq, A.; Moura, A.; Vales, G.; Peraza, J.; Pizarro-Cerdá, J.; Lecuit, M. Listeria costaricensis sp. nov. Int. J. Syst. Evol. Microbiol. 2018, 68, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, A.; Moura, A.; Vales, G.; Tessaud-Rita, N.; Aguilhon, C.; Lecuit, M. Listeria thailandensis sp. nov. Int. J. Syst. Evol. Microbiol. 2019, 69, 74–81. [Google Scholar] [CrossRef]
- Quereda, J.J.; Leclercq, A.; Moura, A.; Vales, G.; Gómez-Martín, A.; García-Muñoz, A.; Thouvenot, P.; Tessaud-Rita, N.; Bracq-Dieye, H.; Lecuit, M. Listeria valentina sp. nov., isolated from a water trough and the faeces of healthy sheep. Int. J. Syst. Evol. Microbiol. 2020, 70, 5868–5879. [Google Scholar] [CrossRef]
- Carlin, C.R.; Liao, J.; Weller, D.; Guo, X.; Orsi, R.; Wiedmann, M. Listeria cossartiae sp. nov., Listeria immobilis sp. nov., Listeria portnoyi sp. nov. and Listeria rustica sp. nov., isolated from agricultural water and natural environments. Int. J. Syst. Evol. Microbiol. 2021, 71, 004795. [Google Scholar] [CrossRef] [PubMed]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control). The European Union One Health 2019 Zoonoses Report. EFSA J. 2021, 19, e06406. [Google Scholar]
- Shourav, H.A.; Hasan, M.; Ahmed, S. Antibiotic susceptibility pattern of Listeria spp. isolated from cattle farm environment in Bangladesh. J. Agric. Food Res. 2020, 2, 100082. [Google Scholar] [CrossRef]
- Rugna, G.; Carra, E.; Bergaminia, F.; Franzinia, G.; Faccini, S.; Gattuso, A.; Morgantia, M.; Baldi, D.; Naldia, S.; Serraino, A.; et al. Distribution, virulence, genotypic characteristics and antibiotic resistance of Listeria monocytogenes isolated over one-year monitoring from two pig slaughterhouses and processing plants and their fresh hams. Int. J. Food Microbiol. 2021, 336, 108912. [Google Scholar] [CrossRef] [PubMed]
- Kraśniewska, K.; Kosakowska, O.; Pobiega, K.; Gniewosz, M. The influence of two-component mixtures from Spanish origanum oil with Spanish marjoram oil or coriander oil on antilisterial activity and sensory quality of a fresh cut vegetable mixture. Foods 2020, 9, 1740. [Google Scholar] [CrossRef]
- FDA. Control of Listeria monocytogenes in Ready-To-Eat Foods: Guidance for Industry. U.S. Department of Health and Human Services Food and Drug Administration Center for Food Safety and Applied Nutrition 2017. Available online: https://www.fda.gov/files/food/published/Draft-Guidance-for-Industry--Control-of-Listeria-monocytogenes-in-Ready-To-Eat-Foods-%28PDF%29.pdf (accessed on 3 December 2021).
- Waghmare, R.B.; Annapure, U.S. Integrated effect of sodium hypochlorite and modified atmosphere packaging on quality and shelf life of fresh-cut cilantro. Food Packag. Shelf Life 2015, 3, 62–69. [Google Scholar] [CrossRef]
- Henriques, A.R.; Fraqueza, M.J. Biofilm-forming ability and biocide susceptibility of Listeria monocytogenes strains isolated from the ready-to-eat meat-based food products food chain. LWT—Food Sci. Technol. 2017, 81, 180–187. [Google Scholar] [CrossRef]
- Finnegan, M.; Linley, E.; Denyer, S.P.; McDonnell, G.; Simons, C.; Maillard, J.Y. Mode of action of hydrogen peroxide and other oxidizing agents: Differences between liquid and gas forms. J. Antimicrob. Chemother. 2010, 65, 108–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capita, R.; Vicente-Velasco, M.; Rodríguez-Melcón, C.; García-Fernández, C.; Carballo, J.; Alonso-Calleja, C. Effect of low doses of biocides on the antimicrobial resistance and the biofilms of Cronobacter sakazakii and Yersinia enterocolitica. Sci. Rep. 2019, 9, 15905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buzón-Durán, L.; Alonso-Calleja, C.; Riesco-Peláez, F.; Capita, R. Effect of subinhibitory concentrations of biocides on the architecture and viability of MRSA biofilms. Food Microbiol. 2017, 65, 294–301. [Google Scholar] [CrossRef]
- Capita, R.; Riesco-Peláez, F.; Alonso-Hernando, A.; Alonso-Calleja, C. Exposure of Escherichia coli ATCC 12806 to sublethal concentrations of food-grade biocides influences its ability to form biofilm, resistance to antimicrobials, and ultrastructure. Appl. Environ. Microbiol. 2014, 80, 1268–1280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Melcón, C.; Capita, R.; Rodríguez-Jerez, J.; Martínez-Suárez, J.; Alonso-Calleja, C. Effect of low doses of disinfectants on the biofilm-forming ability of Listeria monocytogenes. Foodborne Path. Dis. 2019, 16, 262–268. [Google Scholar] [CrossRef]
- Rodríguez-Melcón, C.; Riesco-Peláez, F.; García-Fernández, C.; Alonso-Calleja, C.; Capita, R. Susceptibility of Listeria monocytogenes planktonic cultures and biofilms to sodium hypochlorite and benzalkonium chloride. Food Microbiol. 2019, 82, 533–540. [Google Scholar] [CrossRef]
- Capita, R.; Alonso-Calleja, C. Antibiotic-resistant bacteria: A challenge for the food industry. Crit. Rev. Food Sci. Nutr. 2013, 53, 11–48. [Google Scholar] [CrossRef]
- CLSI. M100 Performance Standars for Antimicrobial Susceptibility Testing, 29th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2019. [Google Scholar]
- EUCAST. European Committee on Antimicrobial Susceptibility Testing. V. 9.0. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_9.0_Breakpoint_Tables.pdf (accessed on 14 July 2021).
- CLSI. Performance Standards for Antimicrobial Disk and Dilution Susceptibility Test for Bacteria Isolated from Animals. Approved Standard M31-A3; National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2017; Available online: https://www.dbt.univr.it/documenti/OccorrenzaIns/matdid/matdid485539.pdf (accessed on 3 December 2021).
- To, M.S.; Favrin, S.; Romanova, N.; Griffiths, M.W. Postadaptational resistance to benzalkonium chloride and subsequent physicochemical modifications of Listeria monocytogenes. Appl. Environ. Microbiol. 2002, 68, 5258–5264. [Google Scholar] [CrossRef] [Green Version]
- Buzón-Durán, L.; Capita, R.; Alonso-Calleja, C. Antibiotic susceptibility of methicillin-resistant staphylococci (MRS) of food origin: A comparison of agar disc diffusion method and a commercially available miniaturized test. Food Microbiol. 2018, 72, 220–224. [Google Scholar] [CrossRef]
- Capita, R.; Buzón-Durán, L.; Riesco-Peláez, F.; Alonso-Calleja, C. Effect of sub-lethal concentrations of biocides on the structural parameters and viability of the biofilms formed by Salmonella Typhimurium. Foodborne Path. Dis. 2017, 14, 350–356. [Google Scholar] [CrossRef]
- Lundén, J.; Autio, T.; Markkula, A.; Hellström, S.; Korkeala, H. Adaptive and cross-adaptive responses of persistent and nonpersistent Listeria monocytogenes strains to disinfectants. Int. J. Food Microbiol. 2003, 82, 265–272. [Google Scholar] [CrossRef]
- Sawer, I.K.; Berry, M.I.; Ford, J.L. Effect of medium composition, agitation and the presence of EDTA on the antimicrobial activity of cryptolepine. Lett. Appl. Microbiol. 1997, 25, 207–211. [Google Scholar] [CrossRef] [Green Version]
- Dorey, L.; Lees, P. Impact of growth matrix on pharmacodynamics of antimicrobial drugs for pig pneumonia pathogens. BMC Vet. Res. 2017, 13, 192. [Google Scholar] [CrossRef]
- Alonso-Hernando, A.; Capita, R.; Prieto, M.; Alonso-Calleja, C. Adaptation and cross-adaptation of Listeria monocytogenes and Salmonella enterica to poultry decontaminants. J. Microbiol. 2009, 47, 142–146. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Scientific Opinion on the evaluation of the safety and efficacy of peroxyacetic acid solutions for reduction of pathogens on poultry carcasses and meat. EFSA J. 2014, 12, 3599. [Google Scholar]
- Soumet, C.; Ragimbeau, C.; Maris, P. Screening of benzalkonium chloride resistance in Listeria monocytogenes strains isolated during cold smoked fish production. Lett. Appl. Microbiol. 2005, 41, 291–296. [Google Scholar] [CrossRef] [Green Version]
- Smith, K.; Hunter, I.S. Efficacy of common hospital biocides with biofilms of multi-drug resistant clinical isolates. J. Med. Microbiol. 2008, 57, 966–973. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.; Han, J.; Dai, H.; Jia, P. Biocide-tolerance and antibiotic-resistance in community environments and risk of direct transfers to humans: Unintended consequences of community-wide surface disinfecting during COVID-19? Environ. Pollut. 2021, 283, 117074. [Google Scholar] [CrossRef] [PubMed]
- Norwood, D.E.; Gilmour, A. The growth and resistance to sodium hypochlorite of Listeria monocytogenes in a steady-state multispecies biofilm. J. Appl. Microbiol. 2000, 88, 512–520. [Google Scholar] [CrossRef] [Green Version]
- Tamburro, M.; Ripabelli, G.; Vitullo, M.; Dallman, T.J.; Pontello, M.; Amar, C.F.L.; Sammarco, M.L. Gene expression in Listeria monocytogenes exposed to sublethal concentration of benzalkonium chloride. Comp. Immunol. Microbiol. Infect. Dis. 2015, 40, 31–39. [Google Scholar] [CrossRef]
- Poimenidou, S.V.; Chrysadakou, M.; Tzakoniati, A.; Bikouli, V.C.; Nychas, G.J.; Skandamis, P.N. Variability of Listeria monocytogenes strains in biofilm formation on stainless steel and polystyrene materials and resistance to peracetic acid and quaternary ammonium compounds. Int. J. Food Microbiol. 2016, 237, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Gawande, H.M.; Dhotre, A.V.; Shendurse, A.M.; Khodwe, N.M. Peroxyacetic acid: A potent food industry sanitizer. Indian Food Industry Mag 2013, 32, 26–30. [Google Scholar]
- Kitis, M. Disinfection of wastewater with peracetic acid: A review. Environ. Int. 2004, 30, 47–55. [Google Scholar] [CrossRef]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. Available online: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf (accessed on 14 July 2021).
- OECD. Antimicrobial Resistance. Tackling the Burden in the European Union. Available online: https://www.oecd.org/health/health-systems/AMR-Tackling-the-Burden-in-the-EU-OECD-ECDC-Briefing-Note-2019.pdf (accessed on 14 July 2021).
- Charpentier, E.; Courvalin, P. Antibiotic Resistance in Listeria spp. Antimicrob. Agents Chemother. 1999, 43, 2103–2108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alonso-Hernando, A.; Prieto, M.; García-Fernández, C.; Alonso-Calleja, C.; Capita, R. Increase over time in the prevalence of multiple antibiotic resistance among isolates of Listeria monocytogenes from poultry in Spain. Food Control 2012, 23, 37–41. [Google Scholar] [CrossRef]
- Álvarez-Fernández, E.; Domínguez-Rodríguez, J.; Capita, R.; Alonso-Calleja, C. Influence of housing systems on microbial load and antimicrobial resistance patterns of Escherichia coli isolates from eggs produced for human consumption. J. Food Prot. 2012, 75, 847–853. [Google Scholar] [CrossRef]
- Aras, K.; Ardiç, M. Occurrence and antibiotic susceptibility of Listeria species in turkey meats. Korean J. Food Sci. Anim. Resour. 2015, 35, 669–673. [Google Scholar] [CrossRef] [Green Version]
- Capita, R.; Felices-Mercado, A.; García-Fernández, C.; Alonso-Calleja, C. Characterization of Listeria monocytogenes originating from the Spanish meat-processing chain. Foods 2019, 8, 542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, F.T.; Vieira, B.S.; Vallim, D.C.; Carvalho, L.A.; Carvalho, R.C.T.; Pereira, R.C.L.; Figueiredo, E.E.S. Genetic similarity, antibiotic resistance and disinfectant susceptibility of Listeria monocytogenes isolated from chicken meat and chicken-meat processing environment in Mato Grosso, Brazil. LWT 2019, 109, 77–82. [Google Scholar] [CrossRef]
- WHO (World Health Organization). Critically Important Antimicrobials for Human Medicine, 6th ed.; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- OIE. World Organization for Animal Health. OIE List of Antimicrobial Agents of Veterinary Importance; World Organization for Animal Health: Paris, France, 2018. [Google Scholar]
- Wiggins, G.L.; Albrittony, L.W.; Feeley, J.C. Antibiotic susceptibility of clinical isolates of Listeria monocytogenes. Antimicrob. Agents Chemother. 1978, 13, 854–860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fallah, A.A.; Saei-Dehkordi, S.S.; Rahnama, M.; Tahmasby, H.; Mahzounieh, M. Prevalence and antimicrobial resistance patterns of Listeria species isolated from poultry products marketed in Iran. Food Control 2012, 28, 327–332. [Google Scholar] [CrossRef]
- Cufaoglu, G.; Ambarcioglu, P.; Ayaz, N.D. Meta-analysis of the prevalence of Listeria spp. and antibiotic resistant L. monocytogenes isolates from foods in Turkey. LWT 2021, 144, 111210. [Google Scholar] [CrossRef]
- Molina-González, D.; Alonso-Calleja, C.; Alonso-Hernando, A.; Capita, R. Effect of sub-lethal concentrations of biocides on the susceptibility to antibiotics of multi-drug resistant Salmonella enterica strains. Food Control 2014, 40, 329–334. [Google Scholar] [CrossRef]
- Olaimat, A.N.; Al-Holy, M.A.; Shahbaz, H.M.; Al-Nabusli, A.A.; Abu Goush, M.H.; Osaili, T.M.; Ayyash, M.M.; Holley, R.A. Emergence of antibiotic resistance in Listeria monocytogenes isolated from food products: A comprehensive review. Comprehen. Rev. Food Sci. Food Saf. 2018, 17, 1277–1292. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, G.; Saigal, S.; Elongavan, A. Action and resistance mechanisms of antibiotics: A guide for clinicians. J. Anaesthesiol Clin. Pharmacol. 2017, 33, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Fernández, E.; Alonso-Calleja, C.; García-Fernández, C.; Capita, R. Prevalence and antimicrobial resistance of Salmonella serotypes isolated from poultry in Spain: Comparison between 1993 and 2006. Int. J. Food Microbiol. 2012, 153, 281–287. [Google Scholar] [CrossRef]
- Rodríguez-Melcón, C.; Alonso-Calleja, C.; Capita, R. Architecture and viability of the biofilms formed by nine Listeria strains on various hydrophobic and hydrophilic materials. Appl. Sci. 2019, 9, 5256. [Google Scholar] [CrossRef] [Green Version]
- Chiu, J.; Han, G.; McCrystal, K.; Zuo, M. Macrolide structures can confer differential susceptibility in Escherichia coli K30 deletions of group 1 capsule assembly genes. J. Exp. Microbiol. Immunol. 2017, 3, 50–56. [Google Scholar]
- Jones, I.A.; Joshi, L.T. Biocide use in the antimicrobial era: A review. Molecules 2021, 26, 2276. [Google Scholar] [CrossRef] [PubMed]
- Loughlin, M.F.; Jones, M.V.; Lambert, P.A. Pseudomonas aeruginosa cells adapted to benzalkonium chloride show resistance to other membrane-active agents but not to clinically relevant antibiotics. J. Antimicrob. Chemother. 2002, 49, 631–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braoudaki, M.; Hilton, A.C. Adaptive resistance to biocides in Salmonella enterica and Escherichia coli O157 and cross-resistance to antimicrobial agents. J. Clin. Microbiol. 2004, 42, 73–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Species | Year of Description | Reference |
|---|---|---|
| Listeria monocytogenes | 1940 | [4] |
| Listeria innocua | 1983 | [5] |
| Listeria seeligeri | 1983 | [6] |
| Listeria welshimeri | 1983 | [6] |
| Listeria ivanovii | 1984 | [7] |
| Listeria grayi | 1992 | [8] |
| Listeria marthii | 2010 | [9] |
| Listeria rocourtiae | 2010 | [10] |
| Listeria fleischmannii | 2013 | [11] |
| Listeria weihenstephanensis | 2013 | [12] |
| Listeria aquatica | 2014 | [13] |
| Listeria cornellensis | 2014 | [13] |
| Listeria floridensis | 2014 | [13] |
| Listeria grandensis | 2014 | [13] |
| Listeria riparia | 2014 | [13] |
| Listeria booriae | 2015 | [14] |
| Listeria newyorkensis | 2015 | [14] |
| Listeria goaensis | 2018 | [15] |
| Listeria costaricensis | 2018 | [16] |
| Listeria thailandensis | 2019 | [17] |
| Listeria valentina | 2020 | [18] |
| Listeria cossartiae | 2021 | [19] |
| Listeria farberi | 2021 | [19] |
| Listeria immobilis | 2021 | [19] |
| Listeria portnoyi | 2021 | [19] |
| Listeria rustica | 2021 | [19] |
| Strain | Biocide | ||
|---|---|---|---|
| SH | BC | PAA | |
| ATCC 19111 | 1750 (2250) | 0.25 (1.50) | 1650 (1800) |
| ATCC 19112 | 2250 (2250) | 0.50 (0.50) | 1500 (1550) |
| ATCC 19114 | 3500 (3900) | 2.00 (4.00) | 1050 (1150) |
| ATCC 19117 | 3500 (3500) | 0.75 (3.00) | 1700 (1750) |
| ATCC 13932 | 3500 (3700) | 4.00 (5.00) | 1100 (1250) |
| STCC 936 | 3500 (3900) | 3.00 (5.00) | 1050 (1150) |
| STCC 937 | 4000 (4500) | 20.00 (20.00) | 1600 (1650) |
| STCC 938 | 4500 (4500) | 19.00 (19.00) | 1400 (1600) |
| Strain | Antibiotic | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| AMP | KF | FOX | E | C | CN | TE | VA | FOS | |
| ATCC 19111 | 7 (10) | 150 (150) | 170 (180) | 0.05 (4) | 20 (70) | 45 (50) | 15 (30) | 80 (90) | 350 (350) |
| ATCC 19112 | 11 (11) | 140 (140) | 160 (170) | 0.10 (5) | 4 (10) | 45 (45) | 14 (18) | 45 (50) | 160 (160) |
| ATCC 19114 | 9 (9) | 80 (90) | 140 (180) | 0.05 (5) | 20 (70) | 20 (20) | 8 (11) | 20 (20) | 230 (290) |
| ATCC 19117 | 1 (5) | 8 (20) | 150 (200) | 0.20 (5) | 20 (55) | 4 (15) | 2 (10) | 2 (3) | 430 (450) |
| ATCC 13932 | 9 (9) | 70 (70) | 160 (160) | 0.20 (5) | 35 (60) | 20 (20) | 11 (11) | 20 (20) | 250 (260) |
| STCC 936 | 5 (6) | 8 (9) | 20 (70) | 0.20 (5) | 5 (30) | 3 (5) | 2 (3) | 20 (20) | 170 (180) |
| STCC 937 | 15 (15) | 150 (150) | 170 (200) | 0.20 (5) | 50 (70) | 50 (50) | 15 (30) | 35 (40) | 240 (280) |
| STCC 938 | 13 (20) | 150 (160) | 150 (150) | 0.20 (5) | 5 (9) | 100 (100) | 15 (30) | 50 (60) | 220 (230) |
| CUT-OFF POINTS S ≤ - R > | 1–1 | 0.12–0.50 * | 4–8 | 1–1 | 8–8 | 1–1 | 1–2 | 2–2 | 32–32 |
| Biocides (MIC) | Antibiotics (MIC) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SH | BC | PAA | AMP | KF | FOX | E | C | CN | TE | VA | FOS | |
| SH | - | |||||||||||
| BC | 0.715 *** | - | ||||||||||
| PAA | −0.284 | 0.144 | - | |||||||||
| AMP | 0.273 | 0.734 *** | −0.016 | - | ||||||||
| KF | −0.182 | 0.483 * | 0.385 | 0.809 *** | - | |||||||
| FOX | −0.169 | 0.185 | 0.568 ** | 0.387 | 0.640 *** | - | ||||||
| E | 0.706 *** | 0.507 * | −0.018 | 0.000 | −0.348 | −0.265 | - | |||||
| C | 0.196 | 0.348 | 0.164 | 0.292 | 0.147 | 0.451 * | 0.194 | - | ||||
| CN | 0.220 | 0.696 *** | 0.297 | 0.732 *** | 0.829 *** | 0.438 * | 0.006 | −0.119 | - | |||
| TE | −0.166 | 0.471 * | 0.319 | 0.818 *** | 0.977 *** | 0.678 *** | −0.268 | 0.229 | 0.801 *** | - | ||
| VA | −0.513 * | 0.135 | 0.367 | 0.412 * | 0.808 *** | 0.342 | −0.492 * | 0.141 | 0.646 *** | 0.774 *** | - | |
| FOS | −0.155 | −0.235 | 0.590 ** | −0.576 ** | −0.230 | 0.380 | −0.011 | 0.271 | −0.236 | −0.231 | −0.080 | - |
| Biocides (MBC) | Antibiotics (MBC) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SH | BC | PAA | AMP | KF | FOX | E | C | CN | TE | VA | FOS | |
| SH | - | |||||||||||
| BC | 0.785 *** | - | ||||||||||
| PAA | −0.312 | 0.171 | - | |||||||||
| AMP | 0.374 | 0.798 *** | 0.289 | - | ||||||||
| KF | −0.128 | 0.435* | 0.471 * | 0.821 *** | - | |||||||
| FOX | 0.144 | 0.079 | 0.598 ** | 0.162 | 0.430 * | - | ||||||
| E | 0.601 ** | 0.301 | −0.475 * | 0.052 | −0.341 | −0.157 | - | |||||
| C | 0.047 | −0.094 | 0.027 | −0.342 | −0.104 | 0.479 * | −0.354 | - | ||||
| CN | 0.140 | 0.631 *** | 0.506 * | 0.937 *** | 0.833 *** | 0.209 | −0.158 | −0.414 * | - | |||
| TE | −0.017 | 0.566 ** | 0.698 *** | 0.799 *** | 0.913 *** | 0.473 * | −0.453 * | 0.017 | 0.854 *** | - | ||
| VA | −0.426 * | 0.126 | 0.517 ** | 0.549 ** | 0.807 *** | 0.108 | −0.749 *** | −0.127 | 0.694 *** | 0.805 *** | - | |
| FOS | −0.043 | −0.132 | 0.510 * | −0.325 | −0.210 | 0.618 ** | −0.325 | 0.633 *** | −0.178 | 0.063 | −0.156 | - |
| Biocides | Antibiotics | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SH | BC | PAA | AMP | KF | FOX | E | C | CN | TE | VA | FOS |
| 0.967 *** | 0.995 *** | 0.979 *** | 0.850 *** | 0.996 *** | 0.905 *** | 0.571 ** | 0.767 *** | 0.993 *** | 0.868 *** | 0.997 *** | 0.974 *** |
| Biocides | Antibiotics | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SH | BC | PAA | AMP | KF | FOX | E | C | CN | TE | VA | FOS | |
| HYD | −0.504 * | −0.580 ** | −0.389 | 0.017 | 0.116 | 0.103 | −0.725 *** | −0.365 | −0.198 | 0.117 | 0.140 | −0.328 |
| (−0.532 **) | (−0.616 **) | (−0.387) | (−0.186) | (0.113) | (0.006) | (−0.082) | (−0.097) | (−0.155) | (−0.237) | (0.099) | (−0.313) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Melcón, C.; Alonso-Calleja, C.; García-Fernández, C.; Carballo, J.; Capita, R. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) for Twelve Antimicrobials (Biocides and Antibiotics) in Eight Strains of Listeria monocytogenes. Biology 2022, 11, 46. https://doi.org/10.3390/biology11010046
Rodríguez-Melcón C, Alonso-Calleja C, García-Fernández C, Carballo J, Capita R. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) for Twelve Antimicrobials (Biocides and Antibiotics) in Eight Strains of Listeria monocytogenes. Biology. 2022; 11(1):46. https://doi.org/10.3390/biology11010046
Chicago/Turabian StyleRodríguez-Melcón, Cristina, Carlos Alonso-Calleja, Camino García-Fernández, Javier Carballo, and Rosa Capita. 2022. "Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) for Twelve Antimicrobials (Biocides and Antibiotics) in Eight Strains of Listeria monocytogenes" Biology 11, no. 1: 46. https://doi.org/10.3390/biology11010046
APA StyleRodríguez-Melcón, C., Alonso-Calleja, C., García-Fernández, C., Carballo, J., & Capita, R. (2022). Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) for Twelve Antimicrobials (Biocides and Antibiotics) in Eight Strains of Listeria monocytogenes. Biology, 11(1), 46. https://doi.org/10.3390/biology11010046