Single Point Insulin Sensitivity Estimator (SPISE) As a Prognostic Marker for Emerging Dysglycemia in Children with Overweight or Obesity
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Desing and Study Population
2.2. Anthropometric and Laboratory Assessment
2.3. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. SPISE Index Is Dependent of Age, but Not of Sex during Childhood
3.3. Cross-Sectional Association of SPISE Score with Established Markers of Insulin Resistance
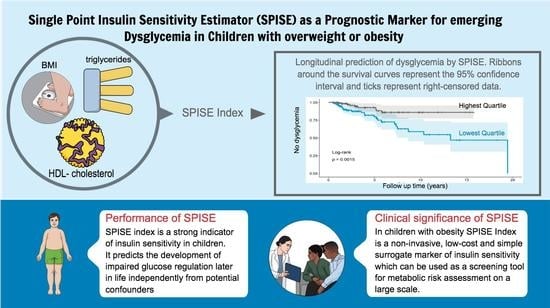
3.4. Longitudinal Prediction of Dysglycemia
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Outcome | N (%) |
|---|---|
| Dysglycemia (total) Intake of antidiabetics ≥2/3 Prediabetes criteria positive * ≥2/3 Diabetes criteria positive ** | 79 (100%) |
| 50 (63.29%) 29 (36.71%) 2 (2.53%) |
References
- Garrido-Miguel, M.; Oliveira, A.; Cavero-Redondo, I.; Álvarez-Bueno, C.; Pozuelo-Carrascosa, D.P.; Soriano-Cano, A.; Martínez-Vizcaíno, V. Prevalence of Overweight and Obesity among European Preschool Children: A Systematic Review and Meta-Regression by Food Group Consumption. Nutrients 2019, 11, 1698. [Google Scholar] [CrossRef] [Green Version]
- Berenson, G.S.; Bogalusa Heart Study Group. Health consequences of obesity. Pediatr. Blood Cancer 2012, 58, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, D.; Dee, A.; Perry, I.J. The lifetime costs of overweight and obesity in childhood and adolescence: A systematic review. Obes. Rev. 2018, 19, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Gepstein, V.; Weiss, R. Obesity as the Main Risk Factor for Metabolic Syndrome in Children. Front. Endocrinol. 2019, 10, 568. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Zuo, J.; Zhou, J.; Cai, J.; Chen, C.; Xiang, E.; Li, H.; Cheng, X.; Chen, P. Childhood obesity leads to adult type 2 diabetes and coronary artery diseases: A 2-sample mendelian randomization study. Medicine 2019, 98, e16825. [Google Scholar] [CrossRef]
- Valaiyapathi, B.; Gower, B.; Ashraf, A.P. Pathophysiology of Type 2 Diabetes in Children and Adolescents. Curr. Diabetes Rev. 2020, 16, 220–229. [Google Scholar] [CrossRef]
- Levy-Marchal, C.; Arslanian, S.; Cutfield, W.; Sinaiko, A.; Druet, C.; Marcovecchio, M.L.; Chiarelli, F.; ESPE-LWPES-ISPAD-APPES-APEG-SLEP-JSPE; The Insulin Resistance in Children Consensus Conference Group. Insulin resistance in children: Consensus, perspective, and future directions. J. Clin. Endocrinol. Metab. 2010, 95, 5189–5198. [Google Scholar] [CrossRef] [Green Version]
- Brown, R.J.; Yanovski, J.A. Estimation of insulin sensitivity in children: Methods, measures and controversies. Pediatr. Diabetes 2014, 15, 151–161. [Google Scholar] [CrossRef] [Green Version]
- Cutfield, W.S.; Jefferies, C.A.; Jackson, W.E.; Robinson, E.M.; Hofman, P.L. Evaluation of HOMA and QUICKI as measures of insulin sensitivity in prepubertal children. Pediatr. Diabetes 2003, 4, 119–125. [Google Scholar] [CrossRef]
- Kurtoğlu, S.; Hatipoğlu, N.; Mazıcıoğlu, M.; Kendirici, M.; Keskin, M.; Kondolot, M. Insulin resistance in obese children and adolescents: HOMA-IR cut-off levels in the prepubertal and pubertal periods. J. Clin. Res. Pediatr. Endocrinol. 2010, 2, 100–106. [Google Scholar] [CrossRef]
- Conwell, L.S.; Batch, J.A. Oral glucose tolerance test in children and adolescents: Positives and pitfalls. J. Paediatr. Child Health 2004, 40, 620–626. [Google Scholar] [CrossRef]
- Tam, C.S.; Xie, W.; Johnson, W.D.; Cefalu, W.T.; Redman, L.M.; Ravussin, E. Defining insulin resistance from hyperinsulinemic-euglycemic clamps. Diabetes Care 2012, 35, 1605–1610. [Google Scholar] [CrossRef] [Green Version]
- Mostafa, S.A.; Davies, M.J.; Morris, D.H.; Yates, T.; Srinivasan, B.T.; Webb, D.; Brady, E.; Khunti, K. The association of the triglyceride-to-HDL cholesterol ratio with insulin resistance in White European and South Asian men and women. PLoS ONE 2012, 7, e50931. [Google Scholar] [CrossRef] [Green Version]
- Katz, A.; Nambi, S.S.; Mather, K.; Baron, A.D.; Follmann, D.A.; Sullivan, G.; Quon, M.J. Quantitative insulin sensitivity check index: A simple, accurate method for assessing insulin sensitivity in humans. J. Clin. Endocrinol. Metab. 2000, 85, 2402–2410. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [Green Version]
- McLaughlin, T.; Abbasi, F.; Cheal, K.; Chu, J.; Lamendola, C.; Reaven, G. Use of metabolic markers to identify overweight individuals who are insulin resistant. Ann. Intern. Med. 2003, 139, 802–809. [Google Scholar] [CrossRef]
- Bridges, K.G.; Jarrett, T.; Thorpe, A.; Baus, A.; Cochran, J. Use of the triglyceride to HDL cholesterol ratio for assessing insulin sensitivity in overweight and obese children in rural Appalachia. J. Pediatr. Endocrinol. Metab. 2016, 29, 153–156. [Google Scholar] [CrossRef]
- Paulmichl, K.; Hatunic, M.; Højlund, K.; Jotic, A.; Krebs, M.; Mitrakou, A.; Porcellati, F.; Tura, A.; Bergsten, P.; Forslund, A.; et al. Modification and Validation of the Triglyceride-to-HDL Cholesterol Ratio as a Surrogate of Insulin Sensitivity in White Juveniles and Adults without Diabetes Mellitus: The Single Point Insulin Sensitivity Estimator (SPISE). Clin. Chem. 2016, 62, 1211–1219. [Google Scholar] [CrossRef] [Green Version]
- Koutny, F.; Stein, R.; Kiess, W.; Weghuber, D.; Körner, A. Elevated transaminases potentiate the risk for emerging dysglycemia in children with overweight and obesity. Pediatr. Obes. 2021, 16, e12822. [Google Scholar] [CrossRef]
- Kromeyer-Hauschild, K.; Wabitsch, M.; Kunze, D.; Geller, F.; Geiß, H.C.; Hesse, V.; von Hippel, A.; Jaeger, U.; Johnsen, D.; Korte, W.; et al. Perzentilen für den Body Mass Index für das Kindes- und Jugendalter unter Heranziehung verschiedener deutscher Stichproben (Centiles for body mass index for children and adolescents derived from distinct independent German cohorts). Monatsschr. Kinderheilkd. 2001, 149, 807–818. [Google Scholar] [CrossRef]
- Coleman, L.; Coleman, J. The measurement of puberty: A review. J. Adolesc. 2002, 25, 535–550. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes. Diabetes Care 2020, 43, S14–S31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barchetta, I.; Dule, S.; Bertoccini, L.; Cimini, F.A.; Sentinelli, F.; Bailetti, D.; Marini, G.; Barbonetti, A.; Loche, S.; Cossu, E.; et al. The single-point insulin sensitivity estimator (SPISE) index is a strong predictor of abnormal glucose metabolism in overweight/obese children: A long-term follow-up study. J. Endocrinol. Investig. 2022, 45, 43–51. [Google Scholar] [CrossRef]
- Cederholm, J.; Zethelius, B. SPISE and other fasting indexes of insulin resistance: Risks of coronary heart disease or type 2 diabetes. Comparative cross-sectional and longitudinal aspects. Upsala J. Med. Sci. 2019, 124, 265–272. [Google Scholar] [CrossRef] [Green Version]
- Correa-Burrows, P.; Blanco, E.; Gahagan, S.; Burrows, R. Validity assessment of the single-point insulin sensitivity estimator (spise) for diagnosis of cardiometabolic risk in post-pubertal hispanic adolescents. Sci. Rep. 2020, 10, 14399. [Google Scholar] [CrossRef] [PubMed]
- Furthner, D.; Anderwald, C.H.; Bergsten, P.; Forslund, A.; Kullberg, J.; Ahlström, H.; Manell, H.; Ciba, I.; Mangge, H.; Maruszczak, K.; et al. Single Point Insulin Sensitivity Estimator in Pediatric Non-Alcoholic Fatty Liver Disease. Front. Endocrinol. 2022, 13, 830012. [Google Scholar] [CrossRef] [PubMed]
- Kelsey, M.M.; Zeitler, P.S. Insulin Resistance of Puberty. Curr. Diabetes Rep. 2016, 16, 64. [Google Scholar] [CrossRef]
- Behiry, E.G.; El Nady, N.M.; AbdEl Haie, O.M.; Mattar, M.K.; Magdy, A. Evaluation of TG-HDL Ratio Instead of HOMA Ratio as Insulin Resistance Marker in Overweight and Children with Obesity. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 676–682. [Google Scholar] [CrossRef]
- Ma, M.; Liu, H.; Yu, J.; He, S.; Li, P.; Ma, C.; Zhang, H.; Xu, L.; Ping, F.; Li, W.; et al. Triglyceride is independently correlated with insulin resistance and islet beta cell function: A study in population with different glucose and lipid metabolism states. Lipids Health Dis. 2020, 19, 121. [Google Scholar] [CrossRef]
- Klöting, N.; Fasshauer, M.; Dietrich, A.; Kovacs, P.; Schön, M.R.; Kern, M.; Stumvoll, M.; Blüher, M. Insulin-sensitive obesity. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E506–E515. [Google Scholar] [CrossRef] [PubMed]
- Sagesaka, H.; Sato, Y.; Someya, Y.; Tamura, Y.; Shimodaira, M.; Miyakoshi, T.; Hirabayashi, K.; Koike, H.; Yamashita, K.; Watada, H.; et al. Type 2 Diabetes: When Does It Start? J. Endocr. Soc. 2018, 2, 476–484. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Tobin, J.D.; Andres, R. Glucose clamp technique: A method for quantifying insulin secretion and resistance. Am. J. Physiol. 1979, 237, E214–E223. [Google Scholar] [CrossRef] [PubMed]
- Tagi, V.M.; Giannini, C.; Chiarelli, F. Insulin Resistance in Children. Front. Endocrinol. 2019, 10, 342. [Google Scholar] [CrossRef] [PubMed]



| Clinical Characteristics | Baseline Cohort | Follow-Up Cohort at Baseline | ||
|---|---|---|---|---|
| N | Value | N | Value | |
| N male (%) | 2107 | 1043 (49.5) | 591 | 274 (46.36) |
| Age range, years | 2107 | 5.01–18.44 | 591 | 5.01–17.56 |
| Mean age (SD), years | 2107 | 11.91 (±3.05) | 591 | 11.47 (±2.83) |
| Mean BMI (SD), kg/m2 | 2107 | 29.41 (±5.84) | 591 | 28.6 (±5.38) |
| Mean BMI SDS (SD) | 2107 | 2.48 (±0.57) | 591 | 2.43 (±0.56) |
| Pubertal stage,% | 2107 | 100 | 591 | 100 |
| 1 | 603 | 28.62 | 191 | 32.32 |
| 2 | 416 | 19.72 | 130 | 22.0 |
| 3 | 238 | 11.30 | 65 | 11.0 |
| 4 | 213 | 10.10 | 58 | 9.81 |
| 5 | 514 | 24.39 | 124 | 20.98 |
| N/A | 123 | 5.84 | 23 | 3.89 |
| Mean fasting glucose (SD), mmol/L | 2107 | 5.21 (±0.58) | 591 | 5.16 (±0.44) |
| Mean 2 h glucose (SD), mmol/L | 1896 | 6.64 (±1.27) | 515 | 6.6 (±1.14) |
| Mean HbA1c (SD),% | 1868 | 5.31 (±1.22) | 515 | 5.23 (±0.33) |
| Mean TG (SD), mmol/L | 2107 | 1.21 (±0.67) | 591 | 1.18 (±0.64) |
| Mean HDL-C (SD), mmol/L | 2107 | 1.22 (±0.28) | 591 | 1.25 (±0.29) |
| Mean fasting insulin (SD), pmol/L | 2107 | 121.6 (±81.94) | 591 | 110.95 (±68.77) |
| Mean SPISE (SD) | 2107 | 5.71 (±1.73) | 591 | 5.94 (±1.71) |
| Mean HOMA-IR (SD) | 2107 | 4.13 (±3.02) | 591 | 3.69 (±2.36) |
| Mean QUICKI-IR (SD) | 2107 | 4.3 (±0.68) | 591 | 4.22 (±0.63) |
| Univariate Regression | Multiple Regression * | |
|---|---|---|
| Age β-slope (per year) | −0.34 | −0.34 |
| 95% CI | −0.36–(−0.32) | −0.36–(−0.32) |
| p value | <0.001 | <0.001 |
| Sex Δ β-slope (for male) | 0.07 | 0.04 |
| 95% CI | −0.07–0.22 | −0.08–0.16 |
| p value | 0.33 | 0.48 |
| Insulin Index | SPISE | HOMA-IR |
|---|---|---|
| HOMA-IR | −0.49 *** | 1 *** |
| QUICKI-IR | −0.55 *** | 0.88 *** |
| Insulin Index | SPISE | HOMA-IR | QUICKI-IR |
|---|---|---|---|
| p-value, Log-rank lowest vs. highest quartile A, N = 296 B | 0.0015 | 0.01 | 0.01 |
| HR lowest vs. highest quartile A (95% CI), N = 296 B Univariate Adjusted for age and sex | |||
| 3.19 (1.5–6.81) ** | 2.35 (1.2–4.59) * | 2.35 (1.2–4.59) * | |
| 3.47 (1.60–7.51) ** | 2.44 (1.24–4.81) * | 2.44 (1.24–4.81) * | |
| HR continuous (95% CI), N = 591 C Univariate Adjusted for age and sex | 0.84 (0.73–0.96) ** 0.77 (0.66–0.9) ** | 1.13 (1.04–1.24) ** 1.16 (1.05–1.27) ** | 1.66 (1.14–2.41) ** 1.85 (1.23–2.77) ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stein, R.; Koutny, F.; Riedel, J.; Dörr, N.; Meyer, K.; Colombo, M.; Vogel, M.; Anderwald, C.H.; Blüher, M.; Kiess, W.; et al. Single Point Insulin Sensitivity Estimator (SPISE) As a Prognostic Marker for Emerging Dysglycemia in Children with Overweight or Obesity. Metabolites 2023, 13, 100. https://doi.org/10.3390/metabo13010100
Stein R, Koutny F, Riedel J, Dörr N, Meyer K, Colombo M, Vogel M, Anderwald CH, Blüher M, Kiess W, et al. Single Point Insulin Sensitivity Estimator (SPISE) As a Prognostic Marker for Emerging Dysglycemia in Children with Overweight or Obesity. Metabolites. 2023; 13(1):100. https://doi.org/10.3390/metabo13010100
Chicago/Turabian StyleStein, Robert, Florian Koutny, Johannes Riedel, Natascha Dörr, Klara Meyer, Marco Colombo, Mandy Vogel, Christian Heinz Anderwald, Matthias Blüher, Wieland Kiess, and et al. 2023. "Single Point Insulin Sensitivity Estimator (SPISE) As a Prognostic Marker for Emerging Dysglycemia in Children with Overweight or Obesity" Metabolites 13, no. 1: 100. https://doi.org/10.3390/metabo13010100
APA StyleStein, R., Koutny, F., Riedel, J., Dörr, N., Meyer, K., Colombo, M., Vogel, M., Anderwald, C. H., Blüher, M., Kiess, W., Körner, A., & Weghuber, D. (2023). Single Point Insulin Sensitivity Estimator (SPISE) As a Prognostic Marker for Emerging Dysglycemia in Children with Overweight or Obesity. Metabolites, 13(1), 100. https://doi.org/10.3390/metabo13010100