Targeted Metabolic Profiles of the Leaves and Xylem Sap of Two Sugarcane Genotypes Infected with the Vascular Bacterial Pathogen Leifsonia xyli subsp. xyli
Abstract
:1. Introduction
2. Results
2.1. Lxx Titer Is Higher in Inoculated S Plants
2.2. Untargeted Metabolomics Detected Sugars, Organic Acids, and Phenolic Compounds in Sugarcane Leaves and Sap
2.3. Targeted Metabolomics of Leaves and Xylem Sap
2.4. Small Variations in the Composition of the Xylem Sap in Relation to Plant Genotype, Time, and Bacterial Inoculation
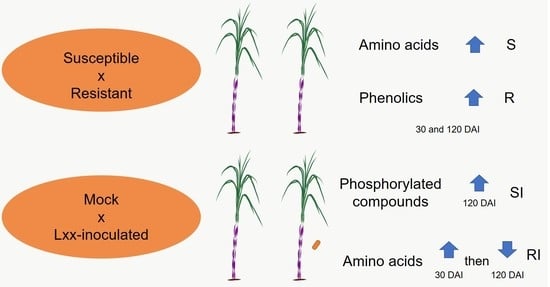
2.5. Resistant and Susceptible Mock-Inoculated Plants Have Distinct Leaf Metabolic Profiles and Change with Time
2.6. Sugarcane Varieties Respond Differently to Pathogen Inoculation
3. Discussion
3.1. The Parasitic Behavior of Leifsonia xyli subsp. xyli Is a Challenge for Omics Studies
3.2. Leaves Had a Greater Number of Identified Features Than the Xylem Sap
3.3. The Susceptible Variety Had Higher Accumulation of Bacterial Growth Enhancers and Fewer Inhibitors
3.4. Sugarcane Varieties Show Distinct Metabolic Responses to Bacterial Inoculation in the Leaves
3.5. Bacterial Inoculation Decreases the Abundance of Abscisic in the Xylem Sap of the Susceptible Variety
3.6. Transcriptomic Data Expand the Connection between Metabolic Changes of the Resistant Genotype Related to Pathogen Inoculation
4. Materials and Methods
4.1. Chemicals
4.2. Bacterial Cultivation
4.3. Plant Inoculation and Bacterial Quantification
4.4. Sample Collection
4.5. Metabolomic Analyses
4.5.1. Untargeted Metabolomics
4.5.2. Targeted Metabolomics
4.6. Statistical Analysis
5. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, W.-F.; Shen, K.; Huang, Y.-K.; Wang, X.-Y.; Yin, J.; Luo, Z.-M.; Zhang, R.-Y.; Shan, H.-L. Incidence of sugarcane ratoon stunting disease in the major cane-growing regions of China. Crop Prot. 2014, 60, 44–47. [Google Scholar] [CrossRef]
- Davis, M.J.; Gillaspie, A.G.; Harris, R.W.; Lawson, R.H. Ratoon stunting disease of sugarcane: Isolation of the causal bacterium. Science 1980, 210, 1365–1367. [Google Scholar] [CrossRef] [Green Version]
- Teakle, D.; Smith, P.; Steindl, D. Association of a small coryneform bacterium with the ratoon stunting disease of sugar-cane. Aust. J. Agric. Res. 1973, 24, 869. [Google Scholar] [CrossRef]
- Croft, B.J. A method for rating sugarcane cultivars for resistance to ratoon stunting disease based on an enzyme-linked immunoassay. Australas. Plant Pathol. 2002, 31, 63. [Google Scholar] [CrossRef]
- Fegan, M.; Croft, B.J.; Teakle, D.S.; Hayward, A.C.; Smith, G.R. Sensitive and specific detection of Clavibacter xyli subsp. xyli, causal agent of ratoon stunting disease of sugarcane, with a polymerase chain reaction-based assay. Plant Pathol. 1998, 47, 495–504. [Google Scholar] [CrossRef]
- Urashima, A.S.; Silva, M.F.; Correia, J.J.; Moraes, M.C.; Sibgh, A.V.; Sainz, M.B. Prevalence and severity of ratoon stunt in commercial Brazilian sugarcane fields. Plant Dis. 2017, 101, 815–821. [Google Scholar] [CrossRef] [Green Version]
- Fu, H.Y.; Sun, S.R.; Wang, J.D.; Ahmad, K.; Wang, H.B.; Chen, R.K.; Gao, S.J. Rapid and quantitative detection of Leifsonia xyli subsp. xyli in sugarcane stalk juice using a real-time fluorescent (TaqMan) PCR assay. Biomed Res. Int. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Urashima, A.S.; Marchetti, L.B.L. Incidence and Severity of Leifsonia xyli subsp. xyli Infection of Sugarcane in Sao Paulo State, Brazil. J. Phytopathol. 2013, 161, 478–484. [Google Scholar] [CrossRef]
- Young, A.J. Seedbed inspections underestimate the overall incidence of ratoon stunting disease. Int. Sugar J. 2016, 118, 254–258. [Google Scholar]
- Grisham, M. Effect of ratoon stunting disease on yield of sugarcane grown in multiple three-year plantings. Phytopathology 1991, 91, 337–340. [Google Scholar] [CrossRef]
- Monteiro-Vitorello, C.B.; Zerillo, M.M.; Van Sluys, M.-A.; Camargo, L.E.A. Genome sequence-based insights into the biology of the sugarcane pathogen Leifsonia xyli subsp. xyli. In Plant Pathogenic Bacteria—Genomics and Molecular Biology; Jackson, R., Ed.; Caister Academic Press: Cambridge, MA, USA, 2009; pp. 135–146. [Google Scholar]
- Quecine, M.C.; Silva, T.M.; Carvalho, G.; Saito, S.; Mondin, M.; Teixeira-Silva, N.S.; Camargo, L.E.A.; Monteiro-Vitorello, C.B. A stable Leifsonia xyli subsp. xyli GFP-tagged strain reveals a new colonization niche in sugarcane tissues. Plant Pathol. 2016, 65, 154–162. [Google Scholar] [CrossRef]
- Young, A.J. Possible origin of ratoon stunting disease following interspecific hybridization of Saccharum species. Plant Pathol. 2016, 65, 1403–1410. [Google Scholar] [CrossRef]
- Cia, M.C.; de Carvalho, G.; Azevedo, R.A.; Monteiro-Vitorello, C.B.; Souza, G.M.; Nishiyama-Junior, M.Y.; Lembke, C.G.; Antunes de Faria, R.S.d.C.; Marques, J.P.R.; Melotto, M.; et al. Novel insights into the early stages of ratoon stunting disease of sugarcane inferred from transcript and protein analysis. Phytopathology 2018, 108, 1455–1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, G.; da Silva, T.G.E.R.; Munhoz, A.T.; Monteiro-Vitorello, C.B.; Azevedo, R.A.; Melotto, M.; Camargo, L.E.A. Development of a qPCR for Leifsonia xyli subsp. xyli and quantification of the effects of heat treatment of sugarcane cuttings on Lxx. Crop Prot. 2016, 80, 51–55. [Google Scholar] [CrossRef] [Green Version]
- Urashima, A.S.; Grachet, N.G. Métodos de detecção de Leifsonia xyli subsp. xyli e efeito da termoterapia na brotação das gemas de diferentes variedades de cana-de-açúcar. Trop. Plant Pathol. 2012, 37, 57–64. [Google Scholar]
- Hoy, J.W.; Grisham, M.P.; Damann, K.E. Spread and increase of ratoon stunting disease of sugarcane and comparison of disease detection methods. Plant Dis. 1999, 83, 1170–1175. [Google Scholar] [CrossRef] [PubMed]
- Harrison, N.A.; Davis, M. Colonization of vascular tissues by Clavibacter xyli subsp. xyli in stalks of sugarcane differing in susceptibility to ratoon stunting disease. Phytopathology 1988, 78, 722–727. [Google Scholar] [CrossRef]
- Gillaspie, A.G., Jr.; Flax, G.; Koike, H. Relationship between numbers of diagnostic bacteria and injury by ratoon stunting disease in sugarcane. Plant Dis. Rep. 1976, 60, 573–575. [Google Scholar]
- McFarlane, S. The relationship between extent of colonisation by Leifsonia xyli subsp. xyli and yield loss in different sugarcane varieties. Proc. S. Afr. Sugar Technol. Assess 2002, 76, 281–284. [Google Scholar]
- Dal-Bianco, M.; Carneiro, M.S.; Hotta, C.T.; Chapola, R.G.; Hoffmann, H.P.; Garcia, A.A.F.; Souza, G.M. Sugarcane improvement: How far can we go? Curr. Opin. Biotechnol. 2012, 23, 265–270. [Google Scholar] [CrossRef]
- Bennett, R.N.; Wallsgrove, R.M. Secondary metabolites in plant defence mechanisms. New Phytol. 1994, 127, 617–633. [Google Scholar] [CrossRef]
- Lattanzio, V.; Lattanzio, V.M.T.; Cardinali, A.; Amendola, V. Role of Phenolics in the Resistance Mechanisms of Plants against Fungal Pathogens and Insects; Research Signpost: Kerala, India, 2006; Volume 661, ISBN 8130800349. [Google Scholar]
- Dixon, R.A. Natural products and plant disease resistance. Nature 2001, 411, 843–847. [Google Scholar] [CrossRef]
- Haapalainen, M.; Mattinen, J.; Metzler, M. The growth of a plant-parasitic bacterium, Clavibacter xyli subsp. cynodontis, is enhanced by xylem fluid components. Physiol. Mol. Plant Pathol. 2000, 56, 147–155. [Google Scholar] [CrossRef]
- Monteiro-Vitorello, C.B.; Camargo, L.E.A.; Van Sluys, M.A.; Kitajima, J.P.; Truffi, D.; Do Amaral, A.M.; Harakava, R.; De Oliveira, J.C.F.; Wood, D.; De Oliveira, M.C.; et al. The genome sequence of the gram-positive sugarcane pathogen Leifsonia xyli subsp. xyli. Mol. Plant-Microbe Interact. 2004, 17, 827–836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, M.J.; Dean, J.L.; Harrison, N.A. Quantitative variability of Clavibacter xyli subsp. xyli populations in sugarcane cultivars differing in resistance to ratoon stunting disease. Phytopathology 1988, 78, 462–468. [Google Scholar] [CrossRef]
- Rossler, L. The effects of ratoon stunting disease on three sugarcane varieties under different irrigation regimes. In Proceedings of the International Society of Sugarcane Technologists, Durban, South Africa, 13–19 June 1974; pp. 250–257. [Google Scholar]
- Ngaruiya, P.N.; Shipton, W.A.; Coventry, R. Ratoon stunting disease of sugarcane as influenced by environmental stressors. In Proceedings of the 2005 Conference of the Australian Society of Sugar Cane Technologists, Bundaberg, QLD, Australia, 3–6 May 2005; Volume 27, pp. 324–333. [Google Scholar]
- Alvarez, S.; Marsh, E.L.; Schroeder, S.G.; Schachtman, D.P. Metabolomic and proteomic changes in the xylem sap of maize under drought. Plant. Cell Environ. 2008, 31, 325–340. [Google Scholar] [CrossRef]
- Coutinho, I.D.; Baker, J.M.; Ward, J.L.; Beale, M.H.; Creste, S.; Cavalheiro, A.J. Metabolite profiling of sugarcane genotypes and identification of flavonoid glycosides and phenolic acids. J. Agric. Food Chem. 2016, 64, 4198–4206. [Google Scholar] [CrossRef]
- Schurr, U. Xylem sap sampling—new approaches to an old topic. Trends Plant Sci. 1998, 3, 293–298. [Google Scholar] [CrossRef]
- Rellán-Álvarez, R. Metabolite profile changes in xylem sap and leaf extracts of strategy I plants in response to iron deficiency and resupply. Front. Plant Sci. 2011, 2, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Radkov, A.D.; Moe, L.A. Bacterial synthesis of d-amino acids. Appl. Microbiol. Biotechnol. 2014, 98, 5363–5374. [Google Scholar] [CrossRef]
- Whitehead, N.A.; Barnard, A.M.L.; Slater, H.; Simpson, N.J.L.; Salmond, G.P.C. Quorum-sensing in Gram-negative bacteria. FEMS Microbiol. Rev. 2001, 25, 365–404. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, W.; Blanot, D.; De Pedro, M.A. Peptidoglycan structure and architecture. FEMS Microbiol. Rev. 2008, 32, 149–167. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Woo, H.M. Deciphering bacterial xylose metabolism and metabolic engineering of industrial microorganisms for use as efficient microbial cell factories. Appl. Microbiol. Biotechnol. 2018, 102, 9471–9480. [Google Scholar] [CrossRef]
- Faria, R.S.C.A.; Cia, M.C.; Monteiro-Vitorello, C.B.; Azevedo, R.A.; Camargo, L.E.A. Characterization of genes responsive to osmotic and oxidative stresses of the sugarcane bacterial pathogen Leifsonia xyli subsp. xyli. Brazilian J. Microbiol. 2020, 51, 77–86. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, H.; Zhu, S.; Ma, C.; Wang, Z. Antibacterial activity and mechanism of action of chlorogenic acid. J. Food Sci. 2011, 76, M398–M403. [Google Scholar] [CrossRef]
- Ngadze, E.; Icishahayo, D.; Coutinho, T.A.; Vand der Waals, J.E. Chlorogenic acid, and total soluble phenols in resistance of potatoes to soft rot. Plant Dis. 2012, 96, 186–192. [Google Scholar] [CrossRef] [Green Version]
- Niggeweg, R.; Michael, A.J.; Martin, C. Engineering plants with increased levels of the antioxidant chlorogenic acid. Nat. Biotechnol. 2004, 22, 746–754. [Google Scholar] [CrossRef]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef]
- Grant, M.R.; Jones, J.D.G. Hormone (dis)harmony moulds plant health and disease. Science 2009, 324, 750–752. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qi, M.; Mei, C. Endogenous salicylic acid protects rice plants from oxidative damage caused by aging as well as biotic and abiotic stress. Plant J. 2004, 40, 909–919. [Google Scholar] [CrossRef]
- Meng, D.; Li, C.; Park, H.J.; González, J.; Wang, J.; Dandekar, A.M.; Turgeon, B.G.; Cheng, L. Sorbitol modulates resistance to Alternaria alternata by regulating the expression of an NLR resistance gene in apple. Plant Cell 2018, 30, 1562–1581. [Google Scholar] [CrossRef] [Green Version]
- Qian, Y.; Tan, D.-X.; Reiter, R.J.; Shi, H. Comparative metabolomic analysis highlights the involvement of sugars and glycerol in melatonin-mediated innate immunity against bacterial pathogen in Arabidopsis. Sci. Rep. 2015, 5, 15815. [Google Scholar] [CrossRef] [Green Version]
- Halder, T.; Upadhyaya, G.; Roy, S.; Biswas, R.; Das, A.; Bagchi, A.; Agarwal, T.; Ray, S. Glycine rich proline rich protein from Sorghum bicolor serves as an antimicrobial protein implicated in plant defense response. Plant Mol. Biol. 2019, 101, 95–112. [Google Scholar] [CrossRef]
- Zhang, Y.; Smith, P.; Maximova, S.N.; Guiltinan, M.J. Application of glycerol as a foliar spray activates the defence response and enhances disease resistance of Theobroma cacao. Mol. Plant Pathol. 2015, 16, 27–37. [Google Scholar] [CrossRef]
- Zhang, X.-Q.; Liang, Y.-J.; Zhu, K.; Wu, C.-X.; Yang, L.-T.; Li, Y.-R. Influence of inoculation of Leifsonia xyli subsp. xyli on photosynthetic parameters and activities of defense enzymes in sugarcane. Sugar Tech 2017, 19, 394–401. [Google Scholar] [CrossRef]
- Rojas, C.M.; Senthil-Kumar, M.; Tzin, V.; Mysore, K.S. Regulation of primary plant metabolism during plant-pathogen interactions and its contribution to plant defense. Front. Plant Sci. 2014, 5, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Chen, M.; Liang, Y.; Xing, Y.; Yang, L.; Chen, M.; Comstock, J.C.; Li, Y.; Yang, L. Morphological and physiological responses of sugarcane to Leifsonia xyli subsp. xyli infection. Plant Dis. 2016, 100, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Bari, R.; Jones, J.D.G. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef]
- Davis, M.J.; Gillaspie JR, A.G.; Vidaver, A.K.; Harris, R.W. Clavibacter: A new genus containing some phytopathogenic coryneform bacteria, including Clavibacter xyli subsp. xyli sp. nov., subsp. nov. and Clavibacter xyli subsp. cynodontis subsp. nov., pathogens that cause ratoon stun. Int. J. Syst. Bacteriol. 1984, 34, 107–117. [Google Scholar]
- Tsogtbaatar, E.; Cocuron, J.-C.; Sonera, M.C.; Alonso, A.P. Metabolite fingerprinting of pennycress (Thlaspi arvense L.) embryos to assess active pathways during oil synthesis. J. Exp. Bot. 2015, 66, 4267–4277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tautenhahn, R.; Patti, G.J.; Rinehart, D.; Siuzdak, G. XCMS Online: A web-based platform to process untargeted metabolomic data. Anal. Chem. 2012, 84, 5035–5039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cocuron, J.-C.; Anderson, B.; Boyd, A.; Alonso, A.P. Targeted metabolomics of Physaria fendleri, an industrial crop producing hydroxy fatty acids. Plant Cell Physiol. 2014, 55, 620–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cocuron, J.-C.; Casas, M.I.; Yang, F.; Grotewold, E.; Alonso, A.P. Beyond the wall: High-throughput quantification of plant soluble and cell-wall bound phenolics by liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2019, 1589, 93–104. [Google Scholar] [CrossRef]
- Cocuron, J.-C.; Alonso, A.P. Liquid chromatography tandem mass spectrometry for measuring 13C-labeling in intermediates of the glycolysis and pentose phosphate pathway. Methods Mol. Biol. 2014, 1090, 131–142. [Google Scholar]
- Xia, J.; Psychogios, N.; Young, N.; Wishart, D.S. MetaboAnalyst: A web server for metabolomic data analysis and interpretation. Nucleic Acids Res. 2009, 37, W652–W660. [Google Scholar] [CrossRef] [Green Version]
- Raboin, L.-M.; Oliveira, K.M.; Lecunff, L.; Telismart, H.; Roques, D.; Butterfield, M.; Hoarau, J.-Y.; D‘Hont, A. Genetic mapping in sugarcane, a high polyploid, using bi-parental progeny: Identification of a gene controlling stalk colour and a new rust resistance gene. Theor. Appl. Genet. 2006, 112, 1382–1391. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro-Moretti, F.R.; Cocuron, J.-C.; Cia, M.C.; Cataldi, T.R.; Labate, C.A.; Alonso, A.P.; Camargo, L.E.A. Targeted Metabolic Profiles of the Leaves and Xylem Sap of Two Sugarcane Genotypes Infected with the Vascular Bacterial Pathogen Leifsonia xyli subsp. xyli. Metabolites 2021, 11, 234. https://doi.org/10.3390/metabo11040234
Castro-Moretti FR, Cocuron J-C, Cia MC, Cataldi TR, Labate CA, Alonso AP, Camargo LEA. Targeted Metabolic Profiles of the Leaves and Xylem Sap of Two Sugarcane Genotypes Infected with the Vascular Bacterial Pathogen Leifsonia xyli subsp. xyli. Metabolites. 2021; 11(4):234. https://doi.org/10.3390/metabo11040234
Chicago/Turabian StyleCastro-Moretti, Fernanda R., Jean-Christophe Cocuron, Mariana C. Cia, Thais R. Cataldi, Carlos A. Labate, Ana Paula Alonso, and Luis E. A. Camargo. 2021. "Targeted Metabolic Profiles of the Leaves and Xylem Sap of Two Sugarcane Genotypes Infected with the Vascular Bacterial Pathogen Leifsonia xyli subsp. xyli" Metabolites 11, no. 4: 234. https://doi.org/10.3390/metabo11040234
APA StyleCastro-Moretti, F. R., Cocuron, J. -C., Cia, M. C., Cataldi, T. R., Labate, C. A., Alonso, A. P., & Camargo, L. E. A. (2021). Targeted Metabolic Profiles of the Leaves and Xylem Sap of Two Sugarcane Genotypes Infected with the Vascular Bacterial Pathogen Leifsonia xyli subsp. xyli. Metabolites, 11(4), 234. https://doi.org/10.3390/metabo11040234