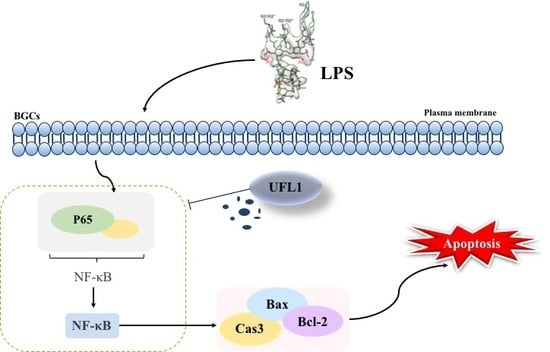
UFL1 Alleviates LPS-Induced Apoptosis by Regulating the NF-κB Signaling Pathway in Bovine Ovarian Granulosa Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cells Treatment
2.3. Cell Viability Assay
2.4. Immunofluorescence
2.5. Cell Transfection
2.6. Total RNA Extraction and Real-Time Fluorescence Quantitative PCR
2.7. Western Blot
2.8. Flow Cytometry for Apoptosis
2.9. HE Staining
2.10. Immunohistochemistry
2.11. Statistical Analysis
3. Results
3.1. The Expression of UFL1 in Cow Ovarian Tissue and bGCs
3.2. The Apoptosis Levels of bGCs under LPS Treatment
3.3. The Expression of UFL1 in LPS-Induced bGCs
3.4. UFL1 Regulated LPS-Induced Apoptosis in bGCs
3.5. UFL1 Reduced the Expression Levels of Genes and Proteins Associated with Apoptosis
3.6. UFL1 Inhibited LPS-Induced Activation of the NF-κB Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Inchaisri, C.; Jorritsma, R.; Vos, P.L.A.M.; Weijden, G.C.V.D.; Hogeveen, H. Economic consequences of reproductive performance in dairy cattle. Theriogenology 2010, 74, 835–846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seegers, H.; Fourichon, C.; Malher, X.; L’Hostis, M. A framework for animal health management. Vet. Res. 1994, 25, 165–173. [Google Scholar]
- McGee, E.A. Initial and cyclic recruitment of ovarian follicles. Endocr. Rev. 2000, 21, 200–214. [Google Scholar]
- Monniaux, D.; Clement, F.; Dalbies-Tran, R.; Estienne, A.; Fabre, S.; Mansanet, C.; Monget, P. The ovarian reserve of primordial follicles and the dynamic reserve of antral growing follicles: What is the link? Boil. Reprod. 2014, 90, 85. [Google Scholar] [CrossRef] [Green Version]
- Deng, W.; Yu, X.; Pang, Y.; Zan, L. Effects of granulosa cells treatments and follocular fluid on cleavage rate of bovine oocyte after in vitro fertilization and culture. Eur. PMC 2008, 41, 393–402. [Google Scholar]
- Hussein, M.R. Apoptosis in the ovary: Molecular mechanisms. Hum. Reprod. Updat. 2005, 11, 162–178. [Google Scholar] [CrossRef]
- Zeuner, A.; Müller, K.; Reguszynski, K.; Jewgenow, K. Apoptosis within bovine follicular cells and its effect on oocyte development during in vitro maturation. Theriogenology 2003, 59, 1421–1433. [Google Scholar] [CrossRef]
- Manabe, N.; Goto, Y.; Matsuda-Minehata, F.; Inoue, N.; Maeda, A.; Sakamaki, K.; Miyano, T. Regulation mechanism of selective atresia in porcine follicles: Regulation of granulosa cell apoptosis during atresia. J. Reprod. Dev. 2004, 50, 493–514. [Google Scholar] [CrossRef] [Green Version]
- Regan, S.L.P.; Knight, P.G.; Yovich, J.L.; Yee, L.; Frank, A.; Arun, D. Granulosa cell apoptosis in the ovarian follicle—a changing view. Front. Endocrinol. 2018, 9, 61. [Google Scholar] [CrossRef]
- Ying, S.; Guo, J.; Dai, Z.; Zhu, H.; Yu, J.; Ma, W.; Li, J.; Akhtar, M.F.; Shi, Z. Time course effect of lipopolysaccharide on Toll-like receptors expression and steroidogenesis in the Chinese goose ovary. Reproduction 2017, 153, 509–518. [Google Scholar] [CrossRef]
- Gindri, P.; de Ávila Castro, N.; Mion, B.; Gasperin, B.G.; Pegoraro, L.M.C.; Rincón, J.A.A.; Vieira, A.D.; Pradieé, J.; Pfeifer, L.F.M.; Corrêa, M.N.; et al. Intrafollicular lipopolysaccharide injection delays ovulation in cows. Anim. Reprod. Sci. 2019, 211, 106226–106233. [Google Scholar] [CrossRef] [PubMed]
- Hossein-Zadeh, N.G. Estimation of genetic and phenotypic relationships between age at first calving and productive performance in iranian holsteins. Trop. Anim. Heal. Prod. 2011, 43, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Berglund, B. Genetic improvement of dairy cow reproductive performance. Reprod. Domest. Anim. 2008, 43, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, K.; Moura, R.F.; Granvik, M.; Igoillo-Esteve, M.; Hohmeier, H.E.; Hendrickx, N.; Newgard, C.B.; Waelkens, E.; Cnop, M.; Schuit, F. Ubiquitin fold modifier 1 (ufm1) and its target ufbp1 protect pancreatic beta cells from er stress-induced apoptosis. PLoS ONE 2011, 6, e18517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, G.; Liu, S.; Wu, Y.; Lei, C.; Zhang, S. Diet-induced bacterial immunogens in the gastrointestinal tract of dairy cows: Impacts on immunity and metabolism. Acta Vet. Scand. 2011, 53, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, S.; Hao, H.; Du, W. Effect and mechanism of LPS on reproductive performance of postpartum dairy cows. Chin. J. Anim. Sci. 2016, 52, 74–79. [Google Scholar]
- Besnard, N.; Horne, E.A.; Whitehead, S.A. Prolactin and lipopolysaccharide treatment increased apoptosis and atresia in rat ovarian follicles. Acta Physiol. Scand. 2001, 172, 17–25. [Google Scholar] [CrossRef]
- Guthrie, H.D.; Garrett, W.M. Apoptosis during folliculogenesis in pigs. Reprod. (Cambridge, England) Suppl. 2001, 58, 17. [Google Scholar]
- Amsterdam, A.; Sasson, R.; Keren-Tal, I.; Aharoni, D.; Dantes, A.; Rimon, E.; Land, A.; Cohen, T.; Dor, Y.; Hirsh, L. Alternative pathways of ovarian apoptosis: Death for life. Biochem. Pharmacol. 2003, 66, 1355–1362. [Google Scholar] [CrossRef]
- Tatsumi, K.; Sou, Y.S.; Tada, N.; Nakamura, E.; Iemura, S.I.; Natsume, T.; Kang, S.H.; Chung, C.H.; Kasahara, M.; Kominami, E.; et al. A novel type of E3 ligase for the Ufm1 conjugation system. J. Biol. Chem. 2010, 285, 5417–5427. [Google Scholar] [CrossRef] [Green Version]
- Cai, Y.; Zhu, G.; Liu, S.; Pan, Z.; Quintero, M.; Poole, C.J.; Lu, C.; Zhu, H.; Islam, B.; Van Riggelen, J.; et al. Indispensable role of the Ubiquitin-fold modifier 1-specific E3 ligase in maintaining intestinal homeostasis and controlling gut inflammation. Cell Discov. 2019, 5, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, T.; Tatsumi, K.; Ozaki, Y.; Kawakami, T.; Suzuki, A.; Ogasahara, K.; Komatsu, M.; Kominami, E.; Tanaka, K.; Yamane, T. Crystal structure of ufc1, the ufm1-conjugating enzyme. Biochem. Biophys. Res. Commun. 2007, 362, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhu, X.; Zhang, Y.; Cai, Y.; Chen, J.; Sivaprakasam, S.; Gurav, A.; Pi, W.; Makala, L.; Wu, J.; et al. Rcad/ufl1, a ufm1 e3 ligase, is essential for hematopoietic stem cell function and murine hematopoiesis. Cell Death Differ. 2015, 22, 1922–1934. [Google Scholar] [CrossRef]
- Wu, J.; Lei, G.; Mei, M.; Tang, Y.; Li, H. A novel C53/LZAP-interacting protein regulates stability of C53/LZAP and DDRGK domain-containing Protein 1 (DDRGK1) and modulates NF-kappaB signaling. J. Biol. Chem. 2010, 285, 15126–15136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, C.; Li, L.; Chen, K.; Wang, G. UFL1 Alleviates Lipopolysaccharide-Induced Cell Damage and Inflammation via Regulation of the TLR4/NF-κB Pathway in Bovine Mammary Epithelial Cells. Oxid. Med. Cell Longev. 2019, 6505373, 17. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Wang, T.; Li, W.; Muhammad, I.; Wang, H.; Sun, X.; Yang, Y.; Li, J.; Xiao, T.; Zhang, X. Baicalin Alleviates Lipopolysaccharide-Induced Liver Inflammation in Chicken by Suppressing TLR4-Mediated NF-κB Pathway. Front. Pharmacol. 2017, 8, 547. [Google Scholar] [CrossRef] [Green Version]
- Bubici, C.; Papa, S.; Pham, C.G.; Zazzeroni, F.; Franzoso, G. Nf-κb and JNK: An intricate affair. Cell Cycle 2004, 3, 1524–1529. [Google Scholar] [CrossRef] [Green Version]
- Crh, R.; Whitfield, C. Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 2002, 71, 635–700. [Google Scholar]
- Herath, S.; Williams, E.J.; Lilly, S.T.; Gilbert, R.O.; Dobson, H.; Bryant, C.E.; Sheldon, I.M. Ovarian follicular cells have innate immune capabilities that modulate their endocrine function. Reproduction 2007, 134, 683–693. [Google Scholar] [CrossRef]
- Xu, M.X.; Sun, X.X.; Li, W.; Xie, G.; Yang, Q.; Qu, Z.W.; Meng, Q.G. LPS at low concentration promotes the fracture healing through regulating the autophagy of osteoblasts via NF-κB signal pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 1569–1579. [Google Scholar]
- Chung, Y. Lipopolysaccharide from prevotella nigrescens stimulates osteoclastogenesis in cocultures of bone marrow mononuclear cells and primary osteoblasts. J. Periodontal. Res. 2006, 41, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, L.; Wei, Z.; Zhang, X.; Wang, Y.; Li, F.; Fu, Y.; Liu, B. Inhibition of histone deacetylase reduces lipopolysaccharide-induced-inflammation in primary mammary epithelial cells by regulating ROS-NF-кB signaling pathways. Int. Immunopharmacol. 2018, 56, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Tilly, J.L.; Kowalski, K.I.; Johnson, A.L.; Hsueh, A.J.W. Involvement of apoptosis in ovarian follicular atresia and postovulatory regression. Endocrinology 1991, 129, 2799–2801. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.Y.; Fang, M.; Yi, J.M.; Jiang, F.; Moeen-Ud-Din, M.; Yang, L.G. Effect of overexpression of inhibin α (1–32) fragment on bovine granulosa cell proliferation, apoptosis, steroidogenesis, and development of co-cultured oocytes. Theriogenology 2008, 70, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Yinghua, Z.; Mingsheng, Z.; Jianchun, W.; Guohua, L.; Honglin, L.; Zhaozhong, H. Transcriptional regulation of the ufm1 conjugation system in response to disturbance of the endoplasmic reticulum homeostasis and inhibition of vesicle trafficking. PLoS ONE 2012, 7, e48587. [Google Scholar]
- Rahman, M.A.; Wang, P.; Zhao, Z.; Wang, D.; Shin, D.M. Systemic Delivery of Bc12-Targeting siRNA by DNA Nanoparticles Suppresses Cancer Cell Growth. Angew. Chem. Int. Ed. Engl. 2017, 56, 16023–16027. [Google Scholar] [CrossRef]
- Zhong, Z.F.; Yu, H.B.; Wang, C.M.; Qiang, W.A.; Wang, S.P.; Zhang, J.M.; Yu, H.; Cui, L.; Wu, T.; Li, D.Q. Corrigendum: Furanodiene Induces Extrinsic and Intrinsic Apoptosis in Doxorubicin-Resistant MCF-7 Breast Cancer Cells via NF-κB-Independent Mechanism. Front. Pharmacol. 2017, 8, 648. [Google Scholar] [CrossRef]
- Luo, J.L.; Kamata, H.; Karin, M. IKK/NF-kappaB signaling: Balancing life and death--a new approach to cancer therapy. J. Clin. Investig. 2005, 115, 2625–2632. [Google Scholar] [CrossRef] [Green Version]
- Karin, M.; Lin, A. NF-kappaB at the crossroads of life and death. Nat. Immunol. 2002, 3, 221–227. [Google Scholar] [CrossRef]
- Lianxu, C.; Hongti, J.; Changlong, Y. NF-kappaBp65-specific siRNA inhibits expression of genes of COX-2, NOS-2 and MMP-9 in rat IL-1beta-induced and TNF-alpha-induced chondrocytes. Osteoarthritis Cartilage 2006, 14, 367–376. [Google Scholar] [CrossRef] [Green Version]
- Baeuerle, P.; Baltimore, D. I kappa b: A specific inhibitor of the nf-kappa b transcription factor. Science 1988, 242, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Hong, J.T. Roles of NF-κB in Cancer and Inflammatory Diseases and Their Therapeutic Approaches. Cells 2016, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Cho, H.J.; Han, S.H.; No, J.G.; Kwon, J.Y.; Kim, H. A Novel LZAP-binding Protein, NLBP, Inhibits Cell Invasion. J. Boil. Chem. 2010, 285, 12232–12240. [Google Scholar] [CrossRef] [PubMed] [Green Version]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Li, C.; Wang, Y.; Li, L.; Han, Z.; Wang, G. UFL1 Alleviates LPS-Induced Apoptosis by Regulating the NF-κB Signaling Pathway in Bovine Ovarian Granulosa Cells. Biomolecules 2020, 10, 260. https://doi.org/10.3390/biom10020260
Wang X, Li C, Wang Y, Li L, Han Z, Wang G. UFL1 Alleviates LPS-Induced Apoptosis by Regulating the NF-κB Signaling Pathway in Bovine Ovarian Granulosa Cells. Biomolecules. 2020; 10(2):260. https://doi.org/10.3390/biom10020260
Chicago/Turabian StyleWang, Xinling, Chengmin Li, Yiru Wang, Lian Li, Zhaoyu Han, and Genlin Wang. 2020. "UFL1 Alleviates LPS-Induced Apoptosis by Regulating the NF-κB Signaling Pathway in Bovine Ovarian Granulosa Cells" Biomolecules 10, no. 2: 260. https://doi.org/10.3390/biom10020260
APA StyleWang, X., Li, C., Wang, Y., Li, L., Han, Z., & Wang, G. (2020). UFL1 Alleviates LPS-Induced Apoptosis by Regulating the NF-κB Signaling Pathway in Bovine Ovarian Granulosa Cells. Biomolecules, 10(2), 260. https://doi.org/10.3390/biom10020260