Rapid Screening of Proanthocyanidins from the Roots of Ephedra sinica Stapf and its Preventative Effects on Dextran-Sulfate-Sodium-Induced Ulcerative Colitis
Abstract
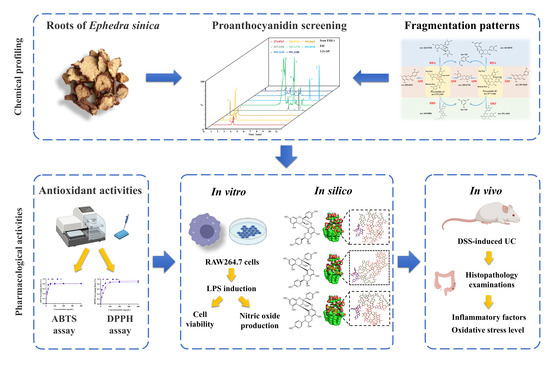
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Plant Materials and Sample Preparation
2.3. Potential Active Constituents: Chemical Profiling of ER and ERE by UPLC-LTQ-Orbitrap
2.4. Antioxidant Activity: The Free Radical Scavenging Activity of ERE
2.4.1. DPPH Assay
2.4.2. ABTS Assay
2.5. In Vitro Studies: Effect of ERE on RAW 264.7 Cells
2.5.1. MTT Assay
2.5.2. Effect of ERE on NO Production in LPS-Stimulated RAW 264.7 Cells
2.6. In Silico Studies: Molecular Docking Study of Dimeric Proanthocyanidins and LPS
2.7. In Vivo Studies: Effect of ERE on DSS-Induced Colitis Mice
2.7.1. Induction of Colitis Mice and Experimental Grouping
2.7.2. Evaluation of Disease Activity Index (DAI), Colon Length and Spleen Index
2.7.3. Histopathological Analysis
2.7.4. Determination of Inflammatory Cytokines and Biochemical Assays
2.8. Statistical Analysis
3. Results
3.1. The Integrated Strategy based on Neutral Loss Filtering and Diagnostic Fragmentation Pattern to Identify Potential PACs
3.2. Potential Active Constituents in ERE and the Antioxidant Activity
3.3. Effects of ERE on Viability and NO Production of LPS-Stimulated RAW 264.7 Cells
3.4. Molecular Docking Study of Dimeric Proanthocyanidins and LPS
3.5. ERE Relieved DSS-Induced Colitis
3.6. ERE Ameliorated Intestinal Damage
3.7. ERE Exerted Intestinal Anti-Inflammatory Effects
3.8. ERE Suppressed MPO Activity in Colon Tissues
3.9. ERE Regulated Oxidative Stress
4. Discussion
5. Conclusions
Supplementary Materials
 means hydrophobic contact;
means hydrophobic contact;  means atoms involved in hydrophobic contact). Figure S3: Computer modeling of the LPS and selected natural compounds binding. (A) Two-dimensional structure of Procyanidin A1; (B) the preferred orientation of Procyanidin A1 in complex with LPS; (C) two-dimensional structure of Procyanidin A2; (D) the preferred orientation of Procyanidin A1 in complex with LPS (black circles mean carbon atoms; red circles mean oxygen atoms; blue circles mean nitrogen atoms; purple circles mean phosphorus atoms; green dashed lines mean hydrogen bonds;
means atoms involved in hydrophobic contact). Figure S3: Computer modeling of the LPS and selected natural compounds binding. (A) Two-dimensional structure of Procyanidin A1; (B) the preferred orientation of Procyanidin A1 in complex with LPS; (C) two-dimensional structure of Procyanidin A2; (D) the preferred orientation of Procyanidin A1 in complex with LPS (black circles mean carbon atoms; red circles mean oxygen atoms; blue circles mean nitrogen atoms; purple circles mean phosphorus atoms; green dashed lines mean hydrogen bonds;  means hydrophobic contact;
means hydrophobic contact;  means atoms involved in hydrophobic contact).
means atoms involved in hydrophobic contact).Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, H.; Cao, G.; Luo, C.; Tan, D.; Vong, C.T.; Xu, Y.; Wang, S.; Lu, H.; Wang, Y.; Jing, W. Emerging pharmacotherapy for inflammatory bowel diseases. Pharmacol. Res. 2022, 178, 106146. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.D.; Lee, J.H.; Lee, Y.M.; Kim, D.K. Puerarin inhibits inflammation and oxidative stress in dextran sulfate sodium-induced colitis mice model. Biomed. Pharmacother. 2020, 124, 109847. [Google Scholar] [CrossRef] [PubMed]
- Alsoud, D.; Verstockt, B.; Fiocchi, C.; Vermeire, S. Breaking the therapeutic ceiling in drug development in ulcerative colitis. Lancet Gastroenterol. Hepatol. 2021, 6, 589–595. [Google Scholar] [CrossRef]
- Veloso, P.M.; Machado, R.; Nobre, C. Mesalazine and inflammatory bowel disease—From well-established therapies to progress beyond the state of the art. Eur. J. Pharm. Biopharm. 2021, 167, 89–103. [Google Scholar] [CrossRef]
- Machado, A.; Geraldi, M.V.; do Nascimento, R.P.; Moya, A.; Vezza, T.; Diez-Echave, P.; Galvez, J.J.; Cazarin, C.B.B.; Marostica Junior, M.R. Polyphenols from food by-products: An alternative or complementary therapy to IBD conventional treatments. Food Res. Int. 2021, 140, 110018. [Google Scholar] [CrossRef]
- Li, C.; Wang, L.; Zhao, J.; Wei, Y.; Zhai, S.; Tan, M.; Guan, K.; Huang, Z.; Chen, C. Lonicera rupicola Hook.f.et Thoms flavonoids ameliorated dysregulated inflammatory responses, intestinal barrier, and gut microbiome in ulcerative colitis via PI3K/AKT pathway. Phytomedicine 2022, 104, 154284. [Google Scholar] [CrossRef]
- Lv, M.; Chen, J.; Gao, Y.; Sun, J.; Zhang, Q.; Zhang, M.; Xu, F.; Zhang, Z. Metabolomics based on liquid chromatography with mass spectrometry reveals the chemical difference in the stems and roots derived from Ephedra sinica. J. Sep. Sci. 2015, 38, 3331–3336. [Google Scholar] [CrossRef]
- Zhang, B.M.; Wang, Z.B.; Xin, P.; Wang, Q.H.; Bu, H.; Kuang, H.X. Phytochemistry and pharmacology of genus Ephedra. Chin. J. Nat. Med. 2018, 16, 811–828. [Google Scholar] [CrossRef]
- Appeldoorn, M.M.; Sanders, M.; Vincken, J.-P.; Cheynier, V.; Le Guernevé, C.; Hollman, P.C.H.; Gruppen, H. Efficient isolation of major procyanidin A-type dimers from peanut skins and B-type dimers from grape seeds. Food Chem. 2009, 117, 713–720. [Google Scholar] [CrossRef]
- Amirshahrokhi, K. Febuxostat attenuates ulcerative colitis by the inhibition of NF-κB, proinflammatory cytokines, and oxidative stress in mice. Int. Immunopharmacol. 2019, 76, 105884. [Google Scholar] [CrossRef]
- Nazima, B.; Manoharan, V.; Miltonprabu, S. Oxidative stress induced by cadmium in the plasma, erythrocytes and lymphocytes of rats. Hum. Exp. Toxicol. 2015, 35, 428–447. [Google Scholar] [CrossRef] [PubMed]
- Sheng, K.; Zhang, G.; Sun, M.; He, S.; Kong, X.; Wang, J.; Zhu, F.; Zha, X.; Wang, Y. Grape seed proanthocyanidin extract ameliorates dextran sulfate sodium-induced colitis through intestinal barrier improvement, oxidative stress reduction, and inflammatory cytokines and gut microbiota modulation. Food Funct. 2020, 11, 7817–7829. [Google Scholar] [CrossRef]
- Cai, X.; Han, Y.; Gu, M.; Song, M.; Wu, X.; Li, Z.; Li, F.; Goulette, T.; Xiao, H. Dietary cranberry suppressed colonic inflammation and alleviated gut microbiota dysbiosis in dextran sodium sulfate-treated mice. Food Funct. 2019, 10, 6331–6341. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Wang, L.; Liu, M.; Wu, X.; Lu, Q.; Liu, R. The underlying mechanism of A-type procyanidins from peanut skin on DSS-induced ulcerative colitis mice by regulating gut microbiota and metabolism. J. Food Biochem. 2022, 46, e14103. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.M.; Wang, L.S.; Cui, Z.C.; Zhao, D.Q.; Liu, Y.H. Dimeric Proanthocyanidins from the Roots of Ephedra sinica. Planta Med. 2008, 74, 1823–1825. [Google Scholar] [CrossRef]
- Kumar, S.; Bhardwaj, V.K.; Singh, R.; Das, P.; Purohit, R. Identification of acridinedione scaffolds as potential inhibitor of DENV-2 C protein: An in silico strategy to combat dengue. J. Cell. Biochem. 2022, 123, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Fan, Y.; Du, L.; Mei, Z.; Fu, Y. In Silico and In Vivo Studies on the Mechanisms of Chinese Medicine Formula (Gegen Qinlian Decoction) in the Treatment of Ulcerative Colitis. Front. Pharmacol. 2021, 12, 665102. [Google Scholar] [CrossRef]
- Jing, J.; Shi, Y.; Zhang, Q.; Wang, J.; Ruan, J. Prediction of Chinese green tea ranking by metabolite profiling using ultra-performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UPLC-Q-TOF/MS). Food Chem. 2017, 221, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Ju, L.; Zhang, J.; Wang, F.; Zhu, D.; Pei, T.; He, Z.; Han, Z.; Wang, M.; Ma, Y.; Xiao, W. Chemical profiling of Houttuynia cordata Thunb. by UPLC-Q-TOF-MS and analysis of its antioxidant activity in C2C12 cells. J. Pharm. Biomed. Anal. 2021, 204, 114271. [Google Scholar] [CrossRef]
- Jiang, Z.-M.; Wang, L.-j.; Pang, H.-q.; Guo, Y.; Xiao, P.-T.; Chu, C.; Guo, L.; Liu, E.H. Rapid profiling of alkaloid analogues in Sinomenii Caulis by an integrated characterization strategy and quantitative analysis. J. Pharm. Biomed. Anal. 2019, 174, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yao, C.; Li, J.; Wang, J.; Yao, S.; Shen, S.; Yang, L.; Zhang, J.; Wei, W.; Bi, Q.; et al. Systematic characterization of chemical constituents in Mahuang decoction by UHPLC tandem linear ion trap-Orbitrap mass spectrometry coupled with feature-based molecular networking. J. Sep. Sci. 2021, 44, 2717–2727. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phuwapraisirisan, P.; Sowanthip, P.; Miles, D.H.; Tip-pyang, S. Reactive radical scavenging and xanthine oxidase inhibition of proanthocyanidins from Carallia brachiata. Phytother. Res. 2006, 20, 458–461. [Google Scholar] [CrossRef]
- Calzada, F.; Cerda-Garcia-Rojas, C.M.; Meckes, M.; Cedillo-Rivera, R.; Bye, R.; Mata, R. Geranins A and B, new antiprotozoal A-type proanthocyanidins from Geranium niveum. J. Nat. Prod. 1999, 62, 705–709. [Google Scholar] [CrossRef]
- Zheng, X.; Yang, Y.; Lu, Y.; Chen, Q. Affinity-Guided Isolation and Identification of Procyanidin B2 from Mangosteen (Garcinia mangostana L.) Rinds and its In Vitro LPS Binding and Neutralization Activities. Plant Foods Hum. Nutr. 2021, 76, 442–448. [Google Scholar] [CrossRef]
- Zang, J.; Ma, S.; Wang, C.; Guo, G.; Zhou, L.; Tian, X.; Lv, M.; Zhang, J.; Han, B. Screening for active constituents in Turkish galls against ulcerative colitis by mass spectrometry guided preparative chromatography strategy: In silico, in vitro and in vivo study. Food Funct. 2018, 9, 5124–5138. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, O.; Ma, N.; Yi, J.; Mi, H.; Cai, S. The preventive effect and underlying mechanism of Rhus chinensis Mill. fruits on dextran sulphate sodium-induced ulcerative colitis in mice. Food Funct. 2021, 12, 9965–9978. [Google Scholar] [CrossRef] [PubMed]
- Esquivel-Alvarado, D.; Alfaro-Viquez, E.; Krueger, C.G.; Vestling, M.M.; Reed, J.D. Classification of proanthocyanidin profiles using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) spectra data combined with multivariate analysis. Food Chem. 2021, 336, 127667. [Google Scholar] [CrossRef]
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Prior, R.L. Screening of Foods Containing Proanthocyanidins and Their Structural Characterization Using LC-MS/MS and Thiolytic Degradation. J. Agric. Food Chem. 2003, 51, 7513–7521. [Google Scholar] [CrossRef]
- Appeldoorn, M.M.; Vincken, J.-P.; Sanders, M.; Hollman, P.C.H.; Gruppen, H. Combined Normal-Phase and Reversed-Phase Liquid Chromatography/ESI-MS as a Tool to Determine the Molecular Diversity of A-type Procyanidins in Peanut Skins. J. Agric. Food Chem. 2009, 57, 6007–6013. [Google Scholar] [CrossRef]
- Ma, Y.; Bao, Y.; Zhang, W.; Ying, X.; Stien, D. Four lignans from Portulaca oleracea L. and its antioxidant activities. Nat. Prod. Res. 2020, 34, 2276–2282. [Google Scholar] [CrossRef]
- Gulcin, I. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [Green Version]
- Mei, Z.; Huang, X.; Zhang, H.; Cheng, D.; Xu, X.; Fang, M.; Hu, J.; Liu, Y.; Liang, Y.; Mei, Y. Chitin derivatives ameliorate DSS-induced ulcerative colitis by changing gut microbiota and restoring intestinal barrier function. Int. J. Biol. Macromol. 2022, 202, 375–387. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham, U.H.; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, C.; Li, Y.; Han, X.; Luo, X.; Chen, L.; Zhang, T.; Wang, N.; Wang, W. Alginate Oligosaccharides Ameliorate DSS-Induced Colitis through Modulation of AMPK/NF-kappaB Pathway and Intestinal Microbiota. Nutrients 2022, 14, 2864. [Google Scholar] [CrossRef] [PubMed]
- Abusaliya, A.; Bhosale, P.B.; Kim, H.H.; Ha, S.E.; Park, M.Y.; Jeong, S.H.; Vetrivel, P.; Park, J.S.; Kim, G.S. Prunetinoside Inhibits Lipopolysaccharide-Provoked Inflammatory Response via Suppressing NF-kappaB and Activating the JNK-Mediated Signaling Pathway in RAW264.7 Macrophage Cells. Int. J. Mol. Sci. 2022, 23, 5442. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, W.; Zheng, F.; Yu, H.; Wei, K. Xanthatin Alleviates LPS-Induced Inflammatory Response in RAW264.7 Macrophages by Inhibiting NF-kappaB, MAPK and STATs Activation. Molecules 2022, 27, 4603. [Google Scholar] [CrossRef]
- Mares, J.; Kumaran, S.; Gobbo, M.; Zerbe, O. Interactions of lipopolysaccharide and polymyxin studied by NMR spectroscopy. J. Biol. Chem. 2009, 284, 11498–11506. [Google Scholar] [CrossRef] [Green Version]
- Uddin, M.J.; Faraone, I.; Haque, M.A.; Rahman, M.M.; Halim, M.A.; Sonnichsen, F.D.; Cicek, S.S.; Milella, L.; Zidorn, C. Insights into the leaves of Ceriscoides campanulata: Natural proanthocyanidins alleviate diabetes, inflammation, and esophageal squamous cell cancer via in vitro and in silico models. Fitoterapia 2022, 158, 105164. [Google Scholar] [CrossRef]
- Hagan, M.; Hayee, B.H.; Rodriguez-Mateos, A. (Poly)phenols in Inflammatory Bowel Disease and Irritable Bowel Syndrome: A Review. Molecules 2021, 26, 1843. [Google Scholar] [CrossRef]
- Nascimento, R.P.D.; Machado, A.; Galvez, J.; Cazarin, C.B.B.; Marostica, M.R., Jr. Ulcerative colitis: Gut microbiota, immunopathogenesis and application of natural products in animal models. Life Sci. 2020, 258, 118129. [Google Scholar] [CrossRef]
- Dinallo, V.; Di Fusco, D.; Di Grazia, A.; Laudisi, F.; Troncone, E.; Di Maggio, G.; Franze, E.; Marafini, I.; Colantoni, A.; Ortenzi, A.; et al. The Deubiquitinating Enzyme OTUD5 Sustains Inflammatory Cytokine Response in Inflammatory Bowel Disease. J. Crohns. Colitis. 2022, 16, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, G.; Franze, E.; Troncone, E.; Maresca, C.; Marafini, I. Interleukin-34 Mediates Cross-Talk Between Stromal Cells and Immune Cells in the Gut. Front. Immunol. 2022, 13, 873332. [Google Scholar] [CrossRef] [PubMed]
- Hansberry, D.R.; Shah, K.; Agarwal, P.; Agarwal, N. Fecal Myeloperoxidase as a Biomarker for Inflammatory Bowel Disease. Cureus 2017, 9, e1004. [Google Scholar] [CrossRef] [Green Version]
- Seril, D.N.; Liao, J.; Yang, G.Y.; Yang, C.S. Oxidative stress and ulcerative colitis-associated carcinogenesis: Studies in humans and animal models. Carcinogenesis 2003, 24, 353–362. [Google Scholar] [CrossRef] [Green Version]
- Bastaki, S.M.A.; Amir, N.; Adeghate, E.; Ojha, S. Lycopodium Mitigates Oxidative Stress and Inflammation in the Colonic Mucosa of Acetic Acid-Induced Colitis in Rats. Molecules 2022, 27, 2774. [Google Scholar] [CrossRef]
- Al-Asmari, A.K.; Abbasmanthiri, R.; Al-Elewi, A.M.; Al-Omani, S.; Al-Asmary, S.; Al-Asmari, S.A. Camel milk beneficial effects on treating gentamicin induced alterations in rats. J. Toxicol. 2014, 2014, 917608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yildiz, G.; Yildiz, Y.; Ulutas, P.A.; Yaylali, A.; Ural, M. Resveratrol Pretreatment Ameliorates TNBS Colitis in Rats. Recent Pat. Endocr. Metab. Immune Drug Discov. 2015, 9, 134–140. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Wang, J.; Zou, L.; Cao, H.; Ni, X.; Xiao, J. Dietary proanthocyanidins on gastrointestinal health and the interactions with gut microbiota. Crit. Rev. Food Sci. Nutr. 2022, 2022, 1–24. [Google Scholar] [CrossRef]
- Kitabatake, M.; Matsumura, Y.; Ouji-Sageshima, N.; Nishioka, T.; Hara, A.; Kayano, S.I.; Ito, T. Persimmon-derived tannin ameliorates the pathogenesis of ulcerative colitis in a murine model through inhibition of the inflammatory response and alteration of microbiota. Sci. Rep. 2021, 11, 7286. [Google Scholar] [CrossRef]
- Tao, W.; Zhang, Y.; Shen, X.; Cao, Y.; Shi, J.; Ye, X.; Chen, S. Rethinking the Mechanism of the Health Benefits of Proanthocyanidins: Absorption, Metabolism, and Interaction with Gut Microbiota. Compr. Rev. Food Sci. Food Saf. 2019, 18, 971–985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Xue, L.; Tata, A.; Song, M.; Neto, C.C.; Xiao, H. Bioactive Components of Polyphenol-Rich and Non-Polyphenol-Rich Cranberry Fruit Extracts and Their Chemopreventive Effects on Colitis-Associated Colon Cancer. J. Agric. Food Chem. 2020, 68, 6845–6853. [Google Scholar] [CrossRef] [PubMed]




 means hydrophobic contact;
means hydrophobic contact;  means atoms involved in hydrophobic contact).
means atoms involved in hydrophobic contact).
 means hydrophobic contact;
means hydrophobic contact;  means atoms involved in hydrophobic contact).
means atoms involved in hydrophobic contact).



| No. | tR (min) | M.F. | [M-H]− m/z | Fragment Ions | Tentative Identification (Connection Sequence for PAC Dimers) |
|---|---|---|---|---|---|
| 273-1 | 3.04 | C15H14O5 | 273.0767 | 229.0878, 205.0870, 189.0565, 187.0766, 161.0612, 147.0459, 137.0249, 135.0457, 123.0460 | (Epi)afzelechin |
| 273-2 | 3.25 | C15H14O5 | 273.0768 | 229.0864, 205.0871, 189.0560, 187.0776, 161.0608, 147.0455, 137.0249, 135.0453, 123.0453 | (Epi)afzelechin |
| 289-1 | 2.49 | C15H14O6 | 289.0716 | 245.0821, 221.0823, 205.0509, 195.0302, 151.0405, 149.0248, 137.0249, 123.0456, 109.0299 | Catechin |
| 305-1 | 0.66 | C15H14O7 | 305.0663 | 287.0561, 269.0457, 179.0352, 137.0249, 125.0249, 109.0299 | (Epi)gallocatechin |
| 305-2 | 1.87 | C15H14O7 | 305.0662 | 287.0561, 269.0461, 179.0353, 137.0249, 125.0249, 109.0299 | (Epi)gallocatechin |
| 527-1 | 10.15 | C30H24O9 | 527.1335 | 401.1150, 391.0930, 379.0910, 323.0941, 307.0618, 273.0771, 253.0509, 229.0873, 125.0249 | Apigeniflavan-A-(epi)afzelechin |
| 543-1 | 6.49 | C30H24O10 | 543.1281 | 417.0970, 407.0764, 273.0771, 269.0459, 248.9584, 212.0738, 174.9561, 146.9662, 112.9859 | (Epi)afzelechin-A-(epi)afzelechin |
| 543-2 | 6.76 | C30H24O10 | 543.1279 | 417.0937, 407.0727, 273.0739, 269.0430, 212.0732, 174.9552, 146.9661, 112.9858, 96.9604 | (Epi)afzelechin-A-(epi)afzelechin |
| 543-3 | 7.19 | C30H24O10 | 543.1281 | 417.0981, 297.0771, 273.0772, 269.0461, 212.0749, 146.9662, 112.9859, 96.9603 | (Epi)afzelechin-A-(epi)afzelechin |
| 543-4 | 8.02 | C30H24O10 | 543.1278 | 417.1088, 407.0768, 273.0768, 269.0456, 212.0748, 174.9560, 146.9661, 129.9760, 112.9858 | (Epi)afzelechin-A-(epi)afzelechin |
| 543-5 | 8.15 | C30H24O10 | 543.1279 | 417.1123, 407.0902, 273.0772, 269.0458, 212.0736, 174.9562, 146.9662, 112.9858, 96.9603 | (Epi)afzelechin-A-(epi)afzelechin |
| 543-6 | 9.32 | C30H24O10 | 543.1281 | 417.0975, 407.0769, 289.0716, 253.0506, 245.0820, 212.0749, 179.0353, 112.9858, 96.9603 | Apigeniflavan-A-(epi)catechin |
| 555-1 | 9.56 | C30H20O11 | 555.0919 | 469.1173, 441.1173, 349.0720, 333.0771, 291.0664, 285.0405, 269.0457, 149.0249 | (Epi)afzelechin-A-kaempferol |
| 555-2 | 11.04 | C30H20O11 | 555.0930 | 469.1157, 441.1154, 349.0714, 333.0772, 291.0664, 285.0403, 269.0456, 149.0248 | (Epi)afzelechin-A-kaempferol |
| 559-1 | 4.95 | C30H24O11 | 559.1233 | 523.1354, 433.1074, 405.1084, 317.0663, 289.0718, 269.0454, 245.0823 | (Epi)afzelechin-A-(epi)catechin |
| 559-2 | 5.50 | C30H24O11 | 559.1230 | 523.1396, 433.1106, 407.0908, 289.0717, 269.0457, 245.0827 | (Epi)afzelechin-A-(epi)catechin |
| 559-3 | 6.16 | C30H24O11 | 559.1231 | 523.1398, 455.1356, 433.1115, 407.0914, 289.0716, 269.0457, 245.0820 | (Epi)afzelechin-A-(epi)catechin |
| 591-1 | 3.72 | C30H24O13 | 591.1127 | 555.1358, 465.1054, 407.0904, 301.0355, 289.0720, 175.0042, 125.0250 | (Epi)gallocatechin-A-(epi)catechin |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, M.; Wang, Y.; Wan, X.; Han, B.; Yu, W.; Liang, Q.; Xiang, J.; Wang, Z.; Liu, Y.; Qian, Y.; et al. Rapid Screening of Proanthocyanidins from the Roots of Ephedra sinica Stapf and its Preventative Effects on Dextran-Sulfate-Sodium-Induced Ulcerative Colitis. Metabolites 2022, 12, 957. https://doi.org/10.3390/metabo12100957
Lv M, Wang Y, Wan X, Han B, Yu W, Liang Q, Xiang J, Wang Z, Liu Y, Qian Y, et al. Rapid Screening of Proanthocyanidins from the Roots of Ephedra sinica Stapf and its Preventative Effects on Dextran-Sulfate-Sodium-Induced Ulcerative Colitis. Metabolites. 2022; 12(10):957. https://doi.org/10.3390/metabo12100957
Chicago/Turabian StyleLv, Mengying, Yang Wang, Xiayun Wan, Bo Han, Wei Yu, Qiaoling Liang, Jie Xiang, Zheng Wang, Yanqing Liu, Yayun Qian, and et al. 2022. "Rapid Screening of Proanthocyanidins from the Roots of Ephedra sinica Stapf and its Preventative Effects on Dextran-Sulfate-Sodium-Induced Ulcerative Colitis" Metabolites 12, no. 10: 957. https://doi.org/10.3390/metabo12100957
APA StyleLv, M., Wang, Y., Wan, X., Han, B., Yu, W., Liang, Q., Xiang, J., Wang, Z., Liu, Y., Qian, Y., & Xu, F. (2022). Rapid Screening of Proanthocyanidins from the Roots of Ephedra sinica Stapf and its Preventative Effects on Dextran-Sulfate-Sodium-Induced Ulcerative Colitis. Metabolites, 12(10), 957. https://doi.org/10.3390/metabo12100957