Spodoptera exigua Multiple Nucleopolyhedrovirus Increases the Susceptibility to Insecticides: A Promising Efficient Way for Pest Resistance Management
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Determination of LC50 of Insecticides
2.3. Determination of LC25 and LC50 of SeMNPV
2.4. Toxicity of Insecticides Combining with SeMNPV
2.5. Field Trial
2.6. Data Analysis
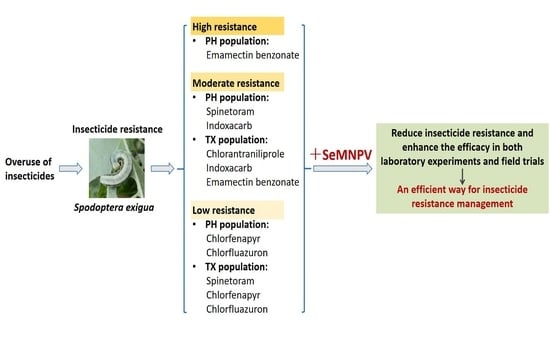
3. Results
3.1. Determination of LC50 of Insecticides
3.2. Determination of LC25 and LC50 of SeMNPV
3.3. Toxicity of Insecticides Combining with SeMNPV against S. exigua
3.4. Field Trial
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Richardson, E.B.; Troczka, B.J.; Gutbrod, O.; Davies, T.G.E.; Nauen, R. Diamide resistance: 10 years of lessons from lepidopteran pests. J. Pest Sci. 2020, 93, 911–928. [Google Scholar] [CrossRef]
- Guerrero, A.; Malo, E.A.; Coll, J.; Quero, C. Semiochemical and natural product-based approaches to control Spodoptera spp. (Lepidoptera: Noctuidae). J. Pest Sci. 2014, 87, 231–247. [Google Scholar] [CrossRef]
- Hafeez, M.; Ullah, F.; Khan, M.M.; Li, X.; Zhang, Z.; Shah, S.; Imran, M.; Assiri, M.A.; Fernández-Grandon, G.M.; Desneux, N.; et al. Metabolic-based insecticide resistance mechanism and ecofriendly approaches for controlling of beet armyworm Spodoptera exigua: A review. Environ. Sci. Pollut. Res. 2021, 29, 1746–1762. [Google Scholar] [CrossRef] [PubMed]
- Devine, G.J.; Furlong, M.J. Insecticide use: Contexts and ecological consequences. Agric. Hum. Values 2007, 24, 281–306. [Google Scholar] [CrossRef]
- Köhler, H.-R.; Triebskorn, R.; Meierbachtol, T.; Harper, J.; Humphrey, N. Wildlife ecotoxicology of pesticides: Can we track effects to the population level and beyond? Science 2013, 341, 759–765. [Google Scholar] [CrossRef]
- Rezende-Teixeira, P.; Dusi, R.G.; Jimenez, P.C.; Espindola, L.S.; Costa-Lotufo, L.V. What can we learn from commercial insecticides? Efficacy, toxicity, environmental impacts, and future developments. Environ. Pollut. 2022, 300, 118983. [Google Scholar] [CrossRef]
- Dermauw, W.; Wybouw, N.; Rombauts, S.; Menten, B.; Vontas, J.; Grbić, M.; Clark, R.M.; Feyereisen, R.; Van Leeuwen, T. A link between host plant adaptation and pesticide resistance in the polyphagous spider mite Tetranychus urticae. Proc. Natl. Acad. Sci. USA 2013, 110, E113–E122. [Google Scholar] [CrossRef]
- Hafeez, M.; Liu, S.; Jan, S.; Shi, L.; Fernández-Grandon, G.M.; Gulzar, A.; Ali, B.; Rehman, M.; Wang, M. Knock-down of gossypol-inducing cytochrome P450 genes reduced deltamethrin sensitivity in Spodoptera exigua (Hübner). Int. J. Mol. Sci. 2019, 20, 2248. [Google Scholar] [CrossRef]
- Zhu, F.; LaVine, L.; O’Neal, S.; LaVine, M.; Foss, C.; Walsh, D. Insecticide resistance and management strategies in urban ecosystems. Insects 2016, 7, 2. [Google Scholar] [CrossRef]
- Berg, J.V.D.; Greyvenstein, B.; du Plessis, H. Insect resistance management facing African smallholder farmers under climate change. Curr. Opin. Insect Sci. 2022, 50, 100894. [Google Scholar] [CrossRef]
- Che, W.; Shi, T.; Wu, Y.; Yang, Y. Insecticide resistance status of field populations of Spodoptera exigua (Lepidoptera: Noctuidae) from China. J. Econ. Èntomol. 2013, 106, 1855–1862. [Google Scholar] [CrossRef]
- Wang, P.; Yang, F.; Wang, Y.; Zhou, L.-L.; Luo, H.-B.; Zhang, S.; Si, S.-Y. Monitoring the resistance of the beet armyworm (Lepidoptera: Noctuidae) to four insecticides in southern China from 2014 to 2018. J. Econ. Èntomol. 2021, 114, 332–338. [Google Scholar] [CrossRef]
- Huang, J.-M.; Zhao, Y.-X.; Sun, H.; Ni, H.; Liu, C.; Wang, X.; Gao, C.-F.; Wu, S.-F. Monitoring and mechanisms of insecticide resistance in Spodoptera exigua (Lepidoptera: Noctuidae), with special reference to diamides. Pestic. Biochem. Physiol. 2021, 174, 104831. [Google Scholar] [CrossRef]
- Su, J.; Sun, X.-X. High level of metaflumizone resistance and multiple insecticide resistance in field populations of Spodoptera exigua (Lepidoptera: Noctuidae) in Guangdong Province, China. Crop. Prot. 2014, 61, 58–63. [Google Scholar] [CrossRef]
- Wang, X.; Xiang, X.; Yu, H.; Liu, S.; Yin, Y.; Cui, P.; Wu, Y.; Yang, J.; Jiang, C.; Yang, Q. Monitoring and biochemical characterization of beta-cypermethrin resistance in Spodoptera exigua (Lepidoptera: Noctuidae) in Sichuan Province, China. Pestic. Biochem. Physiol. 2018, 146, 71–79. [Google Scholar] [CrossRef]
- Haase, S.; Sciocco-Cap, A.; Romanowski, V. Baculovirus insecticides in Latin America: Historical overview, current status and future perspectives. Viruses 2015, 7, 2230–2267. [Google Scholar] [CrossRef]
- Sun, X. History and current status of development and use of viral insecticides in China. Viruses 2015, 7, 306–319. [Google Scholar] [CrossRef]
- Williams, T.; López-Ferber, M.; Caballero, P. Nucleopolyhedrovirus coocclusion technology: A new concept in the development of biological insecticides. Front. Microbiol. 2022, 12, 810026. [Google Scholar] [CrossRef]
- Glare, T.; Caradus, J.; Gelernter, W.; Jackson, T.; Keyhani, N.; Köhl, J.; Marrone, P.; Morin, L.; Stewart, A. Have biopesticides come of age? Trends Biotechnol. 2012, 30, 250–258. [Google Scholar] [CrossRef]
- Lacey, L.A.; Shapiro-Ilan, D.I. Microbial control of insect pests in temperate orchard systems: Potential for incorporation into IPM. Annu. Rev. Èntomol. 2008, 53, 121–144. [Google Scholar] [CrossRef] [Green Version]
- Arthurs, S.; Dara, S.K. Microbial biopesticides for invertebrate pests and their markets in the United States. J. Invertebr. Pathol. 2019, 165, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Lacey, L.A.; Grzywacz, D.; Shapiro-Ilan, D.I.; Frutos, R.; Brownbridge, M.; Goettel, M.S. Insect pathogens as biological control agents: Back to the future. J. Invertebr. Pathol. 2015, 132, 1–41. [Google Scholar] [CrossRef]
- Blissard, G.W.; Theilmann, D.A. Baculovirus entry and egress from insect cells. Annu. Rev. Virol. 2018, 5, 113–139. [Google Scholar] [CrossRef] [PubMed]
- Rosell, G.; Quero, C.; Coll, J.; Guerrero, A. Biorational insecticides in pest management. J. Pestic. Sci. 2008, 33, 103–121. [Google Scholar] [CrossRef]
- Srinivasan, R.; Sevgan, S.; Ekesi, S.; Tamò, M. Biopesticide based sustainable pest management for safer production of vegetable legumes and brassicas in Asia and Africa. Pest Manag. Sci. 2019, 75, 2446–2454. [Google Scholar] [CrossRef] [PubMed]
- Dáder, B.; Aguirre, E.; Caballero, P.; Medina, P. Synergy of lepidopteran nucleopolyhedroviruses AcMNPV and SpliNPV with insecticides. Insects 2020, 11, 316. [Google Scholar] [CrossRef]
- Sarwar, G.; Maan, N.A.; Ayub, M.A.; Shahid, M.R.; Malik, M.A.; Farooq, M. Evaluation of indigenous the nucleopolyhedrovirus (NPV) of Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae) in combination with chlorantraniliprole against Spodoptera species. Egypt. J. Biol. Pest Control. 2021, 31, 58. [Google Scholar] [CrossRef]
- Radmanesh, M. The combined and isolated effect of spinosad and nuclear polyhedrosis virus on the Mediterranean brocade Spodoptera littoralis in laboratory conditions. Biosci. Biotechnol. Res. Commun. 2021, 14, 692–696. [Google Scholar] [CrossRef]
- Yasin, M.; Qazi, M.S.; Wakil, W.; Qayyum, M.A. Evaluation of nuclear polyhedrosis virus (NPV) and emamectin benzoate against Spodoptera litura (F.) (Lepidoptera: Noctuidae). Egypt. J. Biol. Pest Control. 2020, 30, 88. [Google Scholar] [CrossRef]
- Maqsood, S.; Afzal, M.; Aqueel, M.A.; Raza, A.B.M.; Wakil, W.; Babar, M.H. Efficacy of nuclear polyhedrosis virus and flubendiamide alone and in combination against Spodoptera litura F. Pak. J. Zool. 2017, 49, 1783–1788. [Google Scholar] [CrossRef]
- Ahmad, J.N.; Mushtaq, R.; Ahmad, S.J.N.; Malik, M.A.; Manzoor, M.; Tahir, M.; Aslam, Z.; Maqsood, S.; Ahuja, I.; Bones, A.M. Sub-lethal dose reponses of native polyhydroviruses and spinosad for economical and sustainable management of Spodoptera litura in Pakistan. Pak. J. Zool. 2020, 52, 989–999. [Google Scholar] [CrossRef]
- Méndez, W.A.; Valle, J.; Ibarra, J.E.; Cisneros, J.; I Penagos, D.; Williams, T. Spinosad and nucleopolyhedrovirus mixtures for control of Spodoptera frugiperda (Lepidoptera: Noctuidae) in maize. Biol. Control 2002, 25, 195–206. [Google Scholar] [CrossRef]
- Abid, A.D.; Saeed, S.; Zaka, S.M.; Ali, M.; Shahzad, M.S.; Iqbal, M.; Shahzad, U.; Iqbal, N.; Alghanem, S.M. Interaction of HaNPVs with two novel insecticides against Helicoverpa armigera Hubner (Noctuidae: Lepidoptera). Saudi J. Biol. Sci. 2020, 27, 2124–2128. [Google Scholar] [CrossRef]
- Gu, Z.; Li, F.; Hu, J.; Ding, C.; Wang, C.; Tian, J.; Xue, B.; Xu, K.; Shen, W.; Li, B. Sublethal dose of phoxim and Bombyx mori nucleopolyhedrovirus interact to elevate silkworm mortality. Pest Manag. Sci. 2017, 73, 554–561. [Google Scholar] [CrossRef]
- Rabelo, M.M.; Paula-Moraes, S.V.; Pereira, E.J.G.; Siegfried, B.D. Contrasting susceptibility of lepidopteran pests to diamide and pyrethroid insecticides in a region of overwintering and migratory intersection. Pest Manag. Sci. 2020, 76, 4240–4247. [Google Scholar] [CrossRef]
- Allahyari, R.; Aramideh, S.; Michaud, J.P.; Safaralizadeh, M.H.; Rezapanah, M.R. Negative life history impacts for Habrobracon hebetor (Hymneoptera: Braconidae) that develop in bollworm larvae inoculated with Helicoverpa armigera Nucleopolyhedrovirus. J. Econ. Èntomol. 2020, 113, 1648–1655. [Google Scholar] [CrossRef]
- Wang, L.; Cui, L.; Wang, Q.; Chang, Y.; Huang, W.; Rui, C. Sulfoxaflor resistance in Aphis gossypii: Resistance mechanism, feeding behavior and life history changes. J. Pest Sci. 2021, 95, 811–825. [Google Scholar] [CrossRef]
- Ahmad, M.; Farid, A.; Saeed, M. Resistance to new insecticides and their synergism in Spodoptera exigua (Lepidoptera: Noctuidae) from Pakistan. Crop. Prot. 2018, 107, 79–86. [Google Scholar] [CrossRef]
- Zhou, C.; Liu, Y.; Yu, W.; Deng, Z.; Gao, M.; Liu, F.; Mu, W. Resistance of Spodoptera exigua to ten insecticides in Shandong, China. Phytoparasitica 2011, 39, 315–324. [Google Scholar] [CrossRef]
- Tang, T.; Hu, F.; Wang, P.; Fu, W.; Liu, X. Broflanilide effectively controls Helicoverpa armigera and Spodoptera exigua exhibiting diverse susceptibilities to chlorantraniliprole and emamectin benzoate. Pest Manag. Sci. 2020, 77, 1262–1272. [Google Scholar] [CrossRef]
- Williams, T.; Virto, C.; Murillo, R.; Caballero, P. Covert infection of insects by baculoviruses. Front. Microbiol. 2017, 8, 1337. [Google Scholar] [CrossRef] [PubMed]
- Gasque, S.N.; van Oers, M.M.; Ros, V.I. Where the baculoviruses lead, the caterpillars follow: Baculovirus-induced alterations in caterpillar behaviour. Curr. Opin. Insect Sci. 2019, 33, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Avilés, N.; Murillo, R.; Lasa, R.; Pineda, S.; Figueroa, J.I.; Bravo-Patiño, A.; Díaz, O.; Corrales, J.L.; Martínez, A.M. Genetic and biological characterization of four nucleopolyhedrovirus isolates collected in Mexico for the control of Spodoptera exigua (Lepidoptera: Noctuidae). J. Econ. Èntomol. 2017, 110, 1465–1475. [Google Scholar] [CrossRef] [PubMed]
- Cabodevilla, O.; Ibañez, I.; Simón, O.; Murillo, R.; Caballero, P.; Williams, T. Occlusion body pathogenicity, virulence and productivity traits vary with transmission strategy in a nucleopolyhedrovirus. Biol. Control. 2011, 56, 184–192. [Google Scholar] [CrossRef]
- Zamora-Avilés, N.; Alonso-Vargas, J.; Pineda, S.; Isaac-Figueroa, J.; Lobit, P.; Martínez-Castillo, A.M. Effects of a nucleopolyhedrovirus in mixtures with azadirachtin on Spodoptera frugiperda (J. E. Smith) (Lepidoptera: Noctuidae) larvae and viral occlusion body production. Biocontrol Sci. Technol. 2013, 23, 521–534. [Google Scholar] [CrossRef]
- Nathan, S.S.; Kalaivani, K. Combined effects of azadirachtin and nucleopolyhedrovirus (SpltNPV) on Spodoptera litura Fabricius (Lepidoptera: Noctuidae) larvae. Biol. Control 2006, 39, 96–104. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, L.; Wan, N.; Ji, X.; Zhang, H.; Jiang, J. Transcriptomic analysis of the interactions between the Spodoptera exigua midgut and nucleopolyhedrovirus. Pestic. Biochem. Physiol. 2019, 163, 241–253. [Google Scholar] [CrossRef]
- Jakubowska, A.K.; Vogel, H.; Herrero, S. Increase in gut microbiota after immune suppression in baculovirus-infected larvae. PLoS Pathog. 2013, 9, e1003379. [Google Scholar] [CrossRef]
- Balabanidou, V.; Grigoraki, L.; Vontas, J. Insect cuticle: A critical determinant of insecticide resistance. Curr. Opin. Insect Sci. 2018, 27, 68–74. [Google Scholar] [CrossRef]
- Li, X.; Schuler, M.A.; Berenbaum, M.R. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu. Rev. Èntomol. 2007, 52, 231–253. [Google Scholar] [CrossRef]
- Hilliou, F.; Chertemps, T.; Maïbèche, M.; Le Goff, G. Resistance in the genus Spodoptera: Key insect detoxification genes. Insects 2021, 12, 544. [Google Scholar] [CrossRef]
- Guedes, R.N.C.; Walse, S.S.; Throne, J.E. Sublethal exposure, insecticide resistance, and community stress. Curr. Opin. Insect Sci. 2017, 21, 47–53. [Google Scholar] [CrossRef]
- Hafeez, M.; Li, X.; Zhang, Z.; Huang, J.; Wang, L.; Zhang, J.; Shah, S.; Khan, M.; Xu, F.; Fernández-Grandon, G.; et al. De novo transcriptomic analyses revealed some detoxification genes and related pathways responsive to Noposion Yihaogong® 5% EC (Lambda-Cyhalothrin 5%) exposure in Spodoptera frugiperda third-instar larvae. Insects 2021, 12, 132. [Google Scholar] [CrossRef]
- Nawaz, A.; Ali, H.; Sufyan, M.; Gogi, M.D.; Arif, M.J.; Ranjha, M.H.; Arshid, M.; Waseem, M.; Mustafa, T.; Qasim, M.; et al. Comparative bio-efficacy of nuclear polyhedrosis virus (NPV) and Spinosad against American bollwormm, Helicoverpa armigera (Hubner). Rev. Bras. Èntomol. 2019, 63, 277–282. [Google Scholar] [CrossRef]
- Ayyub, M.B.; Nawaz, A.; Arif, M.J.; Amrao, L. Individual and combined impact of nuclear polyhedrosis virus and spinosad to control the tropical armyworm, Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae), in cotton in Pakistan. Egypt. J. Biol. Pest Control 2019, 29, 67. [Google Scholar] [CrossRef]
- Maqsood, S.; Afzal, M.; Aqueel, M.A.; Wakil, W.; Khan, H.A.A. Comparative evaluation of selected biorational insecticides against Spodoptera litura (Fabricius) on cauliflower. Pak. J. Zool. 2018, 50, 1645–1652. [Google Scholar] [CrossRef]
- Abid, A.D.; Saeed, S.; Zaka, S.M.; Shahzad, S.; Ali, M.; Iqbal, M.; Iqbal, N.; Jamal, Z.A. Field evaluation of nucleopolyhedrosis virus and some biorational insecticides against Helicoverpa armigera Hubner (Noctuidae: Lepidoptera). Saudi J. Biol. Sci. 2020, 27, 2106–2110. [Google Scholar] [CrossRef]
- Landwehr, A. Benefits of baculovirus use in IPM strategies for open field and protected vegetables. Front. Sustain. Food Syst. 2021, 4, 593796. [Google Scholar] [CrossRef]


| Type | Common Name | Active Ingredient Content | Supplier |
|---|---|---|---|
| Pyrroles | Chlorfenapyr | 98% | Hangzhou Lancheng Technology Co., Ltd. (Hangzhou, China) |
| Oxadiazine | Indoxacarb | 97% | |
| Amides | Chlorantraniliprole | 96% | |
| Cyantraniliprole | 94% | ||
| Macrolides | Spinosad | 90% | |
| Spinetoram | 94% | ||
| Emamectin benzonate | 70% | ||
| Insect growth regulators | Chlorfluazuron | 96% | |
| Lufenuron | 97% | ||
| Hexaflumuron | 97.6% | ||
| Methoxyfenozide | 98% |
| Insecticide | Treatment |
|---|---|
| Chlorfenapyr and SeMNPV | Water (control) |
| Chlorfenapyr (1800 mL/hm2, recommended dose) | |
| SeMNPV (8.58 × 106 OBs/mL) + chlorfenapyr (900 mL/hm2) | |
| SeMNPV (1.91 × 107 OBs/mL) + chlorfenapyr (900 mL/hm2) | |
| SeMNPV (8.58 × 106 OBs/mL) + chlorfenapyr (1800 mL/hm2) | |
| SeMNPV (1.91 × 107 OBs/mL) + chlorfenapyr (1800 mL/hm2) | |
| Emamectin benzonate and SeMNPV | Water (control) |
| Emamectin benzonate (900 g/hm2, recommended dose) | |
| SeMNPV (8.58 × 106 OBs/mL) + emamectin benzonate (450 g/hm2) | |
| SeMNPV (1.91 × 107 OBs/mL) + emamectin benzonate (450 g/hm2) | |
| SeMNPV (8.58 × 106 OBs/mL) + emamectin benzonate (900 g/hm2) | |
| SeMNPV (1.91 × 107 OBs/mL) + emamectin benzonate (900 g/hm2) |
| Insecticide | Population | LC50 (mg/L) (95% CI) | Slope ± SE | χ2 (df) | RR |
|---|---|---|---|---|---|
| Chlorfenapyr | Lab | 0.537 (0.389–0.728) b | 1.139 ± 0.137 | 1.445 (5) | — |
| PH | 4.198 (3.422–5.189) a | 1.878 ± 0.190 | 2.062 (4) | 7.82 | |
| TX | 3.554 (2.469–5.152) a | 1.713 ± 0.199 | 4.076 (4) | 6.62 | |
| Indoxacarb | Lab | 0.124 (0.056–0.198) c | 1.261 ± 0.225 | 3.354 (4) | — |
| PH | 1.411 (1.129–1.732) b | 1.934 ± 0.185 | 1.936 (5) | 11.38 | |
| TX | 1.977 (1.394–2.860) a | 2.224 ± 0.239 | 5.545 (4) | 15.94 | |
| Chlorantraniliprole | Lab | 0.118 (0.031–0.226) c | 1.145 ± 0.173 | 7.336 (5) | — |
| PH | 0.464 (0.347–0.592) b | 1.556 ± 0.182 | 0.622 (4) | 3.93 | |
| TX | 2.668 (2.188–3.300) a | 1.739 ± 0.176 | 3.014 (4) | 22.61 | |
| Cyantraniliprole | Lab | 1.189 (0.465–1.991) ab | 0.889 ± 0.192 | 3.227 (4) | — |
| PH | 2.571 (2.149–3.410) a | 1.968 ± 0.198 | 4.756 (4) | 2.16 | |
| TX | 1.018 (0.829–1.233) b | 2.470 ± 0.272 | 0.860 (4) | 0.86 | |
| Spinosad | Lab | 0.555 (0.232–0.898) a | 1.570 ± 0.231 | 5.136 (4) | — |
| PH | 0.669 (0.541–0.821) a | 1.869 ± 0.188 | 3.578 (4) | 1.21 | |
| TX | 0.693 (0.552–0.864) a | 1.770 ± 0.181 | 2.835 (4) | 1.25 | |
| Spinetoram | Lab | 0.057 (0.015–0.106) c | 1.220 ± 0.245 | 2.676 (4) | — |
| PH | 0.876 (0.686–1.082) a | 1.853 ± 0.188 | 1.939 (5) | 15.37 | |
| TX | 0.375 (0.175–0.557) b | 2.158 ± 0.331 | 4.763 (4) | 6.58 | |
| Emamectin benzonate | Lab | 0.044 (0.027–0.061) c | 1.440 ± 0.186 | 1.798 (5) | — |
| PH | 1.961 (1.639–2.335) a | 2.528 ± 0.256 | 1.303 (4) | 44.57 | |
| TX | 0.465 (0.370–0.569) b | 1.962 ± 0.204 | 3.627 (4) | 10.57 | |
| Chlorfluazuron | Lab | 0.247 (0.132–0.384) b | 0.816 ± 0.134 | 4.808 (5) | — |
| PH | 1.496 (1.180–1.863) a | 1.703 ± 0.164 | 1.546 (5) | 6.06 | |
| TX | 1.355 (1.074–1.651) a | 2.176 ± 0.240 | 1.923 (4) | 5.49 | |
| Lufenuron | Lab | 0.383 (0.233–0.572) b | 0.888 ± 0.139 | 4.156 (5) | — |
| PH | 1.722 (1.175–2.322) a | 1.281 ± 0.175 | 2.055 (4) | 4.50 | |
| TX | 0.511 (0.336–0.683) b | 2.238 ± 0.268 | 4.333 (4) | 1.33 | |
| Hexaflumuron | Lab | 0.273 (0.163–0.401) b | 0.962 ± 0.142 | 3.630 (5) | — |
| PH | 0.353 (0.271–0.441) b | 1.920 ± 0.217 | 1.949 (4) | 1.29 | |
| TX | 0.568 (0.415–0.757) a | 2.053 ± 0.201 | 4.378 (4) | 2.08 | |
| Methoxyfenozide | Lab | 0.473 (0.271–0.742) b | 1.382 ± 0.167 | 7.451 (5) | — |
| PH | 0.896 (0.591–1.242) a | 1.798 ± 0.202 | 4.191 (4) | 1.89 | |
| TX | 0.890 (0.651–1.153) a | 1.427 ± 0.175 | 1.230 (4) | 1.88 |
| Time | Population | LC25 (×106 OBs/mL) (95% CI) | LC50 (×106 OBs/mL) (95% CI) | Slope ± SE | χ2 (df) |
|---|---|---|---|---|---|
| 48 h | Lab | 1.790 (0.583–3.097) | 4.602 (2.528–7.453) | 1.645 ± 0.178 | 8.627 (4) |
| PH | 8.578 (6.570–10.642) | 19.107 (15.645–23.676) | 1.939 ± 0.196 | 2.944 (4) | |
| TX | - | - | - | - | |
| 72 h | Lab | 1.563 (0.537–2.691) | 4.314 (2.449–6.725) | 1.530 ± 0.177 | 6.446 (4) |
| PH | 4.349 (2.189–6.603) | 11.461 (7.729–16.755) | 1.603 ± 0.175 | 5.023 (4) | |
| TX | 23.643 (15.976–34.291) | 138.900 (85.311–297.972) | 0.877 ± 0.124 | 4.474 (5) | |
| 96 h | Lab | 0.571 (0.022–1.502) | 2.057 (0.410–4.008) | 1.212 ± 0.175 | 8.547 (4) |
| PH | 0.535 (0.131–1.120) | 1.929 (0.849–3.082) | 1.211 ± 0.204 | 2.760 (4) | |
| TX | 2.358 (1.053–3.960) | 14.952 (10.293–21.208) | 0.841 ± 0.111 | 3.804 (5) | |
| 120 h | Lab | 0.182 (0.030–0.441) | 0.717 (0.244–1.262) | 1.135 ± 0.208 | 1.318 (4) |
| PH | 0.229 (0.022–0.660) | 0.979 (0.229–1.942) | 1.070 ± 0.216 | 1.217 (4) | |
| TX | 1.966 (1.162–2.859) | 6.312 (4.642–8.142) | 1.331 ± 0.136 | 3.238 (5) |
| Insecticide | Population | LC50 (mg/L) (95% CI) | Slope ± SE | χ2 (df) | RR a | Fold b |
|---|---|---|---|---|---|---|
| Chlorfenapyr + SeMNPV (LC25) | Lab | 0.319 (0.254–0.392) | 1.808 ± 0.171 | 4.046 (5) | 0.59 | 0.59 |
| PH | 0.319 (0.220–0.424) | 1.391 ± 0.162 | 2.432 (5) | 0.59 | 0.08 | |
| TX | 2.204 (1.889–2.575) | 2.607 ± 0.216 | 2.396 (5) | 4.10 | 0.62 | |
| Indoxacarb + SeMNPV (LC25) | Lab | 0.239 (0.182–0.323) | 1.345 ± 0.173 | 3.189 (4) | 1.93 | 1.93 |
| PH | 0.488 (0.358–0.659) | 1.630 ± 0.153 | 5.319 (5) | 3.94 | 0.35 | |
| TX | 0.577 (0.491–0.677) | 2.240 ± 0.175 | 4.433 (5) | 4.65 | 0.29 | |
| Chlorantraniliprole + SeMNPV (LC25) | Lab | 0.334 (0.258–0.420) | 1.591 ± 0.160 | 4.333 (5) | 2.83 | 2.83 |
| PH | 0.048 (0.027–0.071) | 1.242 ± 0.170 | 4.273 (5) | 0.41 | 0.10 | |
| TX | 1.065 (0.791–1.437) | 1.818 ± 0.146 | 7.389 (5) | 9.03 | 0.40 | |
| Cyantraniliprole + SeMNPV (LC25) | Lab | 1.107 (0.839–1.520) | 1.208 ± 0.135 | 1.971 (5) | 0.93 | 0.93 |
| PH | 0.381 (0.274–0.514) | 1.742 ± 0.169 | 5.575 (5) | 0.32 | 0.15 | |
| TX | 0.572 (0.435–0.734) | 1.278 ± 0.147 | 1.904 (4) | 0.48 | 0.56 | |
| Spinosad + SeMNPV (LC25) | Lab | 0.699 (0.516–1.022) | 1.183 ± 0.140 | 4.620 (5) | 1.26 | 1.26 |
| PH | 0.557 (0.432–0.740) | 1.354 ± 0.141 | 2.798 (5) | 1.00 | 0.83 | |
| TX | 0.465 (0.358–0.599) | 1.180 ± 0.120 | 4.339 (5) | 0.84 | 0.67 | |
| Spinetoram + SeMNPV (LC25) | Lab | 0.035 (0.019–0.051) | 1.277 ± 0.177 | 3.374 (5) | 0.61 | 0.61 |
| PH | 0.270 (0.215–0.332) | 1.826 ± 0.178 | 1.841 (5) | 4.74 | 0.31 | |
| TX | 0.142 (0.109–0.175) | 1.890 ± 0.200 | 1.327 (5) | 2.49 | 0.38 | |
| Emamectin benzonate + SeMNPV (LC25) | Lab | 0.094 (0.044–0.174) | 0.525 ± 0.117 | 0.905 (5) | 2.14 | 2.14 |
| PH | 0.125 (0.086–0.166) | 1.562 ± 0.187 | 0.976 (5) | 2.84 | 0.06 | |
| TX | 0.379 (0.306–0.458) | 1.936 ± 0.184 | 0.669 (5) | 8.61 | 0.81 | |
| Chlorfluazuron + SeMNPV (LC25) | Lab | 0.049 (0.021–0.082) | 0.797 ± 0.131 | 2.381 (5) | 0.20 | 0.20 |
| PH | 0.237 (0.177–0.303) | 1.602 ± 0.169 | 3.559 (5) | 0.96 | 0.16 | |
| TX | 1.080 (0.715–1.625) | 2.168 ± 0.234 | 7.577 (4) | 4.37 | 0.80 | |
| Lufenuron + SeMNPV (LC25) | Lab | 0.361 (0.218–0.634) | 1.102 ± 0.137 | 7.050 (5) | 0.94 | 0.94 |
| PH | 0.367 (0.240–0.506) | 1.221 ± 0.149 | 2.862 (5) | 0.96 | 0.21 | |
| TX | 1.099 (0.856–1.436) | 1.391 ± 0.146 | 3.014 (5) | 2.87 | 2.16 | |
| Hexaflumuron + SeMNPV (LC25) | Lab | 0.145 (0.088–0.217) | 0.823 ± 0.127 | 2.456 (5) | 0.53 | 0.53 |
| PH | 0.130 (0.087–0.176) | 1.508 ± 0.186 | 1.393 (5) | 0.48 | 0.37 | |
| TX | 0.415 (0.297–0.570) | 1.880 ± 0.192 | 4.703 (4) | 1.52 | 0.73 | |
| Methoxyfenozide + SeMNPV (LC25) | Lab | 0.171 (0.138–0.209) | 2.071 ± 0.206 | 2.946 (5) | 0.36 | 0.36 |
| PH | 0.141 (0.085–0.203) | 1.159 ± 0.153 | 4.133 (5) | 0.30 | 0.16 | |
| TX | 0.739 (0.617–0.883) | 2.376 ± 0.217 | 2.938 (5) | 1.56 | 0.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Zhang, J.; Lin, Y.; Li, X.; Liu, M.; Hafeez, M.; Huang, J.; Zhang, Z.; Chen, L.; Ren, X.; et al. Spodoptera exigua Multiple Nucleopolyhedrovirus Increases the Susceptibility to Insecticides: A Promising Efficient Way for Pest Resistance Management. Biology 2023, 12, 260. https://doi.org/10.3390/biology12020260
Zhou S, Zhang J, Lin Y, Li X, Liu M, Hafeez M, Huang J, Zhang Z, Chen L, Ren X, et al. Spodoptera exigua Multiple Nucleopolyhedrovirus Increases the Susceptibility to Insecticides: A Promising Efficient Way for Pest Resistance Management. Biology. 2023; 12(2):260. https://doi.org/10.3390/biology12020260
Chicago/Turabian StyleZhou, Shuxing, Jinming Zhang, Ya Lin, Xiaowei Li, Min Liu, Muhammad Hafeez, Jun Huang, Zhijun Zhang, Limin Chen, Xiaoyun Ren, and et al. 2023. "Spodoptera exigua Multiple Nucleopolyhedrovirus Increases the Susceptibility to Insecticides: A Promising Efficient Way for Pest Resistance Management" Biology 12, no. 2: 260. https://doi.org/10.3390/biology12020260
APA StyleZhou, S., Zhang, J., Lin, Y., Li, X., Liu, M., Hafeez, M., Huang, J., Zhang, Z., Chen, L., Ren, X., Dong, W., & Lu, Y. (2023). Spodoptera exigua Multiple Nucleopolyhedrovirus Increases the Susceptibility to Insecticides: A Promising Efficient Way for Pest Resistance Management. Biology, 12(2), 260. https://doi.org/10.3390/biology12020260