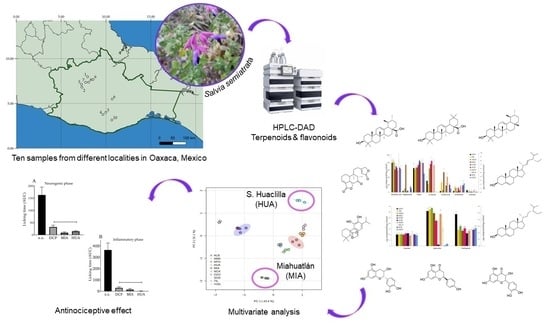
Variation in Terpenoid and Flavonoid Content in Different Samples of Salvia semiatrata Collected from Oaxaca, Mexico, and Its Effects on Antinociceptive Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drugs and Reagents
2.2. Site Descriptions and Plant Material
2.3. Preparations of the Extracts
2.4. Analysis of Phenols and Terpenoids via High-Performance Liquid Chromatography (HPLC)
2.5. Pharmacological Evaluations
2.5.1. Animals
2.5.2. Formaline Test
2.6. Statistical Analysis
3. Results
3.1. Quantification of Terpenoids and Flavonoids from S. semiatrata via HPLC-DAD
3.2. Principal Component Analysis (PCA) of Secondary Metabolite Content
3.3. Canonical Correspondence Analysis (CCA) between Climatic Variables and Secondary Metabolite Content
3.4. Vegetation Type and Variation in Terpenoids and Flavonoids
3.5. Evaluation of the Antinociceptive Effect
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ramamoorthy, T.P.; Elliott, M. Lamiaceae de México: Diversidad, distribución, endemismo y evolución. In Diversidad biológica de México: Orígenes y Distribución; Ramamoorthy, T.P., Bye, R., Lot, A., Fa, J., Eds.; Instituto de Biología, UNAM: Mexico City, Mexico, 1998; pp. 501–526. [Google Scholar]
- Martinez-Gordillo, M.; Fragoso-Martínez, I.; García-Peña, M.; Montiel, O. Genera of Lamiaceae from Mexico, diversity and endemism. Rev. Mex. Biodivers. 2013, 84, 30–86. [Google Scholar] [CrossRef] [Green Version]
- Argueta, A.; Cano, L.; Rodarte, M. Atlas de las Plantas de la Medicina Tradicional Mexicana; Instituto Nacional Indigenista: Mexico City, Mexico, 1994; p. 1193.
- Ortiz-Mendoza, N.; Aguirre-Hernández, E.; Fragoso-Martínez, I.; González-Trujano, M.E.; Basurto-Peña, F.A.; Martínez-Gordillo, M.J. A Review on the Ethnopharmacology and Phytochemistry of the Neotropical Sages (Salvia Subgenus Calosphace; Lamiaceae) Emphasizing Mexican Species. Front. Pharmacol. 2022, 13, 867892. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.B.; Ni, Z.Y.; Shi, Q.W.; Dong, M.; Kiyota, H.; Gu, Y.C.; Cong, B. Constituents from Salvia species and their biological activities. Chem. Rev. 2012, 112, 5967–6026. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Pérez, G.; González-Trujano, M.E.; Martínez-Gordillo, M.J.; Miguel-Chávez, R.; Basurto-Peña, F.A.; Dorazco González, A.; Aguirre-Hernández, E. Amarisolide A and pedalitin as bioactive compounds in the antinociceptive effects of Salvia circinata (Lamiaceae). Bot. Sci. 2019, 97, 355–365. [Google Scholar] [CrossRef] [Green Version]
- Cuevas-Morales, C.; Zavala-Ocampo, L.M.; San Miguel-Chávez, R.; González-Trujano, M.E.; Basurto-Peña, F.A.; Muñoz-Ocotero, V.; Aguirre-Hernández, E. Pharmacological Evaluation of the Antinociceptive Activity and Phytochemical Analysis of Active Extracts of Salvia purpurea Cav. Bot. Sci. 2022, 100, 383–396. [Google Scholar] [CrossRef]
- Ortiz-Mendoza, N.; Zavala-Ocampo, L.M.; Martínez-Gordillo, M.J.; González-Trujano, M.E.; Basurto-Peña, F.A.; Bazany-Rodríguez, I.J.; Rivera-Chávez, J.A.; Dorazco-González, A.; Aguirre-Hernández, E. Antinociceptive and anxiolytic-like effects of a neo-clerodane diterpene from Salvia semiatrata aerial parts. Pharm. Biol. 2020, 58, 620–629. [Google Scholar] [CrossRef]
- Anaya-Alvarado, A. Evaluación de la Actividad Analgésica de Salvia involucrata y Análisis Químico del Extracto Active. Bachelor’s Thesis, Biologist, UNAM, México, Ciudad de Mexico, Mexico, 2022. [Google Scholar]
- Githinji, C.G.; Mbugua, P.M.; Kanui, T.I.; Kariuki, D.K. Phytochemical and analgesic evaluation of Mondia whytei (hook.f) root. J. Pharmacogn. Phytother. 2012, 4, 26–32. [Google Scholar] [CrossRef]
- Rodrigues, M.R.A.; Kanazawa, L.K.S.; Neves, T.L.M.D.; Silva, C.F.D.; Horst, H.; Pizzolatti, M.G.; Werner, M.F.D.P. Antinociceptive and anti-inflammatory potential of extract and isolated compounds from the leaves of Salvia officinalis in mice. J. Ethnopharmacol. 2012, 139, 519–526. [Google Scholar] [CrossRef]
- Hirota, B.C.K.; Paula, C.D.S.; De Oliveira, V.B.; Da Cunha, J.M.; Schreiber, A.K.; Ocampos, F.M.; Miguel, M.D. Phytochemical and antinociceptive, anti-inflammatory, and antioxidant studies of Smilax larvata (Smilacaceae). Evid. -Based Complement. Altern. Med. 2016, 2016, 9894610. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Zhou, Q.; Yao, Y.; Li, X.; Zhang, J.; Su, G.; Deng, A. Inhibitory effect of gardenblue blueberry (Vaccinium ashei Reade) anthocyanin extracts on lipopolysaccharide-stimulated inflammatory response in RAW 264.7 cells. J. Zhejiang Univ. Sci. B 2016, 17, 425–436. [Google Scholar] [CrossRef] [Green Version]
- do Nascimento, J.E.T.; de Morais, S.M.; de Lisboa, D.S.; de Oliveira Sousa, M.; Santos, S.A.A.R.; Magalhães, F.E.A.; Campos, A.R. The orofacial antinociceptive effect of kaempferol-3-O-rutinoside, isolated from the plant Ouratea fieldingiana, on adult zebrafish (Danio rerio). Biomed. Pharmacother. 2018, 107, 1030–1036. [Google Scholar] [CrossRef]
- Forouzanfar, F.; Hosseinzadeh, H. Medicinal herbs in the treatment of neuropathic pain: A review. Iran. J. Basic Med. Sci. 2018, 21, 347–358. [Google Scholar] [CrossRef]
- Islam, S.; Shajib, M.S.; Rashid, R.B. Antinociceptive activities of Artocarpus lacucha Buch-ham (Moraceae) and its isolated phenolic compound, catechin, in mice. BMC Complement. Altern. Med. 2019, 19, 214. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Ma, C.; Chen, Y.; Li, X.; Chen, J. Cytotoxic Neo-clerodane diterpenoids from Scutellaria barbata D.DON. Chem. Biodivers. 2019, 16, e1800499. [Google Scholar] [CrossRef]
- González-Chávez, M.; Alonso-Castro, A.; Zapata-Morales, J.; Arana-Argáez, V.; Torres-Romero, J.; Medina-Rivera, Y.; Sánchez-Mendoza, E.; Pérez-Gutiérrez, S. Anti-inflammatory and antinociceptive effects of tilifodiolide, isolated from Salvia tiliifolia Vahl (Lamiaceae). Drug Dev. Res. 2018, 79, 165–172. [Google Scholar] [CrossRef]
- Javed, F.; Jabeen, Q.; Aslam, N.; Awan, A.M. Pharmacological evaluation of analgesic, anti-inflammatory and antipyretic activities of ethanolic extract of Indigofera argentea Burm. f. J. Ethnopharmacol. 2020, 259, 112966. [Google Scholar] [CrossRef]
- Wink, M. Introduction: Biochemistry, Physiology and Ecological Functions of Secondary Metabolites. In Annual Plant Reviews Biochemistry of Plant Secondary Metabolism; Wink, M., Ed.; Wiley Online Library: Hoboken, NJ, USA, 2010; Volume 40. [Google Scholar]
- Aguiñiga, I.; Cadena-Iñiguez, J.; Santiago-Osorio, E.; Gómez-García, G.; Mendoza-Núñez, V.M.; Rosado-Pérez, J.; Ruíz-Ramos, M.; Cisneros, V.; Ledesma-Martínez, E.; Delgado-Bordonave, A.; et al. Chemical analyses and in vitro and in vivo toxicity of fruit methanol extract of Sechium edule var. nigrum spinosum. Pharm. Biol. 2017, 55, 1638–1645. [Google Scholar] [CrossRef] [Green Version]
- Carrera, F.P.; Noceda, C.; Maridueña-Zavala, M.G.; Cevallos-Cevallos, J.M. Metabolomomics, a powerful tool for understanding Plant abiotic stress. Agronomy 2021, 11, 824. [Google Scholar] [CrossRef]
- Nakabayashi, R.; Yonekura-Sakakibara, K.; Urano, K.; Suzuki, M.; Yamada, Y.; Nishizawa, T.; Matsuda, F.; Kojima, M.; Sakakibara, H.; Shinozaki, K. Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. Plant J. 2014, 77, 367–379. [Google Scholar] [CrossRef] [Green Version]
- Arbona, V.; Manzi, M.; de Ollas, C.; Gómez-Cadenas, A. Metabolomics as a tool to investigate abiotic stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 4885–4911. [Google Scholar] [CrossRef] [Green Version]
- Sülsen, V.P.; Lizarraga, E.; Mamadalieva, N.Z.; Lago, J.H.G. Potential of Terpenoids and Flavonoids from Asteraceae as Anti-Inflammatory, Antitumor, and Antiparasitic Agents. Evid. -Based Complement. Altern. Med. 2017, 2017, 6196198. [Google Scholar] [CrossRef] [PubMed]
- Bano, A.; Qadri, T.A.; Mahnoor; Khan, N. Bioactive metabolites of plants and microbes and their role in agricultural sustainability and mitigation of plant stress. S. Afr. J. Bot. 2023, 159, 98–109. [Google Scholar] [CrossRef]
- Anjali; Kumar, S.; Korra, T.; Thakur, R.; Arutselvan, R.; Kashyap, A.S.; Nehela, Y.; Chaplygin, V.; Minkina, T.; Keswani, C. Role of plant secondary metabolites in defence and transcriptional regulation in response to biotic stress. Plant Stress 2023, 8, 100154. [Google Scholar] [CrossRef]
- Kalaycıoğlu, Z.; Uzaşçı, S.; Dirmenci, T.; Erim, F.B. α-Glucosidase enzyme inhibitory effects and ursolic and oleanolic acid contents of fourteen Anatolian Salvia species. J. Pharm. Biomed. Anal. 2018, 155, 284–287. [Google Scholar] [CrossRef]
- Jash, S.; Gorai, D.; Roy, R. Salvia genus and triterpenoids. Int. J. Pharm. Sci. Res. 2016, 7, 4710–4732. [Google Scholar]
- Lu, Y.; Foo, Y. Polyphenolics of Salvia—A review. Phytochemistry 2002, 59, 117–140. [Google Scholar] [CrossRef]
- Türkmen, M.; Kaya, D.A.; Ayanoğlu, F. Variations in essential oil main components of native grown Salvia aramiensis Rech fil. genotypes depending on years. In Proceedings of the ICAMS Proceedings of the International Conference on Advanced Materials and Systems, Bucharest, Romania, 27 October 2022; pp. 241–246. [Google Scholar] [CrossRef]
- Talebi, S.M.; Behzadpour, A.; Matsyura, A. Morphological and essential oil variations among Iranian populations of Salvia chloroleuca (Lamiaceae). Biosyst. Divers. 2019, 27, 233–237. [Google Scholar] [CrossRef]
- Myrtaj, B.; Dervishi, A.; Nuro, A.; Salihila, J.; Peci, D. Climate influence and essential oils composition of Salvia officinalis in populations of southern Albania. Agric. For. 2022, 68, 123–134. [Google Scholar] [CrossRef]
- Ben Farhat, M.; Sotomayor, J.A.; Jordán, M.J. Salvia verbenaca L. essential oil: Variation of yield and composition according to collection site and phenophase. Biochem. Syst. Ecol. 2019, 82, 35–43. [Google Scholar] [CrossRef]
- Duletić-Laušević, S.; Aradski, A.A.; Živković, J.; Gligorijević, N.; Šavikin, K.; Radulović, S.; Marin, P.D. Evaluation of bioactivities and phenolic composition of extracts of Salvia officinalis L. (Lamiaceae) collected in montenegro. Bot. Serbica 2019, 43, 47–58. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Yang, D.; Liang, Z.; Liu, J.; Yan, K.; Zhu, Y.; Yang, S. Climatic factors control the geospatial distribution of active ingredients in Salvia miltiorrhiza Bunge in China. Sci. Rep. 2019, 9, 904. [Google Scholar] [CrossRef] [Green Version]
- He, C.; Han, T.; Liu, C.; Sun, P.; Liao, D.; Li, X. Deciphering the effects of genotype and climatic factors on the performance, active ingredients and rhizosphere soil properties of Salvia miltiorrhiza. Front. Plant Sci. 2023, 14, 860. [Google Scholar] [CrossRef]
- Holopainen, J.K.; Virjamo, V.; Ghimire, R.P.; Blande, J.D.; Julkunen-Tiitto, R.; Kivimäenpää, M. Climate Change Effects on Secondary Compounds of Forest Trees in the Northern Hemisphere. Front. Plant Sci. 2018, 9, 1445. [Google Scholar] [CrossRef] [Green Version]
- Zarenezhad, E.; Abdulabbas, H.T.; Kareem, A.S.; Kouhpayeh, S.A.; Barbaresi, S.; Najafipour, S.; Mazarzaei, A.; Sotoudeh, M.; Ghasemian, A. Protective role of flavonoids quercetin and silymarin in the viral-associated inflammatory bowel disease: An updated review. Arch. Microbiol. 2023, 205, 252. [Google Scholar] [CrossRef]
- de-Almeida, S.C.X.; da-Silva, A.C.F.; Sousa, N.R.T.; Amorim, I.H.F.; Leite, B.G.; Neves, K.T.R.; Costa, J.G.M.; Felipe, C.F.B.; de-Barros-Viana, G.S. Antinociceptive and anti-inflammatory activities of a triterpene-rich fraction from Himatanthus drasticus. Braz. J. Med. Biol. 2019, 52, e7798. [Google Scholar] [CrossRef]
- Moreno-Pérez, G.F.; González-Trujano, M.E.; Hernández-León, A.; Valle-Dorado, M.G.; Valdés-Cruz, A.; Alvarado-Vásquez, N.; Aguirre-Hernández, E.; Salgado-Ceballos, H.; Pellicer, F. Antihyperalgesic and Antiallodynic Effects of Amarisolide A and Salvia amarissima Ortega in Experimental Fibromyalgia-Type Pain. Metabolites 2022, 13, 59. [Google Scholar] [CrossRef]
- Rodríguez-Hahn, L.; Esquivel, B.; Cárdenas, J. Neo-Clerodane Diterpenoids from American Salvia Species. In Phytochemistry of Medicinal Plants. Recent Advances in Phytochemistry; Arnason, J.T., Mata, R., Romeo, J.T., Eds.; Springer: Boston, MA, USA, 1995. [Google Scholar]
- Lovell, K.; Prevatt-Smith, K.; Lozama, A.; Prisinzano, E. Synthesis of Neoclerodane diterpenes and their pharmacological effects. In Chemistry of Opioids; Nagase, H., Ed.; Springer: New York, NY, USA, 2011. [Google Scholar]
- Safe, S.; Jayaraman, A.; Chapkin, R.S.; Howard, M.; Mohankumar, K.; Shrestha, R. Flavonoids: Structure-function and mechanisms of action and opportunities for drug development. Toxicol. Res. 2021, 37, 147–162. [Google Scholar] [CrossRef]
- Liu, J. Pharmacology of olenolic acid and ursolic acid. J. Ethnopharmacol. 1995, 49, 57–68. [Google Scholar] [CrossRef]







| Location | Voucher Number FCME | Vegetation Types | E (masl) | AP (mm) | AAT (°C) |
|---|---|---|---|---|---|
| APO | 181853 | DF | 1968 | 597.8 | 14.0 |
| TIL | 181854 | QF | 2220 | 708.6 | 18.5 |
| AMA | 181855 | DF | 1629 | 943.6 | 24.6 |
| MIA | 181856 | PQF | 1556 | 1002.6 | 19.8 |
| YOD | 181857 | Agr | 2303 | 1008.8 | 17.3 |
| ALB | 181858 | XS | 1749 | 570.4 | 23.3 |
| NOX | 181859 | QF | 2130 | 689.4 | 20.9 |
| HUA | 181860 | Agr | 2168 | 597.8 | 15.7 |
| SOS | 181861 | PQF | 1920 | 1104.8 | 18.6 |
| OZO | 181862 | DF | 2371 | 816.5 | 17.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz-Mendoza, N.; San Miguel-Chávez, R.; Martínez-Gordillo, M.J.; Basurto-Peña, F.A.; Palma-Tenango, M.; Aguirre-Hernández, E. Variation in Terpenoid and Flavonoid Content in Different Samples of Salvia semiatrata Collected from Oaxaca, Mexico, and Its Effects on Antinociceptive Activity. Metabolites 2023, 13, 866. https://doi.org/10.3390/metabo13070866
Ortiz-Mendoza N, San Miguel-Chávez R, Martínez-Gordillo MJ, Basurto-Peña FA, Palma-Tenango M, Aguirre-Hernández E. Variation in Terpenoid and Flavonoid Content in Different Samples of Salvia semiatrata Collected from Oaxaca, Mexico, and Its Effects on Antinociceptive Activity. Metabolites. 2023; 13(7):866. https://doi.org/10.3390/metabo13070866
Chicago/Turabian StyleOrtiz-Mendoza, Nancy, Rubén San Miguel-Chávez, Martha Juana Martínez-Gordillo, Francisco Alberto Basurto-Peña, Mariana Palma-Tenango, and Eva Aguirre-Hernández. 2023. "Variation in Terpenoid and Flavonoid Content in Different Samples of Salvia semiatrata Collected from Oaxaca, Mexico, and Its Effects on Antinociceptive Activity" Metabolites 13, no. 7: 866. https://doi.org/10.3390/metabo13070866
APA StyleOrtiz-Mendoza, N., San Miguel-Chávez, R., Martínez-Gordillo, M. J., Basurto-Peña, F. A., Palma-Tenango, M., & Aguirre-Hernández, E. (2023). Variation in Terpenoid and Flavonoid Content in Different Samples of Salvia semiatrata Collected from Oaxaca, Mexico, and Its Effects on Antinociceptive Activity. Metabolites, 13(7), 866. https://doi.org/10.3390/metabo13070866