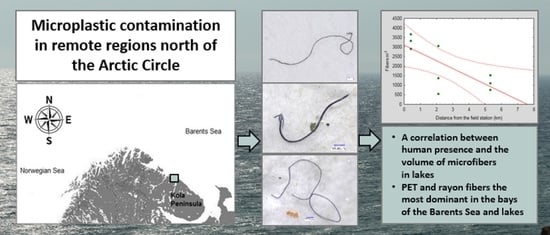
First Evidence of Microplastic Occurrence in the Marine and Freshwater Environments in a Remote Polar Region of the Kola Peninsula and a Correlation with Human Presence
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. The Study Sites and Sample Collection and Preparation
2.2. Negative Control Samples
2.3. Identification of MPs
2.4. Statistical Analyses
3. Results
3.1. MP Debris in the Surface Water of the Barents Sea
3.2. MP Contamination in the Surface Water of Lakes: Significant Correlation with Human Activity
3.3. Method-Driven Contamination–Negative Control
3.4. Polymers Identified
4. Discussion
4.1. Anthropogenic Effect on the Microplastic Concentration
4.2. PET and Rayon Microfibers Dominate in Arctic Waters
4.3. Potential Risks to Organisms from Microplastic Pollution–Implications for Polar Animals
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cole, M.; Lindeque, P.; Halsband, C.; Galloway, T.S. Microplastics as contaminantsin the marine environment: A review. Mar. Pollut. Bull. 2011, 62, 2588–2597. [Google Scholar] [CrossRef] [PubMed]
- Van Cauwenberghe, L.; Janssen, C.R. Microplastics in bivalves cultured for human consumption. Environ. Pollut. 2014, 193, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Zhu, C.; Wang, C.; Gu, C. Occurrence and ecological impacts of microplastics in soil systems: A review. Bull. Environ. Contam. Toxicol. 2019, 102, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Danopoulos, E.; Twiddy, M.; Rotchell, J.M. Microplastic contamination of drinking water: A systematic review. PLoS ONE 2020, 15, e0236838. [Google Scholar] [CrossRef] [PubMed]
- Kanhai, D.K.; Gardfeldt, K.; Krumpen, T.; Thompson, R.C.; O’Connor, I. Microplastics in sea ice and seawater beneath ice floes from the Arctic Ocean. Sci. Rep. 2020, 19, 5004. [Google Scholar] [CrossRef]
- Szymańska, M.; Obolewski, K. Microplastics as contaminants in freshwater environments: A multidisciplinary review. Ecohydrol. Hydrobiol. 2020, 20, 333–345. [Google Scholar] [CrossRef]
- Bessa, F.; Ratcliffe, N.; Otero, V.; Sobral, P.; Marques, J.C.; Waluda, C.M.; Trathan, P.N.; Xavier, J.C. Microplastics in gentoo penguins from the Antarctic region. Sci. Rep. 2019, 9, 14191. [Google Scholar] [CrossRef]
- Nelms, S.E.; Barnett, J.; Brownlow, A.; Davison, N.J.; Deaville, R.; Galloway, T.S.; Lindeque, P.K.; Santillo, D.; Godley, B.J. Microplastics in marine mammals stranded around the British coast: Ubiquitous but transitory? Sci. Rep. 2019, 9, 1075. [Google Scholar] [CrossRef]
- Sequeira, I.F.; Prata, J.C.; Da Costa, J.P.; Duarte, A.C.; Rocha-Santos, T. Worldwide contamination of fish with microplastics: A brief global overview. Mar. Pollut. Bull. 2020, 160, 111681. [Google Scholar] [CrossRef]
- Pastorino, P.; Prearo, M.; Di Blasio, A.; Barcelò, D.; Anselmi, S.; Colussi, S.; Alberti, S.; Tedde, G.; Dondo, A.; Ottino, M.; et al. Microplastics Occurrence in the European Common Frog (Rana temporaria) from Cottian Alps (Northwest Italy). Diversity 2022, 14, 66. [Google Scholar] [CrossRef]
- Tan, F.; Yang, H.; Xu, X.; Fang, Z.; Xu, H.; Shi, Q.; Zhang, X.; Wang, G.; Lin, L.; Zhou, S.; et al. Microplastic pollution around remote uninhabited coral reefs of Nansha Islands, South China Sea. Sci. Total Environ. 2020, 725, 138383. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Lei, Z.; Fuad, M.T.I.; Wang, Q.; Sun, S.; Fang, J.K.-H.; Liu, X. Distribution and potential sources of microplastics in sediments in remote lakes of Tibet, China. Sci. Total Environ. 2022, 806, 150526. [Google Scholar] [CrossRef]
- Ambrosini, R.; Azzoni, R.S.; Pittino, F.; Diolaiuti, G.; Franzetti, A.; Parolini, M. First evidence of microplastic contamination in the supraglacial debris of an alpine glacier. Environ. Pollut. 2019, 253, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Velasco, A.D.J.N.; Rard, L.; Blois, W.; Lebrun, D.; Lebrun, F.; Pothe, F.; Stoll, S. Microplastic and fibre contamination in a remote mountain lake in Switzerland. Water 2020, 12, 2410. [Google Scholar] [CrossRef]
- Zhang, K.; Su, J.; Xiong, X.; Wu, X.; Wu, C.; Liu, J. Microplastic pollution of lakeshore sediments from remote lakes in Tibet plateau, China. Environ. Pollut. 2016, 219, 450–455. [Google Scholar] [CrossRef]
- Feng, S.; Lu, H.; Tian, P.; Xue, Y.; Lu, J.; Tang, M.; Feng, W. Analysis of microplastics in a remote region of the Tibetan Plateau: Implications for natural environmental response to human activities. Sci. Total Environ. 2020, 15, 739:140087. [Google Scholar] [CrossRef]
- Yu, X.; Peng, J.; Wang, J.; Wang, K.; Bao, S. Occurrence of microplastics in the beach sand of the Chinese inner sea: The Bohai Sea. Environ. Pollut. 2016, 214, 722–730. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, Q.; Li, Y.; Tan, S.; Kang, Z.; Yu, X.; Shi, H. Microplastic pollution in the Maowei Sea, a typical mariculture bay of China. Sci. Total Environ. 2019, 658, 62–68. [Google Scholar] [CrossRef]
- Jiang, C.; Yin, L.; Wen, X.; Du, C.; Wu, L.; Long, Y.; Liu, Y.; Ma, Y.; Yin, Q.; Zhou, Z.; et al. Microplastics in sediment and surface water of west dongting lake and south dongting lake: Abundance, source and composition. Int. J. Environ. Res. Public Health 2018, 15, 2164. [Google Scholar] [CrossRef]
- Jiang, C.; Yin, L.; Li, Z.; Wen, X.; Luo, X.; Hu, S.; Yang, H.; Long, Y.; Deng, B.; Huang, L.; et al. Microplastic pollution in the rivers of the Tibet plateau. Environ. Pollut. 2019, 249, 91–98. [Google Scholar] [CrossRef]
- González-Pleiter, M.; Edo, C.; Velázquez, D.; Casero-Chamorro, M.C.; Leganés, F.; Quesada, A.; Fernández-Piñas, F.; Rosal, R. First detection of microplastics in the freshwater of an Antarctic Specially Protected Area. Mar. Pollut. Bull. 2020, 161, 111811. [Google Scholar] [CrossRef] [PubMed]
- González-Pleiter, M.; Velázquez, D.; Edo, C.; Carretero, O.; Gago, J.; Barón-Sola, Á.; Hernández, L.E.; Yousef, I.; Quesada, A.; Leganés, F.; et al. Fibers spreading worldwide: Microplastics and other anthropogenic litter in an Arctic freshwater lake. Sci. Total Environ. 2020, 722, 137904. [Google Scholar] [CrossRef]
- Carr, S.A. Sources and dispersive modes of micro-fibers in the environment. Integr. Environ. Assess. Manage. 2017, 13, 466–469. [Google Scholar] [CrossRef] [PubMed]
- Obbard, R.W.; Sadri, S.; Wong, Y.Q.; Khitun, A.A.; Baker, I.; Thompson, R.C. Global warming releases microplastic legacy frozen in Arctic Sea ice. Earth Future 2014, 2, 315–320. [Google Scholar] [CrossRef]
- Lusher, A.L.; Tirelli, V.; O’Connor, I.; Officer, R. Microplastics in Arctic polar waters: The first reported values of particles in surface and sub-surface samples. Sci. Rep. 2015, 5, 14947. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, M.; Mützel, S.; Primpke, S.; Tekman, M.B.; Trachsel, J.; Gerdts, G. White and wonderful? Microplastics prevail in snow from the Alps to the Arctic. Sci. Adv. 2019, 5, 1157. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, T.; Kang, S.; Sillanpää, M. Importance of atmospheric transport for microplastics deposited in remote areas. Environ. Pollut. 2019, 254, 112953. [Google Scholar] [CrossRef]
- Dris, R.; Gasperi, J.; Saad, M.; Mirande, C.; Tassin, B. Synthetic fibers in atmospheric fallout: A source of microplastics in the environment? Mar. Pollut. Bull. 2016, 104, 290–293. [Google Scholar] [CrossRef]
- Kaliszewicz, A.; Winczek, M.; Karaban, K.; Kurzydłowski, D.; Górska, M.; Koselak, W.; Romanowski, J. The contamination of inland waters by microplastic fibres under different anthropogenic pressure: Preliminary study in Central Europe (Poland). Waste Manag. Res. 2020, 38, 1231–1238. [Google Scholar] [CrossRef]
- Lin, C.T.; Chiu, M.C.; Kuo, M.H. Effects of anthropogenic activities on microplastics in deposit–feeders (Diptera: Chironomidae) in an urban river of Taiwan. Sci. Rep. 2021, 11, 400. [Google Scholar] [CrossRef]
- Cole, M.; Lindeque, P.; Goodhead, R.; Cole, M.; Lindeque, P.; Fileman, E.; Halsband, C.; Goodhead, R.; Moger, J.; Galloway, T.S. Microplastic ingestion by zooplankton. Environ. Sci. Technol. 2013, 47, 6646–6655. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Nor, N.H.M.; Hermsen, E.; Kooi, M.; Mintenig, S.M.; De France, J. Microplastics in freshwaters and drinking water: Critical review and assessment of data quality. Water Res. 2019, 155, 410–422. [Google Scholar] [CrossRef]
- Pan, Z.; Guo, H.; Chen, H.; Wang, S.; Sun, X.; Zou, Q.; Zhang, Y.; Lin, H.; Cai, S.; Huang, J. Microplastics in the Northwestern Pacific: Abundance, distribution, and characteristics. Sci. Total Environ. 2019, 650, 1913–1922. [Google Scholar] [CrossRef]
- Obbard, R.W. Microplastics in Polar Regions: The role of long range transport. Curr. Opin. Environ. Sci. Health 2018, 1, 24–29. [Google Scholar] [CrossRef]
- Tekman, M.B.; Wekerle, C.; Lorenz, C.; Primpke, S.; Hasemann, C.; Gerdts, G.; Bergmann, M. Tying up loose ends of microplastic pollution in the Arctic: Distribution from the sea surface, through the water column to deep-sea sediments at the HAUSGARTEN observatory. Environ. Sci. Technol. 2020, 54, 4079–4090. [Google Scholar] [CrossRef]
- Desforges, J.-P.W.; Galbraith, M.; Dangerfield, N.; Ross, P.S. Widespread distribution of microplastics in subsurface seawater in the NE Pacific Ocean. Mar. Pollut. Bull. 2014, 79, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Waller, C.L.; Griffiths, H.J.; Waluda, C.M.; Thorpe, S.E.; Loaiza, I.; Moreno, B.; Pacherres, C.O.; Hughes, K.A. Microplastics in the Antarctic marine system: An emerging area of research. Sci. Total Environ. 2017, 598, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, J.; Sun, C.; Cao, W.; Wang, M.; Jiang, F.; Ju, P. Comparative study of three sampling methods for microplastics analysis in seawater. Sci. Total Environ. 2021, 765, 144495. [Google Scholar] [CrossRef] [PubMed]
- Frère, L.; Paul-Pont, I.; Rinnert, E.; Petton, S.; Jaffre, J.; Bihannic, I.; Soudant, P.; Lambert, C.; Huvet, A. Influence of environmental and anthropogenic factors on the composition, concentration and spatial distribution of microplastics: A case study of the Bay of Brest (Brittany, France). Environ. Pollut. 2017, 225, 211–222. [Google Scholar] [CrossRef]
- Song, Y.K.; Hong, S.H.; Eo, S.; Jang, M.; Han, G.M.; Isobe, A.; Shim, W.J. Horizontal and Vertical Distribution of Microplastics in Korean Coastal Waters. Environ. Sci. Technol. 2018, 52, 12188–12197. [Google Scholar] [CrossRef] [PubMed]
- Dowarah, K.; Devipriya, S.P. Microplastic prevalence in the beaches of Puducherry, India and its correlation with fishing and tourism/recreational activities. Mar. Pollut. Bull. 2019, 148, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.Y.; Hou, L.; Liang, Q.B.; Li, J.C.; Ren, J. Correlation Between Microplastics Pollution and Eutrophication in the Near Shore Waters of Dianchi Lake. Environ. Sci. 2021, 42, 3166–3175. [Google Scholar]
- Gasperi, J.; Dris, R.; Mirande-Bret, C.; Mandin, C.; Langlois, V.; Tassin, B. First overview of microplastics in indoor and outdoor air. In Proceedings of the 15th EuCheMS International Conference on Chemistry and the Environment, Leipzig, Germany, 22–25 September 2015; HAL Science: Lyon, France, 2015. [Google Scholar]
- Liu, Q.; Babanin, A.V.; Zieger, S.; Young, I.R.; Guan, C. Wind and wave climate in the arctic ocean as observed by altimeters. J. Clim. 2016, 29, 7957–7975. [Google Scholar] [CrossRef]
- Marshall, G.J.; Vignols, R.M.; Rees, W.G. Climate Change in the Kola Peninsula, Arctic Russia, during the Last 50 Years from Meteorological Observations. J. Clim. 2016, 29, 6823–6840. [Google Scholar] [CrossRef]
- Fang, C.; Zheng, R.; Zhang, Y.; Hong, F.; Mu, J.; Chen, M.; Song, P.; Lin, L.; Lin, H.; Le, F.; et al. Microplastic Contamination in Benthic Organisms from the Arctic and Sub-arctic Regions. Chemosphere 2018, 209, 298–306. [Google Scholar] [CrossRef]
- Barrows, A.P.W.; Cathey, S.E.; Petersen, C.W. Marine environment microfiber contamination: Global patterns and the diversity of microparticle origins. Environ. Pollut. 2018, 237, 275–284. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Mu, J.; Zhang, S.; Qu, L.; Jin, F.; Fang, C.; Ma, X.; Zhang, W.; Wang, J. Microplastics abundance and characteristics in surface waters from the Northwest Pacific, the Bering Sea, and the Chukchi Sea. Mar. Pollut. Bull. 2019, 143, 58–65. [Google Scholar] [CrossRef]
- Lenaker, P.L.; Corsi, S.R.; Mason, S.A. Spatial Distribution of Microplastics in Surficial Benthic Sediment of Lake Michigan and Lake Erie. Environ. Sci. Technol. 2021, 55, 373–384. [Google Scholar] [CrossRef]
- Ross, P.S.; Chastain, S.; Vassilenko, E.; Etemadifar, A.; Zimmermann, S.; Quesnel, S.-A.; Eert, J.; Solomon, E.; Patankar, S.; Posacka, A.M.; et al. Pervasive distribution of polyester fibres in the Arctic ocean is driven by Atlantic inputs. Nat. Commun. 2021, 12, 106. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, L.; Liu, K.; Li, D. Prevalence of microplastic fibers in the marginal sea water column off southeast China. Sci. Total Environ. 2022, 804, 150138. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Zhang, Y.; Zheng, R.; Hong, F.; Zhang, M.; Zhang, R.; Mou, J.; Mu, J.; Lin, L.; Bo, J. Spatio-temporal variation of microplastic pollution in the sediment from the Chukchi Sea over five years. Sci. Total Environment. 2022, 806, 150530. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Li, J.; Sun, C.; Jiang, F.; Ju, P.; Qu, L.; Zheng, Y.; He, C. Detection of microplastics in local marine organisms using a Multi-Technology System. Anal. Methods 2019, 11, 78–87. [Google Scholar] [CrossRef]
- Lusher, A.L.; McHugh, M.; Thompson, R.C. Occurrence of microplastics in the gastrointestinal tract of pelagic and demersal fish from the English Channel. Mar. Pollut. Bull. 2013, 67, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Steer, M.; Cole, M.; Thompson, R.C.; Lindeque, P.K. Microplastic ingestion in fish larvae in the western English Channel. Environ. Pollut. 2017, 226, 250–259. [Google Scholar] [CrossRef]
- Lusher, A.L.; Hernandez-Milian, G.; O’Brien, J.; Berrow, S.; O’Connor, I.; Officer, R. Microplastic and macroplastic ingestion by a deep diving, oceanic cetacean: The True’s beaked whale Mesoplodon mirus. Environ. Pollut. 2015, 199, 185–191. [Google Scholar] [CrossRef]
- Browne, M.A.; Niven, S.J.; Galloway, T.S.; Rowland, S.J.; Thompson, R.C. Microplastic moves pollutants and additives to worms, reducing functions linked to health and biodiversity. Curr. Biol. 2013, 23, 2388–2392. [Google Scholar] [CrossRef]
- Ellingsen, I.H.; Dalpadado, P.; Slagstad, D.; Loeng, H. Impact of climatic change on the biological production in the Barents Sea. Clim. Change 2008, 87, 155–175. [Google Scholar] [CrossRef]
- Wassmann, P.; Reigstad, M.; Haug, T.; Rudels, B.; Carroll, M.L.; Hop, H.; Gabrielsen, G.W.; Falk-Petersen, S.; Denisenko, S.G.; Arashkevich, E.; et al. Food webs and carbon flux in the Barents Sea. Prog. Oceanogr. 2006, 71, 232–287. [Google Scholar] [CrossRef]
- Kędra, M.; Renaud, P.E.; Andrade, H.; Goszczko, I.; Ambrose, W.G. Benthic community structure, diversity, and productivity in the shallow Barents Sea bank (Svalbard Bank). Mar. Biol. 2013, 160, 805–819. [Google Scholar] [CrossRef]
- Gulliksen, B.; Hop, H.; Nielssen, M. Benthic life. In Ecosystem Barents Sea; Sakshaug, E., Johnsen, G., Kovacs, K., Eds.; Ch 15; Acad. Press: Trondheim, Norway, 2009; pp. 339–372. [Google Scholar]
- Li, S.; Chen, Y.; Gao, Y.; Xia, Z.; Zhan, A. Chemical oxidants affect byssus adhesion in the highly invasive fouling mussel Limnoperna fortunei. Sci. Total Environ. 2018, 646, 1367–1375. [Google Scholar] [CrossRef] [PubMed]
- Boerger, C.M.; Lattin, G.L.; Moore, S.L.; Moore, C.J. Plastic ingestion by planktivorous fishes in the north pacific central gyre. Mar. Pollut. Bull. 2010, 60, 2275–2278. [Google Scholar] [CrossRef] [PubMed]
- de Sȧ, L.C.; Luís, L.G.; Guilhermino, L. Effects of microplastics on juveniles of the common goby (Pomatoschistus microps): Confusion with prey, reduction of the predatory performance and efficiency, and possible influence of developmental conditions. Environ. Pollut. 2015, 196, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Besseling, E.; Foekema, E.M.; van Franeker, J.A.; Leopold, M.F.; Kühn, S.; Bravo Rebolledo, E.L.; Heße, E.; Mielke, L.; IJzer, J.; Kamminga, P.; et al. Microplastic in a macro filter feeder: Humpback whale Megaptera novaeangliae. Mar. Pollut. Bull. 2015, 95, 248–252. [Google Scholar] [CrossRef]
- Farrell, P.; Nelson, K. Trophic level transfer of microplastic: Mytilus edulis (L.) to Carcinus maenas (L.). Environ. Pollut. 2013, 177, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Setӓlӓ, O.; Fleming-Lehtinen, V.; Lehtiniemi, M. Ingestion and transfer of microplastics in the planktonic food web. Environ. Pollut. 2014, 185, 77–83. [Google Scholar] [CrossRef]
- Morgana, S.; Ghigliotti, L.; Estevez-Calvar, N.; Stifanese, R.; Wieckzorek, A.; Doyle, T.; Christiansen, J.S.; Faimali, M.; Garaventa, F. Microplastics in the Arctic: A case study with sub-surface water and fish samples off Northeast Greenland. Environ. Pollut. 2018, 242, 1078–1086. [Google Scholar] [CrossRef]
- Kühn, S.; Schaafsma, F.L.; van Werven, B.; Flores, H.; Bergmann, M.; Egelkraut-Holtus, M.; Tekman, M.B.; van Franeker, J.A. Plastic ingestion by juvenile polar cod (Boreogadus saida) in the Arctic Ocean. Polar Biol. 2018, 41, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Dawson, A.L.; Kawaguchi, S.; King, C.K.; Townsend, K.A.; King, R.; Huston, W.M.; Bengtson Nash, S.M. Turning microplastics into nanoplastics through digestive fragmentation by Antarctic krill. Nat. Commun. 2018, 9, 1001. [Google Scholar] [CrossRef]
- Wright, S.L.; Rowe, D.; Thompson, R.C.; Galloway, T.S. Microplastic ingestion decreases energy reserves in marine worms. Curr. Biol. 2013, 23, R1031–R1033. [Google Scholar] [CrossRef]
- do Sul, J.A.I.; Costa, M.F. The present and future of microplastic pollution in the marine environment. Environ. Pollut. 2014, 185, 352–364. [Google Scholar] [CrossRef]
- Chen, Q.; Yin, D.; Jia, Y.; Schiwy, S.; Legradi, J.; Yang, S.; Hollert, H. Enhanced uptake of BPA in the presence of nanoplastics can lead to neurotoxic effects in adult zebrafish. Sci. Total Environ. 2017, 609, 1312–1321. [Google Scholar] [CrossRef] [PubMed]
- Browne, M.A.; Galloway, T.S.; Thompson, R.C. Microplastic e an emerging contaminant of potential concern? Integr. Environ. Assess. Manag. 2007, 3, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.; McGonigle, D.; Russell, A.E. Lost at sea: Where is all the plastic? Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- Murray, F.; Cowie, P.R. Plastic contamination in the decapod crustacean Nephrops norvegicus (Linnaeus, 1758). Mar. Pollut. Bull. 2011, 62, 1207–1217. [Google Scholar] [CrossRef]
- Watts, A.J.R.; Lewis, C.; Goodhead, R.M.; Beckett, S.J.; Moger, J.; Tyler, C.R.; Galloway, T.S. Uptake and retention of microplastics by the shore crab Carcinus maenas. Environ. Sci. Technol. 2014, 48, 8823–8830. [Google Scholar] [CrossRef]
- Devriese, L.I.; van der Meulen, M.D.; Maes, T.; Bekaert, K.; Paul-Pont, I.; Fr_ere, L.; Robbens, J.; Vethaak, A.D. Microplastic contamination in brown shrimp (Crangon crangon, Linnaeus 1758) from coastal waters of the Southern North Sea and Channel area. Mar. Pollut. Bull. 2015, 98, 179–187. [Google Scholar] [CrossRef] [PubMed]
- von Moos, N.; Burkhardt-Holm, P.; Kohler, A. Uptake and effects of microplastics on cells and tissue of the blue mussel Mytilus edulis L. after an experimental exposure. Environ. Sci. Technol. 2012, 46, 11327–11335. [Google Scholar] [CrossRef]
- Galloway, T.S. Micro- and nano-plastics and human health. In Marine Anthropogenic Litter; Springer: Berlin/Heidelberg, Germany, 2015; pp. 343–366. [Google Scholar]
- Hodgson, D.J. The Impacts of Microplastic Ingestion on Marine Polychaete Worms. Master’s Thesis, The University of Exeter, Exeter, UK, 2018; p. 116. [Google Scholar]
- Foekema, E.M.; De Gruijter, C.; Mergia, M.T.; van Franeker, J.A.; Murk, A.J.; Koelmans, A.A. Plastic in North Sea fish. Envir. Sci. Technol. 2013, 47, 8818–8824. [Google Scholar] [CrossRef]
- Janiec, K. The comparison of freshwater invertebrates of Spitsbergen (Arctic) and King George Island (Antarctic). Pol. Polar Res. 1996, 17, 173–202. [Google Scholar]
- Coulson, S.J.; Convey, P.; Aakra, K.; Aarvik, L.; Ávila-Jiménez, M.L.; Babenko, A.; Biersma, E.M.; Boström, S.; Brittain, J.E.; Carlsson, A.M.; et al. The terrestrial and freshwater invertebrate biodiversity of the archipelagoes of the Barents Sea; Svalbard, Franz Josef Land and Novaya Zemlya. Soil Biol. Biochem. 2014, 68, 440–470. [Google Scholar] [CrossRef]
- Mateos-Cárdenas, A.; Scott, D.T.; Seitmaganbetova, G.; van Pelt Frank, N.A.; John, O.H.; Marcel, A.K.J. Polyethylene microplastics adhere to Lemna minor (L.), yet have no effects on plant growth or feeding by Gammarus duebeni (Lillj.). Sci. Total Environ. 2019, 689, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A.L.; Welden, N.A.; Sobralc, P.; Cole, M. Sampling, isolating and identifying microplastics ingested by fish and invertebrates. Anal. Methods 2017, 9, 1346. [Google Scholar] [CrossRef]
- Luoto, T.P.; Rantala, M.V.; Kivilä, E.H.; Nevalainen, L.; Ojala, A.E.K. Biogeochemical cycling and ecological thresholds in a High Arctic lake (Svalbard). Aquat. Sci. 2019, 81, 34. [Google Scholar] [CrossRef]
- Kögel, T.; Hamilton, B.M.; Granberg, M.E.; Provencher, J.; Hammer, S.; Gomiero, A.; Magnusson, K.; Lusher, A.L. Current efforts on microplastic monitoring in Arctic fish and how to proceed. Arct. Sci. 2022, 1–18. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaliszewicz, A.; Panteleeva, N.; Karaban, K.; Runka, T.; Winczek, M.; Beck, E.; Poniatowska, A.; Olejniczak, I.; Boniecki, P.; Golovanova, E.V.; et al. First Evidence of Microplastic Occurrence in the Marine and Freshwater Environments in a Remote Polar Region of the Kola Peninsula and a Correlation with Human Presence. Biology 2023, 12, 259. https://doi.org/10.3390/biology12020259
Kaliszewicz A, Panteleeva N, Karaban K, Runka T, Winczek M, Beck E, Poniatowska A, Olejniczak I, Boniecki P, Golovanova EV, et al. First Evidence of Microplastic Occurrence in the Marine and Freshwater Environments in a Remote Polar Region of the Kola Peninsula and a Correlation with Human Presence. Biology. 2023; 12(2):259. https://doi.org/10.3390/biology12020259
Chicago/Turabian StyleKaliszewicz, Anita, Ninel Panteleeva, Kamil Karaban, Tomasz Runka, Michał Winczek, Ewa Beck, Agnieszka Poniatowska, Izabella Olejniczak, Paweł Boniecki, Elena V. Golovanova, and et al. 2023. "First Evidence of Microplastic Occurrence in the Marine and Freshwater Environments in a Remote Polar Region of the Kola Peninsula and a Correlation with Human Presence" Biology 12, no. 2: 259. https://doi.org/10.3390/biology12020259
APA StyleKaliszewicz, A., Panteleeva, N., Karaban, K., Runka, T., Winczek, M., Beck, E., Poniatowska, A., Olejniczak, I., Boniecki, P., Golovanova, E. V., & Romanowski, J. (2023). First Evidence of Microplastic Occurrence in the Marine and Freshwater Environments in a Remote Polar Region of the Kola Peninsula and a Correlation with Human Presence. Biology, 12(2), 259. https://doi.org/10.3390/biology12020259