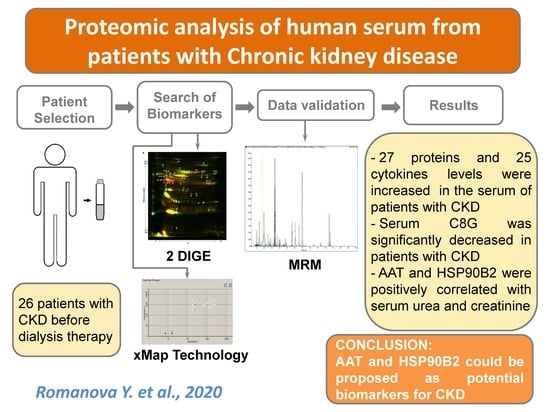
Proteomic Analysis of Human Serum from Patients with Chronic Kidney Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Ethics Statement
2.3. 2D-DIGE of Serum Proteins after Depletion
2.4. Protein Identification
2.5. Preparation of Serum Tryptic Digests
2.6. LC-MRM/MS Analysis of Serum Digests
2.7. Analysis of Serum Levels of Cytokines
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jha, V.; Garcia-Garcia, G.; Iseki, K.; Li, Z.; Naicker, S.; Plattner, B.; Saran, R.; Wang, A.Y.; Yang, C.W. Chronic kidney disease: Global dimension and perspectives. Lancet 2013, 382, 260–272. [Google Scholar] [CrossRef]
- Naghavi, M.; Abajobir, A.A.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abera, S.F.; Aboyans, V.; Adetokunboh, O.; Afshin, A.; Agrawal, A.; et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1151–1210. [Google Scholar] [CrossRef] [Green Version]
- Andrassy, K.M. Comments on ‘KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease’. Kidney Int. 2013, 84, 622–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kriz, W.; LeHir, M. Pathways to nephron loss starting from glomerular diseases-insights from animal models. Kidney Int. 2005, 67, 404–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gentile, G.; Remuzzi, G. Novel Biomarkers for Renal Diseases? None for the Moment (but One). J. Biomol. Screen. 2016, 21, 655–670. [Google Scholar] [CrossRef] [Green Version]
- Lam, C.W. 2. Inflammation, Cytokines and Chemokines in Chronic Kidney Disease. EJIFCC 2009, 20, 12–20. [Google Scholar]
- Carrero, J.J.; Park, S.H.; Axelsson, J.; Lindholm, B.; Stenvinkel, P. Cytokines, atherogenesis, and hypercatabolism in chronic kidney disease: A dreadful triad. Semin. Dial. 2009, 22, 381–386. [Google Scholar] [CrossRef]
- Luczak, M.; Formanowicz, D.; Marczak, Ł.; Suszyńska-Zajczyk, J.; Pawliczak, E.; Wanic-Kossowska, M.; Stobiecki, M. iTRAQ-based proteomic analysis of plasma reveals abnormalities in lipid metabolism proteins in chronic kidney disease-related atherosclerosis. Sci. Rep. 2016, 6, 32511. [Google Scholar] [CrossRef] [Green Version]
- Fourdinier, O.; Schepers, E.; Metzinger-Le Meuth, V.; Glorieux, G.; Liabeuf, S.; Verbeke, F.; Vanholder, R.; Brigant, B.; Pletinck, A.; Diouf, M.; et al. Serum levels of miR-126 and miR-223 and outcomes in chronic kidney disease patients. Sci. Rep. 2019, 9, 4477. [Google Scholar] [CrossRef] [Green Version]
- Fujii, R.; Yamada, H.; Yamazaki, M.; Munetsuna, E.; Ando, Y.; Ohashi, K.; Ishikawa, H.; Shimoda, H.; Sakata, K.; Ogawa, A.; et al. Circulating microRNAs (miR-126, miR-197, and miR-223) are associated with chronic kidney disease among elderly survivors of the Great East Japan Earthquake. BMC Nephrol. 2019, 20, 474. [Google Scholar] [CrossRef]
- Wen, Q.; Zhang, L.; Mao, H.P.; Tang, X.Q.; Rong, R.; Fan, J.J.; Yu, X.Q. Proteomic analysis in peritoneal dialysis patients with different peritoneal transport characteristics. Biochem. Biophys. Res. Commun. 2013, 438, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-P.; Yang, C.-Y.; Liao, C.-C.; Yu, W.-C.; Chi, C.-W.; Lin, C.-H. Plasma Protein Characteristics of Long-Term Hemodialysis Survivors. PLoS ONE 2012, 7, e40232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luczak, M.; Formanowicz, D.; Marczak, Ł.; Pawliczak, E.; Wanic-Kossowska, M.; Figlerowicz, M.; Stobiecki, M. Deeper insight into chronic kidney diseaserelated atherosclerosis: Comparative proteomic studies of blood plasma using 2DE and mass spectrometry. J. Transl. Med. 2015, 13, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glorieux, G.; Mullen, W.; Duranton, F.; Filip, S.; Gayrard, N.; Husi, H.; Schepers, E.; Neirynck, N.; Schanstra, J.P.; Jankowski, J.; et al. New insights in molecular mechanisms involved in chronic kidney disease using high-resolution plasma proteome analysis. Nephrol. Dial. Transplant. 2015, 30, 1842–1852. [Google Scholar] [CrossRef] [Green Version]
- Unlu, M.; Morgan, M.E.; Minden, J.S. Difference gel electrophoresis: A single gel method for detecting changes in protein extracts. Electrophoresis 1997, 18, 2071–2077. [Google Scholar] [CrossRef]
- Tannu, N.S.; Hemby, S.E. Two-dimensional fluorescence difference gel electrophoresis for comparative proteomics profiling. Nat. Protoc. 2006, 1, 1732–1742. [Google Scholar] [CrossRef]
- Wray, W.; Boulikas, T.; Wray, V.P.; Hancock, R. Silver staining of proteins in polyacrylamide gels. Anal. Biochem. 1981, 118, 197–203. [Google Scholar] [CrossRef]
- Gharahdaghi, F.; Weinberg, C.R.; Meagher, D.A.; Imai, B.S.; Mische, S.M. Mass spectrometric identification of proteins from silver-stained polyacrylamide gel: A method for the removal of silver ions to enhance sensitivity. Electrophoresis 1999, 20, 601–605. [Google Scholar] [CrossRef]
- Software, M.L. Skyline Targeted Mass Spec Environment. Available online: https://skyline.ms/project/home/software/Skyline/begin.view (accessed on 6 February 2020).
- MacLean, B.; Tomazela, D.M.; Shulman, N.; Chambers, M.; Finney, G.L.; Frewen, B.; Kern, R.; Tabb, D.L.; Liebler, D.C.; MacCoss, M.J. Skyline: An open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics 2010, 26, 966–968. [Google Scholar] [CrossRef] [Green Version]
- R-project. The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 6 February 2020).
- Ihara, H.; Toya, N.; Kakinoki, T.; Tani, A.; Aoki, Y.; Hashizume, N.; Inada, Y.; Nanba, S.; Urayama, T.; Yoshida, M. Reference ranges for serum protein fractions as determined by capillary zone electrophoresis. Jpn. J. Electroph. 2001, 45, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Larsson, A.; Hansson, L.O. Plasma protein fractions in healthy blood donors quantitated by an automated multicapillary electrophoresis system. J. Chromatogr. Sci. 2006, 44, 479–483. [Google Scholar] [CrossRef] [Green Version]
- Domanski, D.; Percy, A.J.; Yang, J.; Chambers, A.G.; Hill, J.S.; Freue, G.V.; Borchers, C.H. MRM-based multiplexed quantitation of 67 putative cardiovascular disease biomarkers in human plasma. Proteomics 2012, 12, 1222–1243. [Google Scholar] [CrossRef]
- Okuda, S.; Watanabe, Y.; Moriya, Y.; Kawano, S.; Yamamoto, T.; Matsumoto, M.; Takami, T.; Kobayashi, D.; Araki, N.; Yoshizawa, A.C.; et al. jPOSTrepo: An international standard data repository for proteomes. Nucleic Acids Res. 2017, 45, D1107–D1111. [Google Scholar] [CrossRef] [Green Version]
- Critselis, E.; Lambers Heerspink, H. Utility of the CKD273 peptide classifier in predicting chronic kidney disease progression. Nephrol. Dial. Transplant. 2016, 31, 249–254. [Google Scholar] [CrossRef] [Green Version]
- Verbeke, F.; Siwy, J.; Van Biesen, W.; Mischak, H.; Pletinck, A.; Schepers, E.; Neirynck, N.; Magalhães, P.; Pejchinovski, M.; Pontillo, C. The urinary proteomics classifier chronic kidney disease 273 predicts cardiovascular outcome in patients with chronic kidney disease. Nephrol. Dial. Transplant. 2019, gfz242, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kronenberg, F.; Kuen, E.; Ritz, E.; Konig, P.; Kraatz, G.; Lhotta, K.; Mann, J.F.; Muller, G.A.; Neyer, U.; Riegel, W.; et al. Apolipoprotein A-IV serum concentrations are elevated in patients with mild and moderate renal failure. J. Am. Soc. Nephrol. 2002, 13, 461–469. [Google Scholar]
- Zimmerhackl, L.B.; Leuk, B.; Hoschutzky, H. The cytoskeletal protein villin as a parameter for early detection of tubular damage in the human kidney. J. Chromatogr. 1991, 587, 81–84. [Google Scholar] [CrossRef]
- Salih, M.; Demmers, J.A.; Bezstarosti, K.; Leonhard, W.N.; Losekoot, M.; van Kooten, C.; Gansevoort, R.T.; Peters, D.J.; Zietse, R.; Hoorn, E.J. Proteomics of Urinary Vesicles Links Plakins and Complement to Polycystic Kidney Disease. J. Am. Soc. Nephrol. 2016, 27, 3079–3092. [Google Scholar] [CrossRef] [Green Version]
- Pang, L.; Li, Q.; Li, Y.; Liu, Y.; Duan, N.; Li, H. Urine proteomics of primary membranous nephropathy using nanoscale liquid chromatography tandem mass spectrometry analysis. Clin. Proteomics 2018, 15, 5. [Google Scholar] [CrossRef] [Green Version]
- Peters, K.E.; Davis, W.A.; Ito, J.; Winfield, K.; Stoll, T.; Bringans, S.D.; Lipscombe, R.J.; Davis, T.M.E. Identification of Novel Circulating Biomarkers Predicting Rapid Decline in Renal Function in Type 2 Diabetes: The Fremantle Diabetes Study Phase II. Diabetes Care 2017, 40, 1548–1555. [Google Scholar] [CrossRef] [Green Version]
- Boes, E.; Fliser, D.; Ritz, E.; König, P.; Lhotta, K.; Mann, J.F.; Müller, G.A.; Neyer, U.; Riegel, W.; Riegler, P.; et al. Apolipoprotein A-IV Predicts Progression of Chronic Kidney Disease: The Mild to Moderate Kidney Disease Study. Am. Soc. Nephrol. 2016, 17, 528–536. [Google Scholar] [CrossRef]
- Stangl, S.; Kollerits, B.; Lamina, C.; Meisinger, C.; Huth, C.; Stöckl, A.; Dähnhardt, D.; Böger, C.A.; Krämer, B.K.; Peters, A.; et al. Association between apolipoprotein A-IV concentrations and chronic kidney disease in two large population-based cohorts: Results from the KORA studies. J. Intern. Med. 2015, 278, 410–423. [Google Scholar] [CrossRef]
- Smajic, J.; Hasic, S.; Rasic, S. High-density lipoprotein cholesterol, apolipoprotein E and atherogenic index of plasma are associated with risk of chronic kidney disease. Med. Glas. (Zenica) 2018, 15, 115–121. [Google Scholar] [CrossRef]
- Lahrach, H.; Ghalim, N.; Taki, H.; Kettani, A.; Er-Rachdi, L.; Ramdani, B.; Saïle, R. Serum paraoxonase activity, high-sensitivity C-reactive protein, and lipoprotein disturbances in end-stage renal disease patients on long-term hemodialysis. J. Clin. Lipidol. 2008, 2, 43–50. [Google Scholar] [CrossRef]
- Liberopoulos, E.N.; Miltiadous, G.A.; Cariolou, M.; Tselepis, A.D.; Siamopoulos, K.C.; Elisaf, M.S. The influence of serum apolipoprotein E concentration and polymorphism on serum lipid parameters in hemodialysis patients. Am. J. Kidney Dis. 2004, 44, 300–308. [Google Scholar] [CrossRef] [Green Version]
- Naseeb, U.; Shafqat, J.; Jägerbrink, T.; Zarina, S.; Alvestrand, A.; Jörnvall, H.; Axelsson, J. Proteome Patterns in Uremic Plasma. Blood Purif. 2008, 26, 561–568. [Google Scholar] [CrossRef]
- Goek, O.N.; Kottgen, A.; Hoogeveen, R.C.; Ballantyne, C.M.; Coresh, J.; Astor, B.C. Association of apolipoprotein A1 and B with kidney function and chronic kidney disease in two multiethnic population samples. Nephrol. Dial. Transplant. 2012, 27, 2839–2847. [Google Scholar] [CrossRef] [Green Version]
- Calabresi, L.; Simonelli, S.; Conca, P.; Busnach, G.; Cabibbe, M.; Gesualdo, L.; Gigante, M.; Penco, S.; Veglia, F.; Franceschini, G. Acquired lecithin:cholesterol acyltransferase deficiency as a major factor in lowering plasma HDL levels in chronic kidney disease. J. Intern. Med. 2015, 277, 552–561. [Google Scholar] [CrossRef]
- Varela, I.; Tarpey, P.; Raine, K.; Huang, D.; Ong, C.K.; Stephens, P.; Davies, H.; Jones, D.; Lin, M.-L.; Teague, J.; et al. Exome sequencing identifies frequent mutation of the SWI/SNF complex gene PBRM1 in renal carcinoma. Nature 2011, 469, 539–542. [Google Scholar] [CrossRef] [Green Version]
- Borawski, J.; Naumnik, B.; Myśliwiec, M. Serum alpha1-antitrypsin but not complement C3 and C4 predicts chronic inflammation in hemodialysis patients. Ren. Fail. 2003, 25, 589–593. [Google Scholar] [CrossRef] [Green Version]
- Decuypere, J.P.; Ceulemans, L.J.; Wylin, T.; Martinet, W.; Monbaliu, D.; Pirenne, J.; Jochmans, I. Plasmatic Villin 1 Is a Novel In Vivo Marker of Proximal Tubular Cell Injury During Renal Ischemia-Reperfusion. Transplantation 2017, 101, e330–e336. [Google Scholar] [CrossRef]
- Kim, S.H.; Park, W.S.; Park, E.Y.; Park, B.; Joo, J.; Joung, J.Y.; Seo, H.K.; Lee, K.H.; Chung, J. The prognostic value of BAP1, PBRM1, pS6, PTEN, TGase2, PD-L1, CA9, PSMA, and Ki-67 tissue markers in localized renal cell carcinoma: A retrospective study of tissue microarrays using immunohistochemistry. PLoS ONE 2017, 12, e0179610. [Google Scholar] [CrossRef] [Green Version]
- Nagamachi, S.; Ohsawa, I.; Suzuki, H.; Sato, N.; Inoshita, H.; Hisada, A.; Honda, D.; Shimamoto, M.; Shimizu, Y.; Horikoshi, S.; et al. Properdin has an ascendancy over factor H regulation in complement-mediated renal tubular damage. BMC Nephrol. 2014, 15, 82. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.M.; Ren, X.G.; Jiang, Z.H.; Chen, D.J.; Zhao, W.J.; Li, L.J. Lectin-induced renal local complement activation is involved in tubular interstitial injury in diabetic nephropathy. Clin. Chim. Acta 2018, 482, 65–73. [Google Scholar] [CrossRef]
- Choi, Y.W.; Kim, Y.G.; Song, M.; Moon, J.-Y.; Jeong, K.-H.; Lee, T.-W.; Ihm, C.-G.; Park, K.-S.; Lee, S.-H. Potential urine proteomics biomarkers for primary nephrotic syndrome. Clin. Proteom. 2017, 14, 18. [Google Scholar] [CrossRef] [Green Version]
- Wenger, R.H.; Rolfs, A.; Marti, H.H.; Bauer, C.; Gassmann, M. Hypoxia, a novel inducer of acute phase gene expression in a human hepatoma cell line. J. Biol. Chem. 1995, 270, 27865–27870. [Google Scholar] [CrossRef] [Green Version]
- Brunetti, N.D.; Correale, M.; Pellegrino, P.L.; Cuculo, A.; Biase, M.D. Acute phase proteins in patients with acute coronary syndrome: Correlations with diagnosis, clinical features, and angiographic findings. Eur. J. Intern. Med. 2007, 18, 109–117. [Google Scholar] [CrossRef]
- Gilutz, H.; Siegel, Y.; Paran, E.; Cristal, N.; Quastel, M.R. Alpha 1-antitrypsin in acute myocardial infarction. Br. Heart J. 1983, 49, 26–29. [Google Scholar] [CrossRef] [Green Version]
- Borges, D.L.; Lemes, H.P.; de Castro Ferreira, V.; Filho, S.R.F. High-sensitivity C-reactive protein, apolipoproteins, and residual diuresis in chronic kidney disease patients undergoing hemodialysis. Clin. Exp. Nephrol. 2016, 20, 943–950. [Google Scholar] [CrossRef]
- Rasmy, A.; Abozeed, W.; Elsamany, S.; Baiomy, M.E.; Nashwa, A.; Amrallah, A.; Hasaan, E.; Alzahrani, A.; Faris, M.; Alsaleh, K.; et al. Correlation of Preoperative Ki67 and Serum CA15.3 Levels with Outcome in Early Breast Cancers a Multi Institutional Study. Asian Pac. J. Cancer Prev. 2016, 17, 3595–3600. [Google Scholar]
- Chauhan, R.; Lahiri, N. Tissue- and Serum-Associated Biomarkers of Hepatocellular Carcinoma. Biomark. Cancer 2016, 8, 37–55. [Google Scholar] [CrossRef] [Green Version]
- Okumura, F.; Joo-Okumura, A.; Nakatsukasa, K.; Kamura, T. The role of cullin 5-containing ubiquitin ligases. Cell Div. 2016, 11, 1. [Google Scholar] [CrossRef] [Green Version]
- Devor, E.J.; Schickling, B.M.; Reyes, H.D.; Warrier, A.; Lindsay, B.; Goodheart, M.J.; Santillan, D.A.; Leslie, K.K. Cullin-5, a ubiquitin ligase scaffold protein, is significantly underexpressed in endometrial adenocarcinomas and is a target of miR-182. Oncol. Rep. 2016, 35, 2461–2465. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.; Yao, G.D. The role of cullin proteins in gastric cancer. Tumour Biol. 2016, 37, 29–37. [Google Scholar] [CrossRef]
- Nowak, G.; Takacsova-Bakajsova, D.; Megyesi, J. Deletion of protein kinase C-epsilon attenuates mitochondrial dysfunction and ameliorates ischemic renal injury. Am. J. Physiol. Renal Physiol. 2017, 312, F109–F120. [Google Scholar] [CrossRef]
- Gray, M.O.; Karliner, J.S.; Mochly-Rosen, D. A selective epsilon-protein kinase C antagonist inhibits protection of cardiac myocytes from hypoxia-induced cell death. J. Biol. Chem. 1997, 272, 30945–30951. [Google Scholar] [CrossRef] [Green Version]
- Garlid, K.D.; Costa, A.D.; Quinlan, C.L.; Pierre, S.V.; Dos Santos, P. Cardioprotective signaling to mitochondria. J. Mol. Cell. Cardiol. 2009, 46, 858–866. [Google Scholar] [CrossRef] [Green Version]
- Della-Morte, D.; Raval, A.P.; Dave, K.R.; Lin, H.W.; Perez-Pinzon, M.A. Post-ischemic activation of protein kinase C epsilon protects the hippocampus from cerebral ischemic injury via alterations in cerebral blood flow. Neurosci. Lett. 2011, 487, 158–162. [Google Scholar] [CrossRef] [Green Version]
- Morris-Blanco, K.C.; Dave, K.R.; Saul, I.; Koronowski, K.B.; Stradecki, H.M.; Perez-Pinzon, M.A. Protein Kinase C Epsilon Promotes Cerebral Ischemic Tolerance Via Modulation of Mitochondrial Sirt5. Sci. Rep. 2016, 6, 29790. [Google Scholar] [CrossRef] [Green Version]
- Nourbakhsh, M.; Hoffmann, K.; Hauser, H. Interferon-b promoters contain a DNA element that acts as a position-independent silencer on the NF-kappaB site. EMBO J. 1993, 12, 451–459. [Google Scholar] [CrossRef]
- Nourbakhsh, M.; Kalble, S.; Dorrie, A.; Hauser, H.; Resch, K.; Kracht, M. The NF-kappa b repressing factor is involved in basal repression and interleukin (IL)-1-induced activation of IL-8 transcription by binding to a conserved NF-kappa b-flanking sequence element. J. Biol. Chem. 2001, 276, 4501–4508. [Google Scholar] [CrossRef] [Green Version]
- Nauta, A.J.; Raaschou-Jensen, N.; Roos, A.; Daha, M.R.; Madsen, H.O.; Borrias-Essers, M.C.; Ryder, L.P.; Koch, C.; Garred, P. Mannose-binding lectin engagement with late apoptotic and necrotic cells. Eur. J. Immunol. 2003, 33, 2853–2863. [Google Scholar] [CrossRef]
- Lovelace, L.L.; Cooper, C.L.; Sodetz, J.M.; Lebioda, L. Structure of human C8 protein provides mechanistic insight into membrane pore formation by complement. J. Biol. Chem. 2011, 286, 17585–17592. [Google Scholar] [CrossRef] [Green Version]
- Garlisi, C.G.; Falcone, A.; Kung, T.T.; Stelts, D.; Pennline, K.J.; Beavis, A.J.; Smith, S.R.; Egan, R.W.; Umland, S.P. T cells are necessary for Th2 cytokine production and eosinophil accumulation in airways of antigen-challenged allergic mice. Clin. Immunol. Immunopathol. 1995, 75, 75–83. [Google Scholar] [CrossRef]
- Mosmann, T.R.; Coffman, R.L. TH1 and TH2 cells: Different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 1989, 7, 145–173. [Google Scholar] [CrossRef]
- Schrum, S.; Probst, P.; Fleischer, B.; Zipfel, P.F. Synthesis of the CC-chemokines MIP-1alpha, MIP-1beta, and RANTES is associated with a type 1 immune response. J. Immunol. 1996, 157, 3598–3604. [Google Scholar]
- Romanova, Y.D.; Markelova, M.I.; Laikov, A.V.; Fakhrutdinova, L.I.; Hasanova, M.I.; Malanin, S.Y.; Chernov, V.M.; Salafutdinov, I.I.; Khaiboullina, S.F. Cytokine Levels in the Serum of Patients with Chronic Kidney Insufficiency Before and After Hemodialysis. BioNanoScience 2017, 7, 415–418. [Google Scholar] [CrossRef]
- Stangou, M.; Spartalis, M.; Daikidou, D.V.; Kouloukourgiotou, T.; Sampani, E.; Lambropoulou, I.T.; Pantzaki, A.; Papagianni, A.; Efstratiadis, G. Impact of Tauh1 and Tauh2 cytokines in the progression of idiopathic nephrotic syndrome due to focal segmental glomerulosclerosis and minimal change disease. J. Nephropathol. 2017, 6, 187–195. [Google Scholar] [CrossRef]
- Rios, D.R.A.; Pinheiro, M.B.; de Oliveira Junior, W.V.; Braga Gomes, K.; Carvalho, A.T.; Martins-Filho, O.A.; Simoes, E.S.A.C.; Dusse, L.M.S. Cytokine Signature in End-Stage Renal Disease Patients on Hemodialysis. Dis. Markers 2017, 2017, 9678391. [Google Scholar] [CrossRef] [Green Version]
- Bleotu, C.; Chifiriuc, M.C.; Grigore, R.; Grancea, C.; Popescu, C.R.; Anton, G.; Cernescu, C. Investigation of Th1/Th2 cytokine profiles in patients with laryngo-pharyngeal, HPV-positive cancers. Eur. Arch. Otorhinolaryngol. 2013, 270, 711–718. [Google Scholar] [CrossRef]
- Bestetti, R.B.; Dellalibera-Joviliano, R.; Lopes, G.S.; Faria-Jr, M.; Furlan-Daniel, R.; Lopes, K.C.; Batista, D.R. Determination of the Th1, Th2, Th17, and Treg cytokine profile in patients with chronic Chagas heart disease and systemic arterial hypertension. Heart Vessels 2019, 34, 123–133. [Google Scholar] [CrossRef]
- Khan, R.; Gupta, S.; Sharma, A. Circulatory levels of T-cell cytokines (interleukin [IL]-2, IL-4, IL-17, and transforming growth factor-beta) in patients with vitiligo. J. Am. Acad. Dermatol. 2012, 66, 510–511. [Google Scholar] [CrossRef]





| Parameters * | CKD Patients |
|---|---|
| Age (y) | 53.0 ± 16.2 |
| Sex (males/females) | 18/8 |
| eGFR (mL/min/1.73 m2) | 14.7 ± 3.1 |
| Diagnosis of CKD (number of patients) | 26 |
| Chronic glomerulonephritis | 18 |
| Diabetes | 3 |
| Chronic gouty nephropathy | 2 |
| Others or unknown | 3 |
| Body mass index (kg/m2) | 25.8 ± 5.6 |
| Systolic blood pressure (mmHg) | 128.7 ± 23.2 |
| Diastolic blood pressure (mmHg) | 79.2 ± 18.3 |
| Creatinine (mg/dL) | 7.25 ± 3.8 |
| Urea (mg/dL) | 135.0 ± 70.2 |
| Albumin (g/dL) | 3.8 ± 0.5 |
| Total cholesterol (mg/dL) | 177.8 ± 40.2 |
| HDL cholesterol (mg/dL) | 52.1 ± 28.4 |
| LDL cholesterol (mg/dL) | 110.6 ± 45.6 |
| Triglyceride (mg/dL) | 141.9 ± 50.1 |
| n/n | Protein | UniProt Accession Number | Target Peptide Sequence | MRM Transition Q1 | MRM Transition Q3 | Product Ion |
|---|---|---|---|---|---|---|
| 1 | Immunoglobulin superfamily member 22 (IGSF22) | Q8N9C0 | EDSGLILLK | 494.3 | 743.5 | y7 |
| 2 | T-complex protein 1 subunit delta (CCT4) | P50991 | LVIEEAER | 479.8 | 655.4 | b6 |
| 3 | Cullin-5 (CUL5) | Q93034 | EAFQDDPR | 489.2 | 777.4 | y6 |
| 4 | Apolipoprotein A-IV (APOA4) | P06727 | LAPLAEDVR | 492.3 | 589.3 | y5 |
| 5 | Apolipoprotein E (APOE) | P02649 | LGPLVEQGR | 484.8 | 588.3 | y5 |
| 6 | Apolipoprotein A-I (APOA1) | P02647 | QGLLPVLESFK | 615.9 | 819.5 | y7 |
| 7 | Coiled-coil domain-containing protein 43 (CCDC43) | Q96MW1 | LEALGVDR | 436.7 | 446.2 | y4 |
| 8 | Coiled-coil domain-containing protein 171 (CCDC171) | Q6TFL3 | TLQEALEK | 466.3 | 830.5 | y7 |
| 9 | Putative endoplasmin-like protein (HSP90B2) | Q58FF3 | FDDSEK | 370.7 | 478.2 | y4 |
| 10 | Plasminogen (PLG) | P00747 | LSSPAVITDK | 515.8 | 769.4 | b8 |
| 11 | Phospholipase B1 (PLB1) | Q6P1J6 | TETLDLR | 424.2 | 445.2 | b4 |
| 12 | LIM and cysteine-rich domains protein 1 (LMCD1) | Q9NZU5 | YSTLTAR | 406.2 | 465.2 | b4 |
| 13 | Alpha-1-antitrypsin (AAT) | P01009 | LSITGTYDLK | 555.8 | 797.4 | y7 |
| 14 | Villin-1 (VIL1) | P09327 | AFEVPAR | 395.2 | 442.3 | y4 |
| 15 | NF-kappa-B-repressing factor (NKRF) | O15226 | EIPPADIPK | 490.3 | 736.4 | b7 |
| 16 | Amyloid protein-binding protein 2 (APPBP2) | Q92624 | VVVDVLR | 400.3 | 700.4 | y6 |
| 17 | Serine/threonine-protein phosphatase with EF-hands 2 (PPEF2) | O14830 | SLPSSPLR | 428.7 | 472.3 | y4 |
| 18 | Ras GTPase-activating protein 4 (CAPRI) | O43374 | DELDLQR | 444.7 | 531.3 | y4 |
| 19 | Cytoskeleton-associated protein 2-like (CKAP2L) | Q8IYA6 | QFVGETQSR | 526.3 | 776.4 | y7 |
| 20 | Protein kinase C epsilon type (PKCE) | Q02156 | QINQEEFK | 518.2 | 613.2 | b5 |
| 21 | Antigen KI-67 | P46013 | EDSTADDSK | 484.1 | 504.1 | b5 |
| 22 | Complement factor H (CFH) | P08603 | NGFYPATR | 463.2 | 607.3 | y5 |
| 23 | Complement C4 (C4A, C4B) | P0C0L4 P0C0L5 | LTSLSDR | 396.2 | 577.3 | y5 |
| 24 | Ficolin-3 (FCN3) | O75636 | VELEDFNGNR | 596.8 | 722.3 | y6 |
| 25 | C4B-binding protein alpha chain (C4BPA) | P04003 | TWYPEVPK | 510.3 | 569.3 | y5 |
| 26 | Complement C1R subcomponent (C1R) | P00736 | GGGALLGDR | 408.2 | 460.3 | y4 |
| 27 | Complement C1S subcomponent (C1S) | P09871 | LLEVPEGR | 456.8 | 686.3 | y6 |
| 28 | Complement C1q subcomponent subunit C (C1QC) | P02747 | FQSVFTVTR | 542.8 | 623.4 | y5 |
| 29 | Complement С3 (C3) | P01024 | IWDVVEK | 444.7 | 474.3 | y4 |
| 30 | Complement С5 (C5) | P01031 | GTVYNYR | 436.7 | 452.2 | y3 |
| 31 | Complement component C8 alpha chain (C8A) | P07357 | STITYR | 370.7 | 552.3 | y4 |
| 32 | Complement component C8 beta chain (C8B) | P07358 | EYESYSDFER | 662.8 | 672.3 | b5 |
| 33 | Complement component C8 gamma chain (C8G) | P07360 | QLYGDTGVLGR | 589.8 | 678.3 | b6 |
| 34 | Complement С9 (С9) | P02748 | VVEESELAR | 516.3 | 833.4 | y7 |
| 35 | Mannose-binding protein C (MBL2) | P11226 | NAAENGAIQNLIK | 678.4 | 869.4 | b9 |
| 36 | Mannan-binding lectin serine protease 2 (MASP2) | O00187 | WPEPVFGR | 494.3 | 609.3 | b5 |
| 37 | Galectin-3 (Gal-3) | P17931 | LDNNWGR | 437.7 | 671.3 | y6 |
| 38 | Galectin-3-binding protein (M2BP) | Q08380 | VEIFYR | 413.7 | 727.4 | y5 |
| n/n | Protein | Fold Change | SC between Protein and Creatinine | SC between Protein and Urea | Reference | Study Population | Results |
|---|---|---|---|---|---|---|---|
| 1 | APOA4 | 3.4 * ↑ | 0.07 | 0.19 | [32] | 345 CKD patients with type 2 diabetes | Increased plasma level of APOA4 |
| [33] | 177 CKD patients | Increased serum level of APOA4 were significant predictors of disease progression | |||||
| [34] | 6220 participants of general population | Increased serum level of APOA4 were significant predictors of disease progression | |||||
| 2 | APOE | 2.1 ** ↑ | 0.30 | 0.30 | [35] | 117 CKD patients | APOE was a negative predictor of eGFR reduction rate |
| [36] | 109 HD patients | APOE were significantly decreased | |||||
| [8] | 90 CKD patients | Elevated level of APOE in plasma of patients with CKD 1-2 stages | |||||
| [37] | 301 HD patients | HD patients had a significantly lower prevalence of the E4 allele and greater levels of APOE | |||||
| [38] | 7 CKD patients | Increased plasma level of APOE | |||||
| 3 | APOA1 | 1.6 * ↑ | −0.16 | −0.07 | [39] | 17,315 participants of the general population | Higher serum APOA1 was associated with lower prevalence of CKD |
| [40] | 50 patients with CKD and 198 patients on HD therapy | CKD was found to be associated with highly significant reductions in plasma APOA1 | |||||
| [8] | 90 CKD patients | No differences between plasma APOA1 level of patients with CKD 1-2 stages and healthy voluntaries | |||||
| [11] | 76 patients who received initial insertion of PD | APOA1 showed enhanced levels in PD effluents of patients with high transporter | |||||
| 4 | IGSF22 | 4.5 ** ↑ | 0.34 | 0.35 | [41] | 7 patients with clear cell carcinoma | Found in a renal cell carcinoma sample; somatic mutation |
| 5 | HSP90B2 | 4.0 ** ↑ | 0.55 ** | 0.56 ** | - | - | - |
| 6 | AAT | 8.7 ** ↑ | 0.44 * | 0.41 | [12] | 12 non-diabetic ESRD patients | HD patients had altered plasma profiles of AAT isoforms |
| [31] | 63 patients with primary membranous nephropathy | Increased urinary level of AAT | |||||
| [42] | 103 HD patients | Higher serum AAT levels select the HD patients with severe inflammation from those without | |||||
| 7 | VIL1 | 2.6 * ↑ | 0.08 | 0.18 | [43] | 3 patients with AKI after liver transplantation | VIL1 is released in plasma during AKI and shows potential as an early marker for proximal tubular injury |
| [29] | 3 renal transplant recipients | VIL1 concentrations in the urine up to 20 mg/I | |||||
| 8 | Antigen KI-67 | 3.2 * ↑ | 0.31 | 0.31 | [44] | 351 patients with clear cell carcinoma | Ki-67 are significant prognostic factors of clear cell carcinoma |
| 9 | CFH | 2.7 * ↑ | 0.43 * | 0.43 * | [45] | 63 patients with RD | Urinary CFH levels were significantly higher in patients |
| 10 | C4A | 2.8 ** ↑ | 0.42 | 0.45 * | [38] | 7 CKD patients | Increased plasma level of CA4 |
| [13] | 90 patients with CKD | Increased plasma level of CA4 | |||||
| 11 | C4BPA | 4.5 ** ↑ | 0.3 | 0.38 | - | - | - |
| 12 | C1R | 4.1 ** ↑ | 0.48 * | 0.49 * | [14] | 29 patients with CKD | Increased plasma level of C1R |
| 13 | C1S | 2.1 ** ↑ | 0.51 * | 0.51 * | [14] | 29 patients with CKD | Increased plasma level of C1S |
| 14 | C1QC | 3.7 ** ↑ | 0.46 * | 0.50 * | [46] | 62 diabetic patients | No difference |
| 15 | C3 | 4.7 ** ↑ | 0.48 * | 0.50 * | [11] | 76 patients who received initial insertion of PD | C3 showed enhanced expression in PD effluents of patients with high transporter |
| [38] | 7 CKD patients | Increased plasma level of C3 | |||||
| 16 | C5 | 2.2 * ↑ | 0.11 | 0.15 | [45] | 63 patients with RD | Increased urinary MAC (SC5b-9) |
| 17 | C8A | 2.4 ** ↑ | 0.48 ** | 0.47 * | [45] | 63 patients with RD | Increased urinary MAC (SC5b-9) |
| 18 | C8B | 2.7 ** ↑ | 0.25 | 0.38 | [45] | 63 patients with RD | Increased urinary MAC (SC5b-9) |
| 19 | C8G | 3.1 ** ↓ | −0.41 | −0.63 * | [38] | 7 CKD patients | Decreased plasma level of C8G |
| 20 | С9 | 11 ** ↑ | 0.58 ** | 0.62 ** | [45] | 63 patients with RD | Increased urinary MAC (SC5b-9) |
| [47] | 53 patients with different nephropathy | Urinary C9 was elevated in MCD, MN and FSGS groups compared with in IgA nephropathy and healthy controls | |||||
| 21 | MBL2 | 3.4 ** ↑ | 0.18 | 0.18 | [46] | 62 diabetic patients | MBL was found to increase with the progression of DN |
| 22 | CUL5 | 3.3 ** ↑ | 0.23 | 0.29 | - | - | - |
| 23 | PKCE | 3.2 ** ↑ | 0.27 | 0.28 | - | - | - |
| 24 | CCDC43 | 2.2 * ↑ | 0.18 | 0.22 | - | - | - |
| 25 | CDC171 | 3.1 ** ↑ | 0.33 | 0.38 | - | - | - |
| 26 | CAPRI | 2.1 * ↑ | 0.18 | 0.23 | - | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romanova, Y.; Laikov, A.; Markelova, M.; Khadiullina, R.; Makseev, A.; Hasanova, M.; Rizvanov, A.; Khaiboullina, S.; Salafutdinov, I. Proteomic Analysis of Human Serum from Patients with Chronic Kidney Disease. Biomolecules 2020, 10, 257. https://doi.org/10.3390/biom10020257
Romanova Y, Laikov A, Markelova M, Khadiullina R, Makseev A, Hasanova M, Rizvanov A, Khaiboullina S, Salafutdinov I. Proteomic Analysis of Human Serum from Patients with Chronic Kidney Disease. Biomolecules. 2020; 10(2):257. https://doi.org/10.3390/biom10020257
Chicago/Turabian StyleRomanova, Yulia, Alexander Laikov, Maria Markelova, Rania Khadiullina, Alfiz Makseev, Milausha Hasanova, Albert Rizvanov, Svetlana Khaiboullina, and Ilnur Salafutdinov. 2020. "Proteomic Analysis of Human Serum from Patients with Chronic Kidney Disease" Biomolecules 10, no. 2: 257. https://doi.org/10.3390/biom10020257
APA StyleRomanova, Y., Laikov, A., Markelova, M., Khadiullina, R., Makseev, A., Hasanova, M., Rizvanov, A., Khaiboullina, S., & Salafutdinov, I. (2020). Proteomic Analysis of Human Serum from Patients with Chronic Kidney Disease. Biomolecules, 10(2), 257. https://doi.org/10.3390/biom10020257