The Associations of Maternal Health Characteristics, Newborn Metabolite Concentrations, and Child Body Mass Index among US Children in the ECHO Program
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Populations
2.2. Newborn Screening Metabolic Data Collection
2.3. Maternal Health Characteristics, Child BMI, and Covariate Ascertainment
2.4. Statistical Analysis
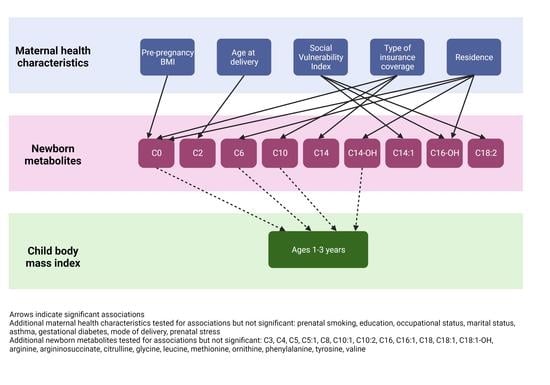
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marciniak, A.; Patro-Małysza, J.; Kimber-Trojnar, Ż.; Marciniak, B.; Oleszczuk, J.; Leszczyńska-Gorzelak, B. Fetal programming of the metabolic syndrome. Taiwan. J. Obstet. Gynecol. 2017, 56, 133–138. [Google Scholar] [CrossRef]
- Rinaudo, P.; Wang, E. Fetal programming and metabolic syndrome. Ann. Rev. Physiol. 2012, 74, 107–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, E.J.; Kim, Y.J. What is fetal programming?: A lifetime health is under the control of in utero health. Obstet. Gynecol. Sci. 2017, 60, 506–519. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, K.; Lillycrop, K.A.; Silver, M.J. Fetal programming and epigenetics. Curr. Opin. Endocr. Metab. Res. 2020, 13, 1–6. [Google Scholar] [CrossRef]
- Fiehn, O. Metabolomics—The link between genotypes and phenotypes. Plant Mol. Biol. 2002, 48, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Gallois, A.; Mefford, J.; Ko, A.; Vaysse, A.; Julienne, H.; Ala-Korpela, M.; Laakso, M.; Zaitlen, N.; Pajukanta, P.; Aschard, H. A comprehensive study of metabolite genetics reveals strong pleiotropy and heterogeneity across time and context. Nat. Commun. 2019, 10, 4788. [Google Scholar] [CrossRef] [Green Version]
- de Nava, A.S.L.; Raja, A. Physiology, Metabolism. [Updated 2021 Sep 20]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK546690/ (accessed on 12 December 2022).
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. HMDB 4.0: The human metabolome database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef]
- McCann, M.R.; George De la Rosa, M.V.; Rosania, G.R.; Stringer, K.A. L-Carnitine and Acylcarnitines: Mitochondrial Biomarkers for Precision Medicine. Metabolites 2021, 11, 51. [Google Scholar] [CrossRef]
- Bröer, S.; Bröer, A. Amino acid homeostasis and signalling in mammalian cells and organisms. Biochem. J. 2017, 474, 1935–1963. [Google Scholar] [CrossRef] [Green Version]
- Larkin, E.K.; Gebretsadik, T.; Moore, M.L.; Anderson, L.J.; Dupont, W.D.; Chappell, J.D.; Minton, P.A.; Peebles, R.S., Jr.; Moore, P.E.; Valet, R.S.; et al. Objectives, design and enrollment results from the Infant Susceptibility to Pulmonary Infections and Asthma Following RSV Exposure Study (INSPIRE). BMC Pulm. Med. 2015, 15, 45. [Google Scholar] [CrossRef] [Green Version]
- Sauder, K.A.; Stamatoiu, A.V.; Leshchinskaya, E.; Ringham, B.M.; Glueck, D.H.; Dabelea, D. Cord Blood Vitamin D Levels and Early Childhood Blood Pressure: The Healthy Start Study. J. Am. Heart Assoc. 2019, 8, e011485. [Google Scholar] [CrossRef] [Green Version]
- National Institutes of Health: Newborn Screening. Available online: https://www.nichd.nih.gov/health/topics/newborn (accessed on 10 June 2022).
- CLSI. Newborn Screening by Tandem Mass Spectrometry, 2nd ed.; CLSI Guideline NBS04; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- Feuchtbaum, L.; Carter, J.; Dowray, S.; Currier, R.J.; Lorey, F. Birth prevalence of disorders detectable through newborn screening by race/ethnicity. Genet. Med. 2012, 14, 937–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Onis, M.; Onyango, A.; Borghi, E.; Siyam, A.; Blössner, M.; Lutter, C. Worldwide implementation of the WHO Child Growth Standards. Public Health Nutr. 2012, 15, 1603–1610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flegal, K.M.; Cole, T.J. Construction of LMS parameters for the Centers for Disease Control and Prevention 2000 growth charts. In National Health Statistics Reports; National Center for Health Statistics: Hyattsville, MA, USA, 2013; pp. 1–3. [Google Scholar]
- UpToDate: Measurement of Growth in Children. Available online: https://www.uptodate.com/contents/measurement-of-growth-in-children?search=calculator-cdc-nchs-infant-weight-for-length-percentiles-less-than§ionRank=2&usage_type=default&anchor=H3361608930&source=machineLearning&selectedTitle=1~150&display_rank=1#H3361608930 (accessed on 11 November 2022).
- van Buuren, S.; Groothuis-Oudshoorn, K. mice: Multivariate Imputation by Chained Equations in R. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef] [Green Version]
- Dambrova, M.; Makrecka-Kuka, M.; Kuka, J.; Vilskersts, R.; Nordberg, D.; Attwood, M.M.; Smesny, S.; Sen, Z.D.; Guo, A.C.; Oler, E.; et al. Acylcarnitines: Nomenclature, Biomarkers, Therapeutic Potential, Drug Targets, and Clinical Trials. Pharmacol. Rev. 2022, 74, 506–551. [Google Scholar] [CrossRef]
- McCarthy, M.E.; Oltman, S.P.; Rogers, E.E.; Ryckman, K.; Jelliffe-Pawlowski, L.L.; Danilack, V.A. The independent and combined influences of small for gestational age and socioeconomic status on newborn metabolite levels. J. Matern Fetal. Neonatal. Med. 2022, 35, 6192–6198. [Google Scholar] [CrossRef]
- Lowe, W.L., Jr.; Bain, J.R.; Nodzenski, M.; Reisetter, A.C.; Muehlbauer, M.J.; Stevens, R.D.; Ilkayeva, O.R.; Lowe, L.P.; Metzger, B.E.; Newgard, C.B.; et al. Maternal BMI and Glycemia Impact the Fetal Metabolome. Diabetes Care 2017, 40, 902–910. [Google Scholar] [CrossRef] [Green Version]
- Ryckman, K.K.; Shchelochkov, O.A.; Cook, D.E.; Berberich, S.L.; Copeland, S.; Dagle, J.M.; Murray, J.C. The influence of maternal disease on metabolites measured as part of newborn screening. J. Matern. Fetal. Neonatal. Med. 2013, 26, 1380–1383. [Google Scholar] [CrossRef] [Green Version]
- Handakas, E.; Keski-Rahkonen, P.; Chatzi, L.; Alfano, R.; Roumeliotaki, T.; Plusquin, M.; Maitre, L.; Richiardi, L.; Brescianini, S.; Scalbert, A.; et al. Cord blood metabolic signatures predictive of childhood overweight and rapid growth. Int. J. Obes. 2021, 45, 2252–2260. [Google Scholar] [CrossRef]
- Cao, T.; Zhao, J.; Hong, X.; Wang, G.; Hu, F.B.; Wang, X.; Liang, L. Cord Blood Metabolome and BMI Trajectory from Birth to Adolescence: A Prospective Birth Cohort Study on Early Life Biomarkers of Persistent Obesity. Metabolites 2021, 11, 739. [Google Scholar] [CrossRef]
- Schooneman, M.G.; Vaz, F.M.; Houten, S.M.; Soeters, M.R. Acylcarnitines: Reflecting or inflicting insulin resistance? Diabetes 2013, 62, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makrecka-Kuka, M.; Sevostjanovs, E.; Vilks, K.; Volska, K.; Antone, U.; Kuka, J.; Makarova, E.; Pugovics, O.; Dambrova, M.; Liepinsh, E. Plasma acylcarnitine concentrations reflect the acylcarnitine profile in cardiac tissues. Sci. Rep. 2017, 7, 17528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giesbertz, P.; Ecker, J.; Haag, A.; Spanier, B.; Daniel, H. An LC-MS/MS method to quantify acylcarnitine species including isomeric and odd-numbered forms in plasma and tissues. J. Lipid Res. 2015, 56, 2029–2039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schooneman, M.G.; Houtkooper, R.H.; Hollak, C.E.M.; Wanders, R.J.A.; Vaz, F.M.; Soeters, M.R.; Houten, S.M. The impact of altered carnitine availability on acylcarnitine metabolism, energy expenditure and glucose tolerance in diet-induced obese mice. Biochim. Biophys. Acta 2016, 1862, 1375–1382. [Google Scholar] [CrossRef]
- L-Acetylcarnitine (HMDB0000201). Available online: https://hmdb.ca/metabolites/HMDB0000201 (accessed on 27 July 2022).
- Bayrampour, H.; Heaman, M.; Duncan, K.A.; Tough, S. Advanced maternal age and risk perception: A qualitative study. BMC Pregnancy Childbirth 2012, 12, 100. [Google Scholar] [CrossRef] [Green Version]
- García-Blanco, A.; Monferrer, A.; Grimaldos, J.; Hervás, D.; Balanzá-Martínez, V.; Diago, V.; Vento, M.; Cháfer-Pericás, C. A preliminary study to assess the impact of maternal age on stress-related variables in healthy nulliparous women. Psychoneuroendocrinology 2017, 78, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Levenson, D.; Romero, R.; Garcia-Flores, V.; Miller, D.; Xu, Y.; Sahi, A.; Hassan, S.S.; Gomez-Lopez, N. The effects of advanced maternal age on T-cell subsets at the maternal-fetal interface prior to term labor and in the offspring: A mouse study. Clin. Exp. Immunol. 2020, 201, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.V.L.; Menezes, M.C.; Oliveira, C.D.L.; Mingoti, S.A.; Jaime, P.C.; Caiaffa, W.T.; Lopes, A.C.S. Does access to healthy food vary according to socioeconomic status and to food store type? an ecologic study. BMC Public Health 2019, 19, 775. [Google Scholar] [CrossRef]
- Williamson, V.G.; Dilip, A.; Dillard, J.R.; Morgan-Daniel, J.; Lee, A.M.; Cardel, M.I. The Influence of Socioeconomic Status on Snacking and Weight among Adolescents: A Scoping Review. Nutrients 2020, 12, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yau, Y.H.; Potenza, M.N. Stress and eating behaviors. Minerva Endocrinol. 2013, 38, 255–267. [Google Scholar]
- Flanagan, J.L.; Simmons, P.A.; Vehige, J.; Willcox, M.D.P.; Garrett, Q. Role of carnitine in disease. Nutr. Metab. 2010, 7, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddam, A.; McLarnan, S.; Kupsco, A. Environmental Chemical Exposures and Mitochondrial Dysfunction: A Review of Recent Literature. Curr. Environ. Health Rep. 2022, 9, 631–649. [Google Scholar] [CrossRef] [PubMed]
- De Jesús, V.R.; Mei, J.V.; Cordovado, S.K.; Cuthbert, C.D. The Newborn Screening Quality Assurance Program at the Centers for Disease Control and Prevention: Thirty-five Year Experience Assuring Newborn Screening Laboratory Quality. Int. J. Neonatal. Screen 2015, 1, 13–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Cohort | ||||
|---|---|---|---|---|
| Maternal Health Characteristic | INSPIRE | MARCH | Healthy Start | p-Value a |
| Sample size | 1920 | 365 | 1207 | |
| Prenatal smoking, n (%) | 345 (18) | 41 (11) | 90 (7) | <0.001 * |
| Missing, n (%) | 2 (0) | 47 (13) | 0 (0) | |
| Pre-pregnancy BMI b, mean (SD) | 27 (7) | 29 (8) | 26 (6) | <0.001 * |
| Missing, n (%) | 72 (4) | 43 (12) | 0 (0) | |
| Education, n (%) | <0.001 * | |||
| <High school | 153 (8) | 31 (8) | 166 (14) | |
| High school degree | 524 (27) | 68 (19) | 215 (18) | |
| Some college b | 572 (30) | 121 (33) | 271 (22) | |
| ≥College degree b | 670 (35) | 100 (27) | 555 (46) | |
| Missing, n (%) | 1 (0) | 45 (12) | 0 (0) | |
| Occupational status, n (%) | 0.001 * | |||
| Not employed | 669 (35) | 81 (22) | 388 (32) | |
| Employed | 1251 (65) | 239 (65) | 682 (57) | |
| Missing, n (%) | 0 (0) | 45 (12) | 137 (11) | |
| Marital status, n (%) | <0.001 * | |||
| Not married | 816 (43) | 177 (48) | 447 (37) | |
| Married | 1104 (58) | 142 (39) | 755 (63) | |
| Missing, n (%) | 0 (0) | 46 (13) | 5 (0) | |
| Age at delivery (years) b, mean (SD) | 27 (5) | 29 (6) | 28 (6) | <0.001 * |
| Missing, n (%) | 0 (0) | 71 (19) | 10 (1) | |
| Asthma, n (%) | 372 (19) | 67 (18) | 197 (16) | 0.05 |
| Missing, n (%) | 1 (0) | 46 (13) | 1 (0) | |
| Gestational diabetes, n (%) | 126 (7) | 23 (6) | 47 (4) | 0.01 * |
| Missing, n (%) | 0 (0) | 71 (19) | 96 (8) | |
| C-section, n (%) | 600 (31) | 105 (29) | 250 (21) | <0.001 * |
| Missing, n (%) | 0 (0) | 71 (19) | 27 (2) | |
| Cohort | ||||
|---|---|---|---|---|
| Infant Characteristic | INSPIRE | MARCH | Healthy Start | p-Value a |
| Sample Size | 1920 | 365 | 1207 | |
| Birth weight (grams) b, mean (SD) | 3432 (461) | 3225 (577) | 3218 (526) | <0.001 * |
| Missing, n (%) | 0 (0) | 71 (19) | 14 (1) | |
| Gestational age (weeks) b, mean (SD) | 39 (1) | 38 (2) | 39 (2) | <0.001 * |
| Missing, n (%) | 0 (0) | 71 (19) | 9 (1) | |
| Race, n (%) | <0.001 * | |||
| White | 1451 (76) | 195 (53) | 850 (70) | |
| Black | 353 (18) | 94 (26) | 156 (13) | |
| Other | 116 (6) | 46 (13) | 201 (17) | |
| Missing, n (%) | 0 (0) | 30 (8) | 0 (0) | |
| Hispanic ethnicity, n (%) | 161 (8) | 34 (9) | 356 (29) | <0.001 * |
| Missing, n (%) | 4 (0) | 30 (8) | 0 (0) | |
| Male sex, n (%) | 1009 (53) | 137 (38) | 606 (50) | 0.16 |
| Missing, n (%) | 0 (0) | 71 (19) | 24 (2) | |
| Age at enrollment (months) b, mean (SD) | 2 (2) | 0 (0) b | 0 (0) b | <0.001 * |
| Missing, n (%) | 0 (0) | 0 (0) | 0 (0) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Snyder, B.M.; Gebretsadik, T.; Rohrig, N.B.; Wu, P.; Dupont, W.D.; Dabelea, D.M.; Fry, R.C.; Lynch, S.V.; McEvoy, C.T.; Paneth, N.S.; et al. The Associations of Maternal Health Characteristics, Newborn Metabolite Concentrations, and Child Body Mass Index among US Children in the ECHO Program. Metabolites 2023, 13, 510. https://doi.org/10.3390/metabo13040510
Snyder BM, Gebretsadik T, Rohrig NB, Wu P, Dupont WD, Dabelea DM, Fry RC, Lynch SV, McEvoy CT, Paneth NS, et al. The Associations of Maternal Health Characteristics, Newborn Metabolite Concentrations, and Child Body Mass Index among US Children in the ECHO Program. Metabolites. 2023; 13(4):510. https://doi.org/10.3390/metabo13040510
Chicago/Turabian StyleSnyder, Brittney M., Tebeb Gebretsadik, Nina B. Rohrig, Pingsheng Wu, William D. Dupont, Dana M. Dabelea, Rebecca C. Fry, Susan V. Lynch, Cindy T. McEvoy, Nigel S. Paneth, and et al. 2023. "The Associations of Maternal Health Characteristics, Newborn Metabolite Concentrations, and Child Body Mass Index among US Children in the ECHO Program" Metabolites 13, no. 4: 510. https://doi.org/10.3390/metabo13040510
APA StyleSnyder, B. M., Gebretsadik, T., Rohrig, N. B., Wu, P., Dupont, W. D., Dabelea, D. M., Fry, R. C., Lynch, S. V., McEvoy, C. T., Paneth, N. S., Ryckman, K. K., Gern, J. E., Hartert, T. V., & on behalf of Program Collaborators for Environmental Influences on Child Health Outcomes. (2023). The Associations of Maternal Health Characteristics, Newborn Metabolite Concentrations, and Child Body Mass Index among US Children in the ECHO Program. Metabolites, 13(4), 510. https://doi.org/10.3390/metabo13040510