Plant-Based Natural Products for the Discovery and Development of Novel Anthelmintics against Nematodes
Abstract
:1. Introduction
2. Anthelmintic Drugs
2.1. Mechanisms of Action of Anthelmintics
2.2. Resistance to Anthelmintics
3. C. elegans
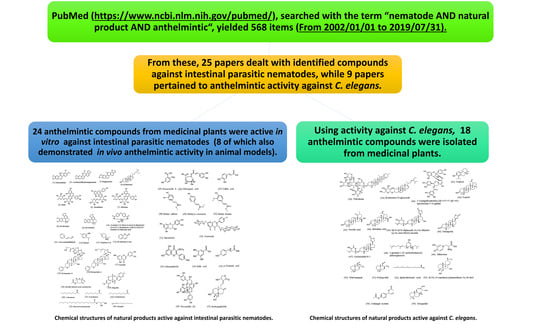
4. Anthelmintic Compounds Derived from Medicinal Plants
4.1. Natural Products Active against Intestinal Parasitic Nematodes
4.2. Natural Products Active against C. elegans
5. Chemistry of Isolated Compounds
5.1. Lipids
5.2. Phenolic Compounds (Including Flavonoids)
5.3. Saponins
6. Suitability for Drug Development
6.1. In Vitro Bioassays
6.2. Potency
6.3. Synergy
6.4. Spectrum
6.5. Toxicity
6.6. Pharmacokinetics
6.7. In Vivo Effects
6.8. Mechanism of Action
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jimenez Castro, P.D.; Howell, S.B.; Schaefer, J.J.; Avramenko, R.W.; Gilleard, J.S.; Kaplan, R.M. Multiple drug resistance in the canine hookworm Ancylostoma caninum: An emerging threat? Parasites Vectors 2019, 12, 576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Little, S.E.; Johnson, E.M.; Lewis, D.; Jaklitsch, R.P.; Payton, M.E.; Blagburn, B.L.; Bowman, D.D.; Moroff, S.; Tams, T.; Rich, L.; et al. Prevalence of intestinal parasites in pet dogs in the United States. Vet. Parasitol. 2009, 166, 144–152. [Google Scholar] [CrossRef]
- Castro, G.A. Helminths: Structure, Classification, Growth, and Development; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996; ISBN 0963117211. [Google Scholar]
- Coghlan, A. Nematode genome evolution. WormBook 2005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cross, J.H. Enteric Nematodes of Humans; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996; ISBN 0963117211. [Google Scholar]
- Bogitsh, B.J.; Burton, J.; Carter, C.E.; Clint, E.; Oeltmann, T.N. Human Parasitology; Academic Press: Cambridge, MA, USA, 2012; ISBN 9780124159846. [Google Scholar]
- WHO. Helminth Control in School Age Children: A Guide for Managers of Control Programmes; WHO: Geneva, Switzerland, 2016. [Google Scholar]
- Sharpe, C.; Thornton, D.J.; Grencis, R.K. A sticky end for gastrointestinal helminths; the role of the mucus barrier. Parasite Immunol. 2018, 40, e12517. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.; Hewitt, G.; Tuffrey, V.; De Silva, N. A review and meta-analysis of the impact of intestinal worms on child growth and nutrition. Matern. Child Nutr. 2008, 4, 118–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.; Xiao, S.-H.; Aroian, R.V. The new anthelmintic tribendimidine is an L-type (Levamisole and Pyrantel) nicotinic acetylcholine receptor agonist. PLoS Negl. Trop. Dis. 2009, 3, e499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.; Georghiou, S.B.; Kelleher, A.J.; Aroian, R.V. Bacillus thuringiensis Cry5B Protein Is highly efficacious as a single-dose therapy against an intestinal roundworm infection in mice. PLoS Negl. Trop. Dis. 2010, 4, e614. [Google Scholar] [CrossRef] [Green Version]
- Bethony, J.; Brooker, S.; Albonico, M.; Geiger, S.M.; Loukas, A.; Diemert, D.; Hotez, P.J. Soil-transmitted helminth infections: Ascariasis, trichuriasis, and hookworm. Lancet 2006, 367, 1521–1532. [Google Scholar] [CrossRef]
- Preston, S.; Jiao, Y.; Baell, J.B.; Keiser, J.; Crawford, S.; Koehler, A.V.; Wang, T.; Simpson, M.M.; Kaplan, R.M.; Cowley, K.J.; et al. Screening of the ‘Open Scaffolds’ collection from compounds Australia identifies a new chemical entity with anthelmintic activities against different developmental stages of the barber’s pole worm and other parasitic nematodes. Int. Parasitol. Drugs Drug Resist. 2017, 7, 286–294. [Google Scholar] [CrossRef] [Green Version]
- Abongwa, M.; Martin, R.J.; Robertson, A.P. A brief review on the mode of action of antinematodal drugs. Acta Vet. 2017, 67, 137–152. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, M.; Misra-Bhattacharya, S. Overcoming drug resistance for macro parasites. Future Microbiol. 2015, 10, 1783–1789. [Google Scholar] [CrossRef] [PubMed]
- Auffenberg, C.; Rosenthal, L.J.; Dresner, N. Levamisole: A common cocaine adulterant with life-threatening side effects. Psychosomatics 2013, 54, 590–593. [Google Scholar] [CrossRef] [PubMed]
- Smout, M.J.; Kotze, A.C.; McCarthy, J.S.; Loukas, A. A novel high throughput assay for anthelmintic drug screening and resistance diagnosis by real-time monitoring of parasite motility. PLoS Negl. Trop. Dis. 2010, 4, e885. [Google Scholar] [CrossRef] [Green Version]
- Weeks, J.C.; Roberts, W.M.; Leasure, C.; Suzuki, B.M.; Robinson, K.J.; Currey, H.; Wangchuk, P.; Eichenberger, R.M.; Saxton, A.D.; Bird, T.D.; et al. Sertraline, Paroxetine, and Chlorpromazine are rapidly acting anthelmintic drugs capable of clinical repurposing. Sci. Rep. 2018, 8, 975. [Google Scholar] [CrossRef] [PubMed]
- Geary, T.G.; Thompson, D.P. Caenorhabditis elegans: How good a model for veterinary parasites? Vet. Parasitol. 2001, 101, 371–386. [Google Scholar] [CrossRef]
- Holden-Dye, L.; Walker, R.J. Anthelmintic drugs and nematicides: Studies in Caenorhabditis elegans. WormBook 2014, 1–29. [Google Scholar] [CrossRef]
- Martin, R.J. γ-Aminobutyric acid- and piperazine-activated single-channel currents from Ascaris suum body muscle. Br. J. Pharmacol. 1985, 84, 445–461. [Google Scholar] [CrossRef]
- Williamson, S.M.; Robertson, A.P.; Brown, L.; Williams, T.; Woods, D.J.; Martin, R.J.; Sattelle, D.B.; Wolstenholme, A.J. The nicotinic acetylcholine receptors of the parasitic nematode Ascaris suum: Formation of two distinct drug targets by varying the relative expression levels of two subunits. PLoS Pathog. 2009, 5, e1000517. [Google Scholar] [CrossRef] [Green Version]
- Taman, A.; El-Beshbishi, S.N.; El Bardicy, S.; Tadros, M.; Ayoub, M.; Mansour, B.; El-Bialy, S. In Vitro screening of BTP-Iso on Schistosoma mansoni and its intermediate host Biomphalaria alexandrina. Asian Pac. J. Trop. Dis. 2016, 6, 946–951. [Google Scholar] [CrossRef]
- Courtot, E.; Charvet, C.L.; Beech, R.N.; Harmache, A.; Wolstenholme, A.J.; Holden-Dye, L.; O’Connor, V.; Peineau, N.; Woods, D.J.; Neveu, C. Functional characterization of a novel class of morantel-sensitive acetylcholine receptors in nematodes. PLoS Pathog. 2015, 11, e1005267. [Google Scholar] [CrossRef] [Green Version]
- Cully, D.F.; Vassilatis, D.K.; Liu, K.K.; Paress, P.S.; Van der Ploeg, L.H.T.; Schaeffer, J.M.; Arena, J.P. Cloning of an avermectin-sensitive glutamate-gated chloride channel from Caenorhabditis elegans. Nature 1994, 371, 707–711. [Google Scholar] [CrossRef] [PubMed]
- Kaminsky, R.; Ducray, P.; Jung, M.; Clover, R.; Rufener, L.; Bouvier, J.; Weber, S.S.; Wenger, A.; Wieland-Berghausen, S.; Goebel, T.; et al. A new class of anthelmintics effective against drug-resistant nematodes. Nature 2008, 452, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Lancheros, E.; Viau, C.; Walter, T.N.; Francis, A.; Geary, T.G. Activity of novel nicotinic anthelmintics in cut preparations of Caenorhabditis elegans. Int. J. Parasitol. 2011, 41, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Robertson, A.P.; Puttachary, S.; Buxton, S.K.; Martin, R.J. Tribendimidine: Mode of action and nAChR subtype selectivity in Ascaris and Oesophagostomum. PLoS Negl. Trop. Dis. 2015, 9, e0003495. [Google Scholar] [CrossRef]
- Guest, M.; Bull, K.; Walker, R.J.; Amliwala, K.; O’Connor, V.; Harder, A.; Holden-Dye, L.; Hopper, N.A. The calcium-activated potassium channel, SLO-1, is required for the action of the novel cyclo-octadepsipeptide anthelmintic, emodepside, in Caenorhabditis elegans. Int. J. Parasitol. 2007, 37, 1577–1588. [Google Scholar] [CrossRef]
- Welz, C.; Krüger, N.; Schniederjans, M.; Miltsch, S.M.; Krücken, J.; Guest, M.; Holden-Dye, L.; Harder, A.; von Samson-Himmelstjerna, G. SLO-1-Channels of parasitic nematodes reconstitute locomotor behaviour and emodepside sensitivity in Caenorhabditis elegans SLO-1 loss of function mutants. PLoS Pathog. 2011, 7, e1001330. [Google Scholar] [CrossRef] [Green Version]
- De Graef, J.; Claerebout, E.; Geldhof, P. Vlaams Diergeneeskundig Tijdschrift; Faculteit van de Diergeneeskunde, Rijksuniversiteit te Gent: Merelbeke, Belgium, 2013; p. 82. [Google Scholar]
- Epe, C.; Kaminsky, R. New advancement in anthelmintic drugs in veterinary medicine. Trends Parasitol. 2013, 29, 129–134. [Google Scholar] [CrossRef]
- Scott, I.; Pomroy, W.E.; Kenyon, P.R.; Smith, G.; Adlington, B.; Moss, A. Lack of efficacy of monepantel against Teladorsagia circumcincta and Trichostrongylus colubriformis. Vet. Parasitol. 2013, 198, 166–171. [Google Scholar] [CrossRef]
- Sales, N.; Love, S. Resistance of Haemonchus sp. to monepantel and reduced efficacy of a derquantel / abamectin combination confirmed in sheep in NSW, Australia. Vet. Parasitol. 2016, 228, 193–196. [Google Scholar] [CrossRef]
- Albonico, M.; Ramsan, M.; Wright, V.; Jape, K.; Haji, H.J.; Taylor, M.; Savioli, L.; Bickle, Q. Soil-transmitted nematode infections and mebendazole treatment in Mafia Island schoolchildren. Ann. Trop. Med. Parasitol. 2002, 96, 717–726. [Google Scholar] [CrossRef]
- Krücken, J.; Fraundorfer, K.; Mugisha, J.C.; Ramünke, S.; Sifft, K.C.; Geus, D.; Habarugira, F.; Ndoli, J.; Sendegeya, A.; Mukampunga, C.; et al. Reduced efficacy of albendazole against Ascaris lumbricoides in Rwandan schoolchildren. Int. J. Parasitol. Drugs Drug Resist. 2017, 7, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Vercruysse, J.; Charlier, J.; Van Dijk, J.; Morgan, E.R.; Geary, T.; von Samson-Himmelstjerna, G.; Claerebout, E. Control of helminth ruminant infections by 2030. Parasitology 2018, 145, 1655–1664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vercruysse, J.; Albonico, M.; Behnke, J.M.; Kotze, A.C.; Prichard, R.K.; McCarthy, J.S.; Montresor, A.; Levecke, B. Is anthelmintic resistance a concern for the control of human soil-transmitted helminths? Int. J. Parasitol. Drugs Drug Resist. 2011, 1, 14–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alum, A.; Rubino, J.R.; Ijaz, M.K. The global war against intestinal parasites—Should we use a holistic approach? Int. J. Infect. Dis. 2010, 14, e732–e738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buckingham, S.D.; Partridge, F.A.; Sattelle, D.B. Automated, high-throughput, motility analysis in Caenorhabditis elegans and parasitic nematodes: Applications in the search for new anthelmintics. Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 226–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katiki, L.M.; Ferreira, J.F.S.; Zajac, A.M.; Masler, C.; Lindsay, D.S.; Chagas, A.C.S.; Amarante, A.F.T. Caenorhabditis elegans as a model to screen plant extracts and compounds as natural anthelmintics for veterinary use. Vet. Parasitol. 2011, 182, 264–268. [Google Scholar] [CrossRef] [Green Version]
- Mathew, M. High Throughput Imaging for Anthelmintic Discovery and Caenorhabditis Elegans Genetic Tools for Target Elucidation. Master’s Thesis, University of British Columbia, Vancouver, BC, Canada, 2016. [Google Scholar]
- Jex, A.R.; Liu, S.; Li, B.; Young, N.D.; Hall, R.S.; Li, Y.; Yang, L.; Zeng, N.; Xu, X.; Xiong, Z.; et al. Ascaris suum draft genome. Nature 2011, 479, 529–533. [Google Scholar] [CrossRef] [Green Version]
- Mathew, M.D.; Mathew, N.D.; Miller, A.; Simpson, M.; Au, V.; Garland, S.; Gestin, M.; Edgley, M.L.; Flibotte, S.; Balgi, A.; et al. Using C. elegans forward and reverse genetics to identify new compounds with anthelmintic activity. PLoS Negl. Trop. Dis. 2016, 10, e0005058. [Google Scholar] [CrossRef]
- Buckingham, S.D.; Sattelle, D.B. Fast, automated measurement of nematode swimming (thrashing) without morphometry. BMC Neurosci. 2009, 10, 84. [Google Scholar] [CrossRef] [Green Version]
- Burns, A.R.; Luciani, G.M.; Musso, G.; Bagg, R.; Yeo, M.; Zhang, Y.; Rajendran, L.; Glavin, J.; Hunter, R.; Redman, E.; et al. Caenorhabditis elegans is a useful model for anthelmintic discovery. Nat. Commun. 2015, 6, 7485. [Google Scholar] [CrossRef]
- Tu, Y. Artemisinin-A gift from Traditional Chinese Medicine to the World (Nobel Lecture). Angew. Chem. Int. Ed. 2016, 55, 10210–10226. [Google Scholar] [CrossRef] [PubMed]
- Weaver, K.J.; May, C.J.; Ellis, B.L. Using a health-rating system to evaluate the usefulness of Caenorhabditis elegans as a model for anthelmintic study. PLoS ONE 2017, 12, e0179376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bull, K.; Cook, A.; Hopper, N.A.; Harder, A.; Holden-Dye, L.; Walker, R.J. Effects of the novel anthelmintic emodepside on the locomotion, egg-laying behaviour and development of Caenorhabditis elegans. Int. J. Parasitol. 2007, 37, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.-Y.; Zhou, S.-F.; Gao, S.-H.; Yu, Z.-L.; Zhang, S.-F.; Tang, M.-K.; Sun, J.-N.; Ma, D.-L.; Han, Y.-F.; Fong, W.-F.; et al. New perspectives on how to discover drugs from herbal medicines: cam’s outstanding contribution to modern therapeutics. Evid. Complementary Altern. Med. 2013, 2013, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [Green Version]
- Balunas, M.J.; Kinghorn, A.D. Drug discovery from medicinal plants. Life Sci. 2005, 78, 431–441. [Google Scholar] [CrossRef]
- Githiori, J.B.; Höglund, J.; Waller, P.J. Ethnoveterinary plant preparations as livestock dewormers: Practices, popular beliefs, pitfalls and prospects for the future. Anim. Health Res. Rev. 2005, 6, 91–103. [Google Scholar] [CrossRef]
- Panda, S.K.; Luyten, W. Antiparasitic activity in Asteraceae with special attention to ethnobotanical use by the tribes of Odisha, India. Parasite 2018, 25, 10. [Google Scholar] [CrossRef] [Green Version]
- Panda, S.K.; da Silva, L.C.N.; Sahal, D.; Leonti, M. Editorial: Ethnopharmacological studies for the development of drugs with special reference to asteraceae. Front. Pharmacol. 2019, 10, 955. [Google Scholar] [CrossRef] [Green Version]
- Ndjonka, D.; Djafsia, B.; Liebau, E. Review on medicinal plants and natural compounds as anti-Onchocerca agents. Parasitol. Res. 2018, 117, 2697–2713. [Google Scholar] [CrossRef]
- Romero-Benavides, J.C.; Ruano, A.L.; Silva-Rivas, R.; Castillo-Veintimilla, P.; Vivanco-Jaramillo, S.; Bailon-Moscoso, N. Medicinal plants used as anthelmintics: Ethnomedical, pharmacological, and phytochemical studies. Eur. J. Med. Chem. 2017, 129, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Tagboto, S.; Townson, S. Antiparasitic properties of medicinal plants and other naturally occurring products. Adv. Parasitol. 2001, 50, 199–295. [Google Scholar] [PubMed]
- Chitwood, D.J. Phytochemical based strategies for nematode control. Annu. Rev. Phytopathol. 2002, 40, 221–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satou, T.; Akao, N.; Matsuhashi, R.; Koike, K.; Fujita, K.; Nikaido, T. Inhibitory effect of isoquinoline alkaloids on movement of second-stage larvae of Toxocara canis. Biol. Pharm. Bull. 2002, 25, 1651–1654. [Google Scholar] [CrossRef] [Green Version]
- Villaseñor, I.M.; Angelada, J.; Canlas, A.P.; Echegoyen, D. Bioactivity studies on β-sitosterol and its glucoside. Phytother. Res. 2002, 16, 417–421. [Google Scholar] [CrossRef]
- Giovanelli, F.; Mattellini, M.; Fichi, G.; Flamini, G.; Perrucci, S. In vitro anthelmintic activity of four plant-derived compounds against sheep gastrointestinal nematodes. Vet. Sci. 2018, 5, 78. [Google Scholar] [CrossRef] [Green Version]
- Vijaya; Yadav, A.K. In Vitro anthelmintic assessment of selected phytochemicals against Hymenolepis diminuta, a zoonotic tapeworm. J. Parasit. Dis. 2016, 40, 1082–1086. [Google Scholar] [CrossRef] [Green Version]
- Bano, S.; Faizi, S.; Lubna; Fayyaz, S.; Iqbal, E.Y. Isolation of Ceramides from Tagetes patula L. Yellow flowers and nematicidal activity of the fractions and pure compounds against cyst nematode, Heterodera zeae. Chem. Biodivers. 2019, 16, e1900092. [Google Scholar]
- Deepak, M.; Dipankar, G.; Prashanth, D.; Asha, M.K.; Amit, A.; Venkataraman, B.V. Tribulosin and β-sitosterol-D-glucoside, the anthelmintic principles of Tribulus terrestris. Phytomedicine 2002, 9, 753–756. [Google Scholar] [CrossRef]
- Barrau, E.; Fabre, N.; Fouraste, I.; Hoste, H. Effect of bioactive compounds from Sainfoin (Onobrychis viciifolia Scop.) on the In Vitro larval migration of Haemonchus contortus: Role of tannins and flavonol glycosides. Parasitology 2005, 131, 531. [Google Scholar] [CrossRef] [Green Version]
- Ayers, S.; Zink, D.; Mohn, K.; Powell, J.; Brown, C.; Murphy, T.; Brand, R.; Pretorius, S.; Stevenson, D.; Thompson, D.; et al. Anthelmintic activity of aporphine alkaloids from Cissampelos capensis. Planta Med. 2007, 73, 296–297. [Google Scholar] [CrossRef] [PubMed]
- Roy, B.; Dasgupta, S.; Manivel, V.; Parameswaran, P.S.; Giri, B.R. Surface topographical and ultrastructural alterations of Raillietina echinobothrida and Ascaridia galli induced by a compound isolated from Acacia oxyphylla. Vet. Parasitol. 2012, 185, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Forbes, W.M.; Gallimore, W.A.; Mansingh, A.; Reese, P.B.; Robinson, R.D. Eryngial (trans -2-dodecenal), a bioactive compound from Eryngium foetidum: Its identification, chemical isolation, characterization and comparison with ivermectin In Vitro. Parasitology 2014, 141, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.R.; Ramsay, A.; Hansen, T.V.A.; Ropiak, H.M.; Mejer, H.; Nejsum, P.; Mueller-Harvey, I.; Thamsborg, S.M. Anthelmintic activity of trans-cinnamaldehyde and A- and B-type proanthocyanidins derived from cinnamon (Cinnamomum verum). Sci. Rep. 2015, 5, 14791. [Google Scholar] [CrossRef] [Green Version]
- Chama, M.A.; Dziwornu, G.A.; Waibel, R.; Osei-Safo, D.; Addae-Mensah, I.; Otchere, J.; Wilson, M. Isolation, characterization, and anthelminthic activities of a novel dichapetalin and other constituents of Dichapetalum filicaule. Pharm. Biol. 2015, 54, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, L.E.; Benincasa, B.I.; Fachin, A.L.; França, S.C.; Contini, S.S.H.T.; Chagas, A.C.S.; Beleboni, R.O. Thymus vulgaris L. essential oil and its main component thymol: Anthelmintic effects against Haemonchus contortus from sheep. Vet. Parasitol. 2016, 228, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Grando, T.H.; de Sá, M.F.; Baldissera, M.D.; Oliveira, C.B.; de Souza, M.E.; Raffin, R.P.; Santos, R.C.V.; Domingues, R.; Minho, A.P.; Leal, M.L.R.; et al. In vitro activity of essential oils of free and nanostructured Melaleuca alternifolia and of terpinen-4-ol on eggs and larvae of Haemonchus contortus. J. Helminthol. 2016, 90, 377–382. [Google Scholar] [CrossRef]
- Wangchuk, P.; Pearson, M.S.; Giacomin, P.R.; Becker, L.; Sotillo, J.; Pickering, D.; Smout, M.J.; Loukas, A. Compounds derived from the Bhutanese Daisy, Ajania nubigena, demonstrate dual anthelmintic activity against Schistosoma mansoni and Trichuris muris. PLoS Negl. Trop. Dis. 2016, 10, e0004908. [Google Scholar] [CrossRef] [Green Version]
- Dilrukshi Herath, H.M.P.; Preston, S.; Hofmann, A.; Davis, R.A.; Koehler, A.V.; Chang, B.C.H.; Jabbar, A.; Gasser, R.B. Screening of a small, well-curated natural product-based library identifies two rotenoids with potent nematocidal activity against Haemonchus contortus. Vet. Parasitol. 2017, 244, 172–175. [Google Scholar] [CrossRef]
- Preston, S.; Korhonen, P.K.; Mouchiroud, L.; Cornaglia, M.; McGee, S.L.; Young, N.D.; Davis, R.A.; Crawford, S.; Nowell, C.; Ansell, B.R.E.; et al. Deguelin exerts potent nematocidal activity via the mitochondrial respiratory chain. FASEB J. 2017, 31, 4515–4532. [Google Scholar] [CrossRef] [Green Version]
- Ortu, E.; Sanna, G.; Scala, A.; Pulina, G.; Caboni, P.; Battacone, G. In Vitro anthelmintic activity of active compounds of the fringed rue Ruta chalepensis against dairy ewe gastrointestinal nematodes. J. Helminthol. 2017, 91, 447–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Son-de Fernex, E.; Alonso-Díaz, M.Á.; Valles-de la Mora, B.; Mendoza-de Gives, P.; González-Cortazar, M.; Zamilpa, A. Anthelmintic effect of 2H-chromen-2-one isolated from Gliricidia sepium against Cooperia punctata. Exp. Parasitol. 2017, 178, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Doligalska, M.; Jóźwicka, K.; Donskow-Łysoniewska, K.; Kalinowska, M. The antiparasitic activity of avenacosides against intestinal nematodes. Vet. Parasitol. 2017, 241, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Jasso Díaz, G.; Hernández, G.T.; Zamilpa, A.; Becerril Pérez, C.M.; Ramírez Bribiesca, J.E.; Hernández Mendo, O.; Sánchez Arroyo, H.; González Cortazar, M.; Mendoza de Gives, P. In Vitro assessment of Argemone mexicana, Taraxacum officinale, Ruta chalepensis and Tagetes filifolia against Haemonchus contortus nematode eggs and infective (L3) larvae. Microb. Pathog. 2017, 109, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Mitre, G.F.; Olmedo-Juárez, A.; Rojo-Rubio, R.; González-Cortázar, M.; Mendoza-de Gives, P.; Hernández-Beteta, E.E.; Reyes-Guerrero, D.E.; López-Arellano, M.E.; Vázquez-Armijo, J.F.; Ramírez-Vargas, G.; et al. Caffeoyl and coumaroyl derivatives from Acacia cochliacantha exhibit ovicidal activity against Haemonchus contortus. J. Ethnopharmacol. 2017, 204, 125–131. [Google Scholar] [CrossRef]
- Soldera-Silva, A.; Seyfried, M.; Campestrini, L.H.; Zawadzki-Baggio, S.F.; Minho, A.P.; Molento, M.B.; Maurer, J.B.B. Assessment of anthelmintic activity and bio-guided chemical analysis of Persea americana seed extracts. Vet. Parasitol. 2018, 251, 34–43. [Google Scholar] [CrossRef]
- Wanderley, L.F.; Soares, A.M.D.S.; Silva, C.R.E.; Figueiredo, I.M.; Ferreira, A.T.S.; Perales, J.; Mota, H.R.O.; Oliveira, J.T.A.; Costa Junior, L.M. A cysteine protease from the latex of Ficus benjamina has in vitro anthelmintic activity against Haemonchus contortus. Rev. Bras. Parasitol. Vet. Braz. J. Vet. Parasitol. 2018, 27, 473–480. [Google Scholar] [CrossRef]
- von Son-de Fernex, E.; Alonso-Díaz Miguel, Á.; Valles-de la Mora, B.; Mendoza-de Gives, P.; Castillo-Gallegos, E.; Zamilpa, A.; González-Cortazar, M. Effect of Gliricidia sepium leaves intake on larval establishment of Cooperia punctata in calves and bio-guided fractionation of bioactive molecules. Vet. Parasitol. 2018, 252, 137–141. [Google Scholar] [CrossRef]
- Payne, S.E.; Flematti, G.R.; Reeder, A.; Kotze, A.C.; Durmic, Z.; Vercoe, P.E. Procyanidin A2 in the Australian plant Alectryon oleifolius has anthelmintic activity against equine cyathostomins In Vitro. Vet. Parasitol. 2018, 249, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Cortes-Morales, J.A.; Olmedo-Juárez, A.; Trejo-Tapia, G.; González-Cortazar, M.; Domínguez-Mendoza, B.E.; Mendoza-de Gives, P.; Zamilpa, A. In Vitro ovicidal activity of Baccharis conferta Kunth against Haemonchus contortus. Exp. Parasitol. 2019, 197, 20–28. [Google Scholar] [CrossRef]
- Sinott, F.A.; Sena-Lopes, Â.; Leal, K.S.; Thais de Oliveira Silva, M.; Cardoso de Freitas, M.; Quintana de Moura, M.; Aires Berne, M.E.; Borsuk, S. Essential oil from Brazilian Red Propolis exhibits anthelmintic activity against larvae of Toxocara cati. Exp. Parasitol. 2019, 200, 37–41. [Google Scholar] [CrossRef] [PubMed]
- García-Hernández, C.; Rojo-Rubio, R.; Olmedo-Juárez, A.; Zamilpa, A.; Mendoza de Gives, P.; Antonio-Romo, I.A.; Aguilar-Marcelino, L.; Arece-García, J.; Tapia-Maruri, D.; González-Cortazar, M. Galloyl derivatives from Caesalpinia coriaria exhibit in vitro ovicidal activity against cattle gastrointestinal parasitic nematodes. Exp. Parasitol. 2019, 200, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, T.; Singh, A.; Kumar, S.; Dhanani, T.; Gajbhiye, N.A.; Koley, T.K.; Maurya, A.; Filgona, J. Ovicidal and larvicidal effects of extracts from leaves of Andrographis paniculata (Burm. f.) Wall.ex Nees against field isolates of human hookworm (Ancylostoma duodenale). J. Ethnopharmacol. 2019, 235, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Ramírez, G.S.; Torres-Acosta, J.F.J.; Sandoval-Castro, C.A.; Borges-Argáez, R.; Cáceres-Farfán, M.; Mancilla-Montelongo, G.; Mathieu, C. Bio-guided fractionation to identify Senegalia gaumeri leaf extract compounds with anthelmintic activity against Haemonchus contortus eggs and larvae. Vet. Parasitol. 2019, 270, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Mukai, D.; Matsuda, N.; Yoshioka, Y.; Sato, M.; Yamasaki, T. Potential anthelmintics: Polyphenols from the tea plant Camellia sinensis L. are lethally toxic to Caenorhabditis elegans. J. Nat. Med. 2008, 62, 155–159. [Google Scholar] [CrossRef]
- Samoylenko, V.; Dunbar, D.C.; Gafur, M.A.; Khan, S.I.; Ross, S.A.; Mossa, J.S.; El-Feraly, F.S.; Tekwani, B.L.; Bosselaers, J.; Muhammad, I. Antiparasitic, nematicidal and antifouling constituents from Juniperus berries. Phytother. Res. 2008, 22, 1570–1576. [Google Scholar] [CrossRef]
- Shai, L.J.; Bizimenyera, E.S.; Bagla, V.; McGaw, L.J.; Eloff, J.N. Curtisia dentata (Cornaceae) leaf extracts and isolated compounds inhibit motility of parasitic and free-living nematodes. Onderstepoort J. Vet. Res. 2009, 76, 249–256. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.-B.; Li, G.-H.; Zheng, L.-J.; Ji, K.-Y.; Lü, H.; Liu, F.-F.; Dang, L.-Z.; Mo, M.-H.; Zhang, K.-Q. Nematicidal Cardenolides from Nerium indicum Mill. Chem. Biodivers. 2009, 6, 431–436. [Google Scholar] [CrossRef]
- Fobofou, S.A.T.; Franke, K.; Sanna, G.; Porzel, A.; Bullita, E.; La Colla, P.; Wessjohann, L.A. Isolation and anticancer, anthelminthic, and antiviral (HIV) activity of acylphloroglucinols, and regioselective synthesis of empetrifranzinans from Hypericum roeperianum. Bioorg. Med. Chem. 2015, 23, 6327–6334. [Google Scholar] [CrossRef]
- Nguyen, B.; Chompoo, J.; Tawata, S. Insecticidal and nematicidal activities of novel mimosine derivatives. Molecules 2015, 20, 16741–16756. [Google Scholar] [CrossRef] [Green Version]
- Van Puyvelde, L.; Liu, M.; Veryser, C.; De Borggraeve, W.M.; Mungarulire, J.; Mukazayire, M.J.; Luyten, W. Active principles of Tetradenia riparia. IV. Anthelmintic activity of 8(14),15-sandaracopimaradiene-7α,18-diol. J. Ethnopharmacol. 2018, 216, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Kipanga, P.; Mai, A.H.; Dhondt, I.; Braeckman, B.P.; De Borggraeve, W.; Luyten, W. Bioassay-guided isolation of three anthelmintic compounds from Warburgia ugandensis Sprague subspecies ugandensis, and the mechanism of action of polygodial. Int. J. Parasitol. 2018, 48, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Veryser, C.; Lu, J.-G.; Wenseleers, T.; De Borggraeve, W.M.; Jiang, Z.-H.; Luyten, W. Bioassay-guided isolation of active substances from Semen Torreyae identifies two new anthelmintic compounds with novel mechanism of action. J. Ethnopharmacol. 2018, 224, 421–428. [Google Scholar] [CrossRef]
- Hirazawa, N.; Oshima, S.; Mitsuboshi, T.; Hata, K. The anthelmintic effect of medium-chain fatty acids against the monogenean Heterobothrium okamotoi in the tiger puffer Takifugu rubripes: Evaluation of doses of caprylic acid at different water temperatures. Aquaculture 2001, 195, 211–223. [Google Scholar] [CrossRef]
- Faizi, S.; Fayyaz, S.; Bano, S.; Yawar Iqbal, E.; Siddiqi, H.; Naz, A. Isolation of Nematicidal Compounds from Tagetes patula L. Yellow Flowers: Structure–Activity Relationship studies against cyst nematode Heterodera zeae infective stage larvae. J. Agric. Food Chem. 2011, 59, 9080–9093. [Google Scholar] [CrossRef]
- Mukherjee, N.; Mukherjee, S.; Saini, P.; Roy, P.; Babu, S.P.S. Phenolics and Terpenoids; the promising new search for anthelmintics: A critical review. Mini Rev. Med. Chem. 2016, 16, 1415–1441. [Google Scholar] [CrossRef]
- Spiegler, V.; Liebau, E.; Hensel, A. Medicinal plant extracts and plant-derived polyphenols with anthelmintic activity against intestinal nematodes. Nat. Prod. Rep. 2017, 34, 627–643. [Google Scholar] [CrossRef]
- Ali, N.; Shah, S.W.A.; Shah, I.; Ahmed, G.; Ghias, M.; Khan, I. Cytotoxic and anthelmintic potential of crude saponins isolated from Achillea wilhelmsii C. Koch and Teucrium Stocksianum Boiss. BMC Complementary Altern. Med. 2011, 11, 106. [Google Scholar] [CrossRef] [Green Version]
- Maestrini, M.; Tava, A.; Mancini, S.; Salari, F.; Perrucci, S. In Vitro anthelmintic activity of saponins derived from Medicago spp. plants against donkey gastrointestinal nematodes. Vet. Sci. 2019, 6, 35. [Google Scholar] [CrossRef] [Green Version]
- Abriola, L.; Hoyer, D.; Caffrey, C.R.; Williams, D.L.; Yoshino, T.P.; Vermeire, J.J. Development and optimization of a high-throughput screening method utilizing Ancylostoma ceylanicum egg hatching to identify novel anthelmintics. PLoS ONE 2019, 14, e0217019. [Google Scholar] [CrossRef]
- Arafa, W.M.; Shokeir, K.M.; Khateib, A.M. Comparing an In Vivo egg reduction test and in vitro egg hatching assay for different anthelmintics against Fasciola species, in cattle. Vet. Parasitol. 2015, 214, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C. Organic synthesis: The art and science of replicating the molecules of living nature and creating others like them in the laboratory. Proc. R. Soc. Math. Phys. Eng. Sci. 2014, 470, 20130690. [Google Scholar] [CrossRef] [PubMed]
- Klongsiriwet, C.; Quijada, J.; Williams, A.R.; Mueller-Harvey, I.; Williamson, E.M.; Hoste, H. Synergistic inhibition of Haemonchus contortus exsheathment by flavonoid monomers and condensed tannins. Int. J. Parasitol. Drugs Drug Resist. 2015, 5, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H.; Ulrich-Merzenich, G. Synergy research: Approaching a new generation of phytopharmaceuticals. Phytomedicine 2009, 16, 97–110. [Google Scholar] [CrossRef]
- Hu, Y.; Platzer, E.G.; Bellier, A.; Aroian, R.V. Discovery of a highly synergistic anthelmintic combination that shows mutual hypersusceptibility. Proc. Natl. Acad. Sci. USA 2010, 107, 5955–5960. [Google Scholar] [CrossRef] [Green Version]
- Keiser, J.; Tritten, L.; Adelfio, R.; Vargas, M. Effect of combinations of marketed human anthelmintic drugs against Trichuris muris in vitro and in vivo. Parasites Vectors 2012, 5, 292. [Google Scholar] [CrossRef] [Green Version]
- Mx, E. (12) International Application Published under the Patent Cooperation Treaty (PCT) (19) World Intellectual Property Organization International Bureau (43) International Publication Date national Publication Number (25) Filing Language. Patent No. WO 2008/062431, 29 May 2008. [Google Scholar]
- Elfawal, M.A.; Savinov, S.N.; Aroian, R.V. Drug Screening for discovery of broad-spectrum agents for soil-transmitted nematodes. Sci. Rep. 2019, 9, 12347. [Google Scholar] [CrossRef] [Green Version]
- Partridge, F.A.; Brown, A.E.; Buckingham, S.D.; Willis, N.J.; Wynne, G.M.; Forman, R.; Else, K.J.; Morrison, A.A.; Matthews, J.B.; Russell, A.J.; et al. An automated high-throughput system for phenotypic screening of chemical libraries on C. elegans and parasitic nematodes. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 8–21. [Google Scholar] [CrossRef]
- Martin, R.J.; Robertson, A.P. Control of nematode parasites with agents acting on neuro-musculature systems: Lessons for neuropeptide ligand discovery. Adv. Exp. Med. Biol. 2010, 692, 138–154. [Google Scholar]
- Committee on Predictive-Toxicology Approaches for Military Assessments of Acute Exposures; Committee on Toxicology; Board on Environmental Studies and Toxicology; Board on Life Sciences; Division on Earth and Life Studies; The National Academies of Sciences, Engineering, and Medicine. Application of Modern Toxicology Approaches for Predicting Acute Toxicity for Chemical Defense; National Academies Press (US): Washington, DC, USA, 2015. Available online: https://www.ncbi.nlm.nih.gov/books/NBK321423/ (accessed on 27 February 2020).
- Turfus, S.C.; Delgoda, R.; Picking, D.; Gurley, B.J. Pharmacokinetics. In Pharmacognosy: Fundamentals, Applications and Strategy; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 495–512. ISBN 9780128020999. [Google Scholar]
- Said, H.M. (Ed.) Physiology of the Gastrointestinal Tract; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Giordani, F.; Morrison, L.J.; Rowan, T.G.; De Koning, H.P.; Barrett, M.P. The animal trypanosomiases and their chemotherapy: A review. Parasitology 2016, 143, 1862–1889. [Google Scholar] [CrossRef]
- Dubois, O.; Allanic, C.; Charvet, C.L.; Guégnard, F.; Février, H.; Théry-Koné, I.; Cortet, J.; Koch, C.; Bouvier, F.; Fassier, T.; et al. Lupin (Lupinus spp.) seeds exert anthelmintic activity associated with their alkaloid content. Sci. Rep. 2019, 9, 9070. [Google Scholar] [CrossRef] [PubMed]
- Sakai, C.; Tomitsuka, E.; Esumi, H.; Harada, S.; Kita, K. Mitochondrial fumarate reductase as a target of chemotherapy: From parasites to cancer cells. Biochim. Biophys. Acta Gen. Subj. 2012, 1820, 643–651. [Google Scholar] [CrossRef] [PubMed]


| Drug Class | Mechanism of Action | Drug Name | Year of Initial Approval | Year of First Resistance Report |
|---|---|---|---|---|
| Heterocyclic compounds | Agonist of the inhibitory GABA-receptor | Piperazine | 1954 | 1966 |
| Benzimidazoles | Inhibiting microtubule polymerisation | Mebendazole | 1972 | 1975 |
| Albendazole | 1972 | 1983 | ||
| Tetrahydropyri-midines | Agonist of nicotinic acetyl-choline receptors | Morantel | 1970 | 1979 |
| Agonist of nicotinic acetyl-choline receptors | Pyrantel | 1974 | 1996 | |
| Imidazothiazoles | Agonist of nicotinic acetyl-choline receptors | Levamisole | 1970 | 1979 |
| Macrocyclic lactones | Allosteric modulators of glutamate-gated chloride channels | Ivermectin | 1981 | 1988 |
| Moxidectin | 1991 | 1995 | ||
| Amino-acetonitrile derivatives | Agonist of nicotinic acetyl-choline receptors | Monepantel | 2009 | 2013 [33] |
| Spiroindole | Antagonist of nicotinic acetyl-choline receptors | Derquantel | 2010 | 2016 [34] |
| Aminophenylamidines | Agonist of nicotinic acetylcholine receptors | Tribendimidine a | 2004 | - |
| Cyclooctadepsipeptide | Activating a SLO-1-depen-dent pathway | Emodepside b | 2005 | - |
| Active Compounds | Plant | Parasite Model | Anthelmintic Activity | Reference | ||
|---|---|---|---|---|---|---|
| In Vitro | In Vivo | Assay | ||||
| Chelerythrine | Chelidonium majus | Toxocara canis | IC50 = 28 μΜ | nd | Mortality after 24 h | [60] |
| 6-Methoxydihydrosanguinarine | Macleaya cordata | IC50 = 18 μΜ | nd | |||
| Sanguinarine | Macleaya cordata | IC50 = 58 μΜ | nd | |||
| β-Sitosterol | Mentha cordifolia | Ascaris suum | 60 mM induced paralysis of worm in 1 h | nd | Paralysis | [61] |
| Rutin | Onobrychis viciifolia | Haemonchus contortus | Migration was reduced by 25% at 1965 μM | nd | Larval migration inhibition for 3 h | [66] |
| Nicotiflorin | Migration was reduced by 30% at 2018 μM | nd | ||||
| Narcissin | Migration was reduced by 35% at 1921 μM | nd | ||||
| (S)-Dicentrine | Cissampelos capensis | H. contortus | EC90 = 6.3 μg/mL (18.5 μM)) in a larval development assay | 25 mg/kg dosed orally resulted in 67 % reduction of worm counts in a mouse model infected by Heligmosomoides polygyrus | Larval development in vitro/ worm counts in vivo | [67] |
| 63(S)-Neolitsine | EC90 = 6.4 μg/mL (19.8 μM) in a larval development assay | nd | ||||
| 12-Amino-7,17-dioxo-2-oxa-8,16-diazatricylo [14.2.2.2 3, 6] tetraicosa-1 (20),3,5,18,21,23-hexaene-12-carboxylic acid | Acacia oxyphylla | Ascaridia galli | 50, 100 and 1000 μg/mL (121, 242 and 2420 μM) induced the death of worms after 30 h, 22 h and 15 h. | nd | Mortality | [68] |
| Eryngial | Eryngium foetidum | Strongyloides stercoralis | LD50 = 461 μM | nd | Larval mortality after 24 h | [69] |
| trans-Cinnamaldehyde | Cinnamomum verum | A. suum | 25.6 μg/mL (193.8 μM) induced larval death within 3 h | Infection was not signify-cantly decreased by daily administration in the diet (1000 mg/d) or as a targeted, encapsulated dose (1000 mg, twice daily) in a pig model | Larval mortality after 12 h in vitro/ larval burden in vivo | [70] |
| Dichapetalin X | Dichapetalum filicaule | Necator americanus | IC50 = 744.4 μM | nd | Egg hatch inhibition assay | [71] |
| Dichapetalin A | IC50 = 277.7 μM | |||||
| Glycerol monostearate | IC50 = 853.4 μM | |||||
| Thymol | Thymus vulgaris | H. contortus | Effective against the three main stages of parasites: IC50 = 2.9 mM against egg hatching; IC50 = 3.3 mM against larval motility; 16.6 mM completely inhibited the movement of adult worms within 8 h | nd | Egg hatching; motility of worms | [72] |
| Terpinen-4-ol | Melaleuca alternifolia | H. contortus | LC50 = 4.1 mM, LC90 = 20.2 mM in egg hatching assay; 22.7 mM induced a 82.4% inhibition of larval migration | nd | Egg hatching; inhibition of larval migration | [73] |
| Luteolin | Ajania nubigena | Trichuris muris | IC50 = 9.7 μg/mL (33.9 μM) | A single oral dose of 100 mg/kg induced a 27.6% reduction of worm burden in a mouse model | Mortality of adult worms after 12 h in vitro/ worm burden in vivo | [74] |
| (3R,6R)-Linalool oxide acetate | IC50 = 20.4 μg/mL (96.1 μM) | nd | ||||
| Deguelin | Mundulea sericea | H. contortus | IC50 = 14.8 μM | nd | Larval mortality after 72 h | [75,76] |
| 2-Decanone | Ruta chalepensis | Teladorsagia spp. (52%), Haemonchus. contortus (25%) and Trichostrongylus spp. (23%) | IC50 = 447.9 μM | nd | Immotile/paralysis after 24 h | [77] |
| 2-Nonanone | IC50 = 1757.5 μM | nd | ||||
| 2-Undecanone | IC50 = 5167.5 μM | Nd | ||||
| 2H-Chromen-2-one | Gliricidia sepium | Cooperia punctata | IC50 = 164.3 μM | nd | Egg hatch inhibition assay | [78] |
| Avenacoside | Avena sativa | Heligmosomoides bakeri | Avenacosides change the molecular pattern of nematode larva proteins and block glycoprotein pump activity. | Mouse model | Larval development assay | [79] |
| Chlorogenic acid | Tagetes filifolia | H. contortus | LC50 248 μg/mL | nd | Egg hatch inhibition assay | [80] |
| Caffeoyl and coumaroyl derivatives | Acacia cochliacantha | H. contortus | With concentration 1 mg/mL several compounds show egg hatch inhibition: caffeic acid (98%), methyl caffeate (88%), methyl-p-coumarate (88%) and methylferulate (75%). Additionally, p-coumaric acid and ferulic acid mixture and methyl ferulate and quercetin also showed 94% egg hatch inhibition. | nd | Egg hatch inhibition assay | [81] |
| Epicatechin, rutin | Persea americana | H. contortus | Epicatechin (EC50 = 10 μg/mL), rutin (EC50 = 30 μg/mL) | Goat | Larval migration inhibition assay | [82] |
| CM-cellulose, a cysteine protease | Ficus benjamina | H. contortus | EC50 value for larval development = 0.22 mg/mL, EC50 value for larval exsheathment = 0.79 mg/mL | Sheep | Larval development and exsheathment inhibition assay | [83] |
| Kaempferol 3-O-rhamnopyranosyl-(1 → 6)-β-D-glucopyranoside-7-O-rhamnopyranoside | Gliricidia sepium | C. punctata | Fully inhibited the C. punctata exsheathment process at 2400 µg/mL in calves | Calves | Larval development and exsheathment inhibition assay | [84] |
| Procyanidin A2 | Alectryon oleifolius | cyathostomins | IC50 = 12.6 μg/mL | nd | Larval migration inhibition assay | [85] |
| Isokaempferide | Baccharis conferta | H. contortus | IC50 = 80 µg/mL | nd | Egg hatching inhibition assay | [86] |
| EO | Brazilian Red Propolis | Toxocara cati | IC50 = 300 μg/mL | In mouse model, at 600 μg/mL after exposure for 48 h, shows larvicidal activity | Larval mortality after 48h (in vitro and in vivo) | [87] |
| Gallic acid | Caesalpinia coriaria | Gastrointestinal nematodes (Cooperia spp, Haemonchus spp., Ostertagia ssp., Trichostrongylus spp. and Oesophagostomum spp.) | The bioactive molecules (gallic acid and unidentified compound) displayed an ovicidal activity of 100% at 1000 µg/mL. | nd | Egg hatching inhibition assay | [88] |
| Andrographolide | Andrographis paniculata | Ancylostoma duodenale | Andrographolide exhibits significant ovicidal and larvicidal activity at 0.125 µg/mL and 19 µg/mL, respectively. | nd | Egg hatching inhibition assay | [89] |
| p-Coumaric acid | Senegalia gaumeri | H. contortus | At 400 μg/mL ovicidal effect of 8.7%, a larvae failing eclosion effect of 2.9%, and 88.4% of the emerged larvae were damaged. | nd | Egg hatching inhibition assay | [90] |
| Active Compounds | Plant | Anthelmintic Activity | Reference | ||
|---|---|---|---|---|---|
| In Vitro | In Vivo | Assay | |||
| Tribulosin | Tribulus terrestris | ED50 = 66.0 μM | nd | Immotile/paralysis after 18 h | [65] |
| β-Sitosterol-D-glucoside | ED50 = 142.1 μM | nd | |||
| (−)-Epigallocatechin-(2β→O→7′,4β→8′)-epicatechin-3′-O-gallate | Camellia sinensis | LC50 = 49 μM | nd | Mortality after 96 h | [91] |
| Totarol | Juniperus procera | 279.3 μM showed strong nematicidal activity | nd | Mortality after 24 h | [92] |
| Lupeol | Curtisia dentata | LC50 = 4.7 μM | nd | Immotile/paralysis after 7 d | [93] |
| Ursolic acid | LC50 = 26.3 μM | nd | |||
| Betulinic acid | LC50 = 153.3 μM | nd | |||
| 3β-O-(β-D-Diginosyl)-14,15α-dihydroxy-5α-card-20(22)-enolide | Nerium indicum | LC50 = 84.9 μM | nd | Mortality after 72 h | [94] |
| Uzarigenin | LC50 = 474.7 μM | nd | |||
| Cardenolide N-1 | LC50 = 80.4 μM | nd | |||
| 3-Geranyl-1-(2′-methylbutanoyl)-phloroglucinol | Hypericum roeperianum | 100 μg/mL (285.3 μM) induced a death percentage of 37% | nd | Mortality after 30 min | [95] |
| Mimosine | Leucaena leucocephala | IC50 = 16.8 μM | nd | Mortality after 48 h | [96] |
| (14),15-Sandaracopimaradiene -7α,18-diol | Tetradenia riparia | IC50 = 5.4 ± 0.9 µg/mL (17.8 ± 2.9 µM). | nd | Motility test using WMicrotracker, | [97] |
| Warburganal, polygodial, alpha-linolenic acid | Warburgia ugandensis | Warburganal (IC50: 28.2 ± 8.6 μM), polygodial (IC50: 13.1 ± 5.3 μM) and α-linolenic acid (IC50: 70.1 ± 17.5 μM) | nd | Motility test using WMicrotracker, | [98] |
| Galangal acetate, miogadial | Semen torreyae | Galangal acetate (IC50: 58.5 ± 8.9 μM) and miogadial (IC50: 25.1 ± 5.4 μM) | nd | Motility test using WMicrotracker, | [99] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Panda, S.K.; Luyten, W. Plant-Based Natural Products for the Discovery and Development of Novel Anthelmintics against Nematodes. Biomolecules 2020, 10, 426. https://doi.org/10.3390/biom10030426
Liu M, Panda SK, Luyten W. Plant-Based Natural Products for the Discovery and Development of Novel Anthelmintics against Nematodes. Biomolecules. 2020; 10(3):426. https://doi.org/10.3390/biom10030426
Chicago/Turabian StyleLiu, Maoxuan, Sujogya Kumar Panda, and Walter Luyten. 2020. "Plant-Based Natural Products for the Discovery and Development of Novel Anthelmintics against Nematodes" Biomolecules 10, no. 3: 426. https://doi.org/10.3390/biom10030426
APA StyleLiu, M., Panda, S. K., & Luyten, W. (2020). Plant-Based Natural Products for the Discovery and Development of Novel Anthelmintics against Nematodes. Biomolecules, 10(3), 426. https://doi.org/10.3390/biom10030426