A Comparative Perspective on Functionally-Related, Intracellular Calcium Channels: The Insect Ryanodine and Inositol 1,4,5-Trisphosphate Receptors
Abstract
:1. Introduction
2. Discovery of RyRs and IP3Rs
| Receptor | Species | Amino Acid (residue) | cDNA Size (bp) | Molecular Weight (kDa) | Reference |
|---|---|---|---|---|---|
| RyRs | Lepidoptera | ||||
| Bombyx mori (Bombycidae) | 5084 | 15,255 * | 575 | [36] | |
| Cnaphalocrocis medinalis (Crambidae) | 5087 | 15,773 | 574 | [37] | |
| Plutella xylostella (Plutellidae) | 5123 | 15,748 | 579 | [38] | |
| 5164 | 16,113 | 584 | [39] | ||
| Ostrinia furnacalis (Crambidae) | 5108 | 16,211 | 577 | [40] | |
| Helicoverpa armigera (Noctuidae) | 5142 | 16,083 | 581 | [41] | |
| Pieris rapae (Pieridae) | 5107 | 15,540 | 578 | [42] | |
| Chilo suppressalis (Crambidae) | 5133 | 16,392 | 581 | [43] | |
| 5133 | 16,102 | 581 | [44] | ||
| 5128 | 15,402 | 580 | [64] | ||
| Spodoptera exigua (Noctuidae) | 5118 | 15,748 | 579 | [45] | |
| Grapholita molesta (Tortricidae) | 5133 | 16,299 | 580 | [46] | |
| Tuta absoluta (Gelechiidae) | 5121 | 16,431 | 579 | [47] | |
| Spodoptera frugiperda | 5109 | 15,330 | 578 | [48] | |
| Diptera | |||||
| Drosophila melanogaster (Drosophilidae) | 5134 | 15,405 * | 581 | [65] | |
| Bactrocera dorsalis (Tephritidae) | 5140 | 15,750 | 582 | [49] | |
| Coleoptera | |||||
| Tribolium castaneum (Tenebrionidae) | 5094 | 15,308 | 577 | [50] | |
| Leptinotarsa decemlineata (Chrysomelidae) | 5128 | 15,792 | 582 | [51] | |
| Hemiptera | |||||
| Laodelphax striatellus (Delphacidae) | 5115 | 15,910 | 579 | [43] | |
| Bemisia tabaci (Aleyrodidae) | 5139 | 15,763 | 581 | [43] | |
| Nilaparvata lugens (Delphacidae) | 5140 | 15,735 | 581 | [52] | |
| Sogatella furcifera (Delphacidae) | 5128 | 15,985 | 579 | [53] | |
| Myzus persicae (Aphididae) | 5101 | 15,306 * | 580 | [54] | |
| Toxoptera citricida (Aphididae) | 5101 | 15,639 | 580 | [55] | |
| Dialeurodes citri (Aleyrodidae) | 5126 | 15,538 | 579 | [56] | |
| IP3Rs | Diptera | ||||
| Drosophila melanogaster (Drosophilidae) | 2833 | 9558 | 319 | [61] | |
| Coleoptera | |||||
| Tribolium castaneum (Tenebrionidae) | 2724 | 8175 * | 309 | [50] | |
| Leptinotarsa decemlineata (Chrysomelidae) | 2736 | 8211 * | 312 | Doğan and Toprak, unpublished | |
| Hemiptera | |||||
| Bemisia tabaci (Aleyrodidae) | 2733 | 8202 * | 311 | [62] | |
| Myzus persicae (Aphididae) | 3790 | 11,373 * | - | [63] | |
| Hymenoptera | |||||
| Bombus terrestris (Apidae) | 2727 | 10,966 | 309 | [63] | |
3. Structure of RyRs and IP3Rs
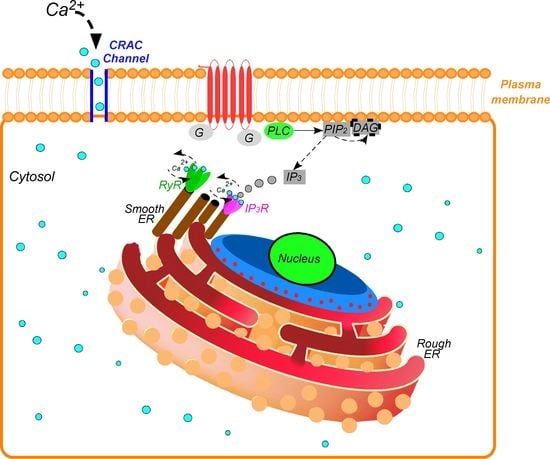
4. Pathway
5. Functions
5.1. Lipid Metabolism
5.2. Muscle Excitation and Contraction in Locomotor Activities
5.3. Visual and Olfactory Sensory Transduction
5.4. Development
6. Potential of RyR and IP3R as Target Sites in Pest Control
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berridge, M.J.; Lipp, P.; Bootman, M.D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000, 1, 11–21. [Google Scholar] [CrossRef]
- Taylor, W.C. Calcium regulation in insects. Adv. Insect Physiol. 1987, 19, 155–186. [Google Scholar] [CrossRef]
- Gu, S.H.; Chow, Y.S.; O’Reilly, D.R. Role of calcium in the stimulation of ecdysteroidogenesis by recombinant prothoracicotropic hormone in the prothoracic glands of the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 1998, 28, 861–867. [Google Scholar] [CrossRef]
- Takeo, S.; Tsuda, M.; Akahori, S.; Matsuo, T.; Aigaki, T. The calcineurin regulator sra plays an essential role in female meiosis in Drosophila. Curr. Biol. 2006, 16, 1435–1440. [Google Scholar] [CrossRef] [Green Version]
- Yoshiga, T.; Yokoyama, N.; Imai, N.; Ohnishi, A.; Moto, K.; Matsumoto, S. cDNA cloning of calcineurin heterosubunits from the pheromone gland of the silkmoth, Bombyx mori. Insect Biochem. Mol. Biol. 2002, 32, 477–486. [Google Scholar] [CrossRef]
- Teets, N.M.; Yi, S.X.; Lee, R.E.; Denlinger, D.L., Jr. Calcium signaling mediates cold sensing in insect tissues. Proc. Natl. Acad. Sci. USA 2013, 110, 9154–9159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bronk, P.; Kuklin, E.A.; Gorur-Shandilya, S.; Liu, C.; Wiggin, T.D.; Reed, M.L.; Marder, E.; Griffith, L.C. Regulation of eag by Ca2+/calmodulin controls presynaptic excitability in Drosophila. J. Neurophysiol. 2018, 119, 1665–1680. [Google Scholar] [CrossRef]
- Bahk, S.; Jones, W.D. Insect odorant receptor trafficking requires calmodulin. BMC Biol. 2016, 14, 83. [Google Scholar] [CrossRef] [Green Version]
- Pallen, C.J.; Steele, J.E. A putative role for calmodulin in corpus cardiacum stimulated trehalose synthesis in fat body of the american cockroach (Periplaneta americana). Insect Biochem. 1988, 18, 577–584. [Google Scholar] [CrossRef]
- Doğan, C.; Hänniger, S.; Heckel, D.G.; Coutu, C.; Hegedus, D.D.; Crubaugh, L.; Groves, R.L.; Mutlu, D.A.; Suludere, Z.; Bayram, Ş.; et al. Characterization of calcium signaling proteins from the fat body of the Colorado Potato Beetle, Leptinotarsa decemlineata (Coleoptera: Chrysomelidae): Implications for diapause and lipid metabolism. Insect Biochem. Mol. Biol. 2021, 133, 103549. [Google Scholar] [CrossRef]
- Doğan, C.; Hänniger, S.; Heckel, D.G.; Coutu, C.; Hegedus, D.D.; Crubaugh, L.; Groves, R.L.; Bayram, Ş.; Toprak, U. Two calcium-binding chaperones from the fat body of the Colorado potato beetle, Leptinotarsa decemlineata (Coleoptera: Chrysomelidae) involved in diapause. Arch. Insect Biochem. Physiol. 2021, 106, e21755. [Google Scholar] [CrossRef]
- Bootman, M.D.; Lipp, P.; Berridge, M.J. The organization and functions of local Ca2+ signales. J. Cell Sci. 2001, 114, 2213–2222. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.M.; Taylor, C.W. IP3 receptors-lessons from analyses ex cellula. J. Cell Sci. 2018, 132, jcs222463. [Google Scholar] [CrossRef] [Green Version]
- Pizzo, P.; Lissandron, V.; Capitanio, P.; Pozzan, T. Ca2+ signalling in the Golgi apparatus. Cell Calcium 2011, 50, 184–192. [Google Scholar] [CrossRef]
- Glitsch, M.D.; Bakowski, D.; Parekh, A.B. Store-operated Ca2+ entry depends on mitochondrial Ca2+ uptake. EMBO J. 2002, 21, 6744–6754. [Google Scholar] [CrossRef]
- Haller, T.; Dietl, P.; Deetjen, P.; Völkl, H. The lysosomal compartment as intracellular calcium store in MDCK cells: A possible involvement in InsP3-mediated Ca2+ release. Cell Calcium 1996, 19, 157–165. [Google Scholar] [CrossRef]
- Fill, M.; Copello, J.A. Ryanodine receptor calcium release channels. Physiol. Rev. 2002, 82, 893–922. [Google Scholar] [CrossRef] [Green Version]
- Kobrinsky, E.; Ondrias, K.; Marks, A.R. Expressed ryanodine receptor can substitute for the inositol 1,4,5-trisphosphate receptor in Xenopus laevis oocytes during progesterone-induced maturation. Dev. Biol. 1995, 172, 531–540. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, S.L.; Serysheva, I.I. Ryanodine receptor structure: Progress and challenges. J. Biol. Chem. 2009, 284, 4047–4051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanner, J.T.; Georgiou, D.K.; Joshi, A.D.; Hamilton, S.L. Ryanodine receptors: Structure, expression, molecular details, and function in calcium release. Cold Spring Harb. Perspect. Biol. 2010, 2, a003996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paknejad, N.; Hite, R.K. Structural basis for the regulation of inositol trisphosphate receptors by Ca2+ and IP3. Nat. Struct. Mol Biol. 2018, 25, 660–668. [Google Scholar] [CrossRef]
- Prole, D.L.; Taylor, C.W. Structure and Function of IP3 Receptors. Cold Spring Harb. Perspect. Biol. 2019, 11, a035063. [Google Scholar] [CrossRef] [Green Version]
- Beutner, G.; Sharma, V.K.; Giovannucci, D.R.; Yule, D.I.; Sheu, S.S. Identification of a ryanodine receptor in rat heart mitochondria. J. Biol. Chem. 2001, 276, 21482–21488. [Google Scholar] [CrossRef] [Green Version]
- Ryu, S.Y.; Beutner, G.; Dirksen, R.T.; Kinnally, K.W.; Sheu, S.S. Mitochondrial ryanodine receptors and other mitochondrial Ca2+ permeable channels. FEBS Lett. 2010, 584, 1948–1955. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Zhang, D.; He, X.; Huang, Y.; Shao, H. Transport of calcium ions into mitochondria. Curr. Genom. 2016, 17, 215–219. [Google Scholar] [CrossRef] [Green Version]
- Roest, G.; La Rovere, R.M.; Bultynck, G.; Parys, J.B. IP3 receptor properties and function at membrane contact sites. Adv. Exp. Med. Biol. 2017, 981, 149–178. [Google Scholar] [CrossRef] [PubMed]
- Cremer, T.; Neefjes, J.; Berlin, I. The journey of Ca2+ through the cell—Pulsing through the network of ER membrane contact sites. J. Cell Sci. 2020, 133, jcs249136. [Google Scholar] [CrossRef] [PubMed]
- Seo, M.D.; Velamakanni, S.; Ishiyama, N.; Stathopulos, P.B.; Rossi, A.M.; Khan, S.A.; Dale, P.; Li, C.; Ames, J.B.; Ikura, M.; et al. Structural and functional conservation of key domains in InsP3 and ryanodine receptors. Nature 2012, 483, 108–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeshima, H.; Nishimura, S.; Matsumoto, T.; Ishida, H.; Kangawa, K.; Minamino, N.; Matsuo, H.; Ueda, M.; Hanaoka, M.; Hirose, T. Primary structure and expression from complementary DNA of skeletal muscle ryanodine receptor. Nature 1989, 339, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Otsu, K.; Willard, H.F.; Khanna, V.K.; Zorzato, F.; Green, N.M.; MacLennan, D.H. Molecular cloning of cDNA encoding the Ca2+ release channel (ryanodine receptor) of rabbit cardiac muscle sarcoplasmic reticulum. J. Biol. Chem. 1990, 265, 13472–13483. [Google Scholar] [CrossRef]
- Hakamata, Y.; Nakai, J.; Takeshima, H.; Imoto, K. Primary structure and distribution of a novel ryanodine receptor/calcium release channel from rabbit brain. FEBS Lett. 1992, 312, 229–235. [Google Scholar] [CrossRef] [Green Version]
- Hasan, G.; Rosbash, M. Drosophila homologs of two mammalian intracellular Ca(2+)-release channels: Identification and expression patterns of the inositol 1,4,5-triphosphate and the ryanodine receptor genes. Development 1992, 116, 967–975. [Google Scholar] [CrossRef]
- Takeshima, H.; Nishi, M.; Iwabe, N.; Miyata, T.; Hosoya, T.; Masai, I.; Hotta, Y. Isolation and characterization of a gene for a ryanodine receptor/calcium release channel in Drosophila melanogaster. FEBS Lett. 1994, 337, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Puente, E.; Suner, M.; Evans, A.D.; McCaffery, A.R.; Windass, J.D. Identification of a polymorphic ryanodine receptor gene from Heliothis virescens (Lepidoptera: Noctuidae). Insect Biochem. Mol. Biol. 2000, 30, 335–347. [Google Scholar] [CrossRef]
- Scott-Ward, T.S.; Dunbar, S.J.; Windass, J.D.; Williams, A.J. Characterization of the ryanodine receptor-Ca2+ release channel from the thoracic tissues of the lepidopteran insect Heliothis virescens. J. Membr. Biol. 2001, 179, 127–141. [Google Scholar] [CrossRef]
- Kato, K.; Kiyonaka, S.; Sawaguchi, Y.; Tohnishi, M.; Masaki, T.; Yasokawa, N.; Mizuno, Y.; Mori, E.; Inoue, K.; Hamachi, I.; et al. Molecular characterization of flubendiamide sensitivity in the lepidopterous ryanodine receptor Ca(2+) release channel. Biochemistry 2009, 48, 10342–10352. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Han, Z.; Zhu, Y.; Xie, Z.; Wang, J.; Liu, Y.; Li, X. Molecular characterization of a ryanodine receptor gene in the rice leaffolder, Cnaphalocrocis medinalis (Guenée). PLoS ONE 2012, 7, e36623. [Google Scholar] [CrossRef]
- Sun, L.; Cui, L.; Rui, C.; Yan, X.; Yang, D.; Yuan, H. Modulation of the expression of ryanodine receptor mRNA from Plutella xylostella as a result of diamide insecticide application. Gene 2012, 511, 265–273. [Google Scholar] [CrossRef]
- Wang, X.; Wu, S.; Yang, Y.; Wu, Y. Molecular cloning, characterization and mRNA expression of a ryanodine receptor gene from diamondback moth, Plutella xylostella. Pestic. Biochem. Physiol. 2012, 102, 204–212. [Google Scholar] [CrossRef]
- Cui, L.; Yang, D.; Yan, X.; Rui, C.; Wang, Z.; Yuan, H. Molecular cloning, characterization and expression profiling of a ryanodine receptor gene in Asian corn borer, Ostrinia furnacalis (Guenée). PLoS ONE 2013, 8, e75825. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Liu, Y.; Gao, J.; Xie, Z.; Huang, L.; Wang, W.; Wang, J. Molecular cloning and mRNA expression of a ryanodine receptor gene in the cotton bollworm, Helicoverpa armigera. Pestic. Biochem. Physiol. 2013, 107, 327–333. [Google Scholar] [CrossRef]
- Wu, S.; Wang, F.; Huang, J.; Fang, Q.; Shen, Z.; Ye, G. Molecular and cellular analyses of a ryanodine receptor from hemocytes of Pieris rapae. Dev. Comp. Immunol. 2013, 41, 1–10. [Google Scholar] [CrossRef]
- Liu, Y.; Shahzad, M.F.; Zhang, L.; Li, F.; Lin, K. Amplifying long transcripts of ryanodine receptors of five agricultural pests by transcriptome analysis and gap filling. Genome 2013, 56, 651–658. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, L.; Chen, Q.; Qin, W.; Huang, S.; Jiang, Y.; Qin, H. Chlorantraniliprole resistance and its biochemical and new molecular target mechanisms in laboratory and field strains of Chilo suppressalis (Walker). Pest Manag. Sci. 2018, 74, 1416–1423. [Google Scholar] [CrossRef]
- Sun, L.; Qiu, G.; Cui, L.; Ma, C.; Yuan, H. Molecular characterization of a ryanodine receptor gene from Spodoptera exigua and its upregulation by chlorantraniliprole. Pestic. Biochem. Physiol. 2015, 123, 56–63. [Google Scholar] [CrossRef]
- Sun, L.N.; Zhang, H.J.; Quan, L.F.; Yan, W.T.; Yue, Q.; Li, Y.Y.; Qiu, G.S. Characterization of the ryanodine receptor gene with a unique 3’-UTR and alternative splice site from the oriental fruit moth. J. Insect Sci. 2016, 16, 16. [Google Scholar] [CrossRef] [Green Version]
- Roditakis, E.; Steinbach, D.; Moritz, G.; Vasakis, E.; Stavrakaki, M.; Ilias, A.; Garcia-Vidal, L. Ryanodine receptor point mutations confer diamide insecticide resistance in tomato leafminer, Tuta absoluta (Lepidoptera: Gelechiidae). Insect Biochem. Mol. Biol. 2017, 80, 11–20. [Google Scholar] [CrossRef]
- Boaventura, D.; Bolzan, A.; Padovez, F.E.; Okuma, D.M.; Omoto, C.; Nauen, R. Detection of a ryanodine receptor target-site mutation in diamide insecticide resistant fall armyworm, Spodoptera frugiperda. Pest Manag. Sci. 2020, 76, 47–54. [Google Scholar] [CrossRef]
- Yuan, G.R.; Shi, W.Z.; Yang, W.J.; Jiang, X.Z.; Dou, W.; Wang, J.J. Molecular characteristics, mRNA expression, and alternative splicing of a ryanodine receptor gene in the oriental fruit fly, Bactrocera dorsalis (Hendel). PLoS ONE 2014, 9, e95199. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Gao, J.; Wang, W.; Huang, L.; Guo, X.; Li, B.; Wang, J. Comparative characterization of two intracellular Ca2+-release channels from the red flour beetle, Tribolium castaneum. Sci. Rep. 2014, 4, 6702. [Google Scholar] [CrossRef] [Green Version]
- Wan, P.J.; Guo, W.Y.; Yang, Y.; Lü, F.G.; Lu, W.P.; Li, G.Q. RNAi suppression of the ryanodine receptor gene results in decreased susceptibility to chlorantraniliprole in Colorado potato beetle Leptinotarsa decemlineata. J. Insect Physiol. 2014, 63, 48–55. [Google Scholar] [CrossRef]
- Wang, J.; Xie, Z.; Gao, J.; Liu, Y.; Wang, W.; Huang, L.; Wang, J. Molecular cloning and characterization of a ryanodine receptor gene in brown planthopper (BPH), Nilaparvata lugens (Stål). Pest Manag. Sci. 2014, 70, 790–797. [Google Scholar] [CrossRef]
- Yang, Y.; Wan, P.J.; Hu, X.X.; Li, G.Q. RNAi mediated knockdown of the ryanodine receptor gene decreases chlorantraniliprole susceptibility in Sogatella furcifera. Pestic. Biochem. Physiol. 2014, 108, 58–65. [Google Scholar] [CrossRef]
- Troczka, B.J.; Williams, A.J.; Bass, C.; Williamson, M.S.; Field, L.M.; Davies, T.G. Molecular cloning, characterisation and mRNA expression of the ryanodine receptor from the peach-potato aphid, Myzus persicae. Gene 2015, 556, 106–112. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.Y.; Jiang, X.Z.; Yuan, G.R.; Shang, F.; Wang, J.J. Molecular Characterization, mRNA expression and alternative splicing of ryanodine receptor gene in the brown citrus aphid, Toxoptera citricida (Kirkaldy). Int. J. Mol. Sci. 2015, 16, 15220–15234. [Google Scholar] [CrossRef] [Green Version]
- Yuan, G.R.; Wang, K.Y.; Mou, X.; Luo, R.Y.; Dou, W.; Wang, J.J. Molecular cloning, mRNA expression and alternative splicing of a ryanodine receptor gene from the citrus whitefly, Dialeurodes citri (Ashmead). Pestic. Biochem. Physiol. 2017, 142, 59–66. [Google Scholar] [CrossRef]
- Supattapone, S.; Worley, P.F.; Baraban, J.M.; Snyder, S.H. Solubilization, purification, and characterization of an inositol trisphosphate receptor. J. Biol. Chem. 1988, 263, 1530–1534. [Google Scholar] [CrossRef]
- Furuichi, T.; Yoshikawa, S.; Miyawaki, A.; Wada, K.; Maeda, N.; Mikoshiba, K. Primary structure and functional expression of the inositol 1,4,5-trisphosphate-binding protein P400. Nature 1989, 342, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Südhof, T.C.; Newton, C.L.; Archer, B.T., 3rd; Ushkaryov, Y.A.; Mignery, G.A. Structure of a novel InsP3 receptor. EMBO J. 1991, 10, 3199–3206. [Google Scholar] [CrossRef] [PubMed]
- Blondel, O.; Takeda, J.; Janssen, H.; Seino, S.; Bell, G.I. Sequence and functional characterization of a third inositol trisphosphate receptor subtype, IP3R-3, expressed in pancreatic islets, kidney, gastrointestinal tract, and other tissues. J. Biol. Chem. 1993, 268, 11356–11363. [Google Scholar] [CrossRef]
- Yoshikawa, S.; Tanimura, T.; Miyawaki, A.; Nakamura, M.; Yuzaki, M.; Furuichi, T.; Mikoshiba, K. Molecular cloning and characterization of the inositol 1,4,5-trisphosphate receptor in Drosophila melanogaster. J. Biol. Chem. 1992, 267, 16613–16619. [Google Scholar] [CrossRef]
- Guo, L.; Liang, P.; Fang, K.; Chu, D. Silence of inositol 1,4,5-trisphosphate receptor expression decreases cyantraniliprole susceptibility in Bemisia tabaci. Pestic. Biochem. Physiol. 2017, 142, 162–169. [Google Scholar] [CrossRef]
- Troczka, B.J.; Richardson, E.; Homem, R.A.; Davies, T.G.E. An analysis of variability in genome organisation of intracellular calcium release channels across insect orders. Gene 2018, 670, 70–86. [Google Scholar] [CrossRef]
- Peng, Y.C.; Sheng, C.W.; Casida, J.E.; Zhao, C.Q.; Han, Z.J. Ryanodine receptor genes of the rice stem borer, Chilo suppressalis: Molecular cloning, alternative splicing and expression profiling. Pestic. Biochem. Physiol. 2017, 135, 69–77. [Google Scholar] [CrossRef]
- Xu, X.; Bhat, M.B.; Nishi, M.; Takeshima, H.; Ma, J. Molecular cloning of cDNA encoding a drosophila ryanodine receptor and functional studies of the carboxyl-terminal calcium release channel. Biophys. J. 2000, 78, 1270–1281. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, S.L. Ryanodine receptors. Cell Calcium 2005, 38, 253–260. [Google Scholar] [CrossRef]
- Mikoshiba, K. The IP3 receptor/Ca2+ channel and its cellular function. Biochem. Soc. Symp. 2007, 74, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Tung, C.C.; Lobo, P.A.; Kimlicka, L.; Van Petegem, F. The amino-terminal disease hotspot of ryanodine receptors forms a cytoplasmic vestibule. Nature 2010, 468, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Yuchi, Z.; Lau, K.; Van Petegem, F. Disease mutations in the ryanodine receptor central region: Crystal structures of a phosphorylation hot spot domain. Structure 2012, 20, 1201–1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, K.; Van Petegem, F. Crystal structures of wild type and disease mutant forms of the ryanodine receptor SPRY2 domain. Nat. Commun. 2014, 5, 5397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuchi, Z.; Yuen, S.M.; Lau, K.; Underhill, A.Q.; Cornea, R.L.; Fessenden, J.D.; Van Petegem, F. Crystal structures of ryanodine receptor SPRY1 and tandem-repeat domains reveal a critical FKBP12 binding determinant. Nat. Commun. 2015, 6, 7947. [Google Scholar] [CrossRef]
- Yan, Z.; Bai, X.; Yan, C.; Wu, J.; Li, Z.; Xie, T.; Peng, W.; Yin, C.; Li, X.; Scheres, S. Structure of the rabbit ryanodine receptor RyR1 at near-atomic resolution. Nature 2015, 517, 50–55. [Google Scholar] [CrossRef] [Green Version]
- Zalk, R.; Clarke, O.B.; des Georges, A.; Grassucci, R.A.; Reiken, S.; Mancia, F.; Hendrickson, W.A.; Frank, J.; Marks, A.R. Structure of a mammalian ryanodine receptor. Nature 2015, 517, 44–49. [Google Scholar] [CrossRef] [Green Version]
- Bosanac, I.; Yamazaki, H.; Matsu-Ura, T.; Michikawa, T.; Mikoshiba, K.; Ikura, M. Crystal structure of the ligand binding suppressor domain of type 1 inositol 1,4,5-trisphosphate receptor. Mol. Cell. 2005, 17, 193–203. [Google Scholar] [CrossRef]
- Chan, J.; Whitten, A.E.; Jeffries, C.M.; Bosanac, I.; Mal, T.K.; Ito, J.; Porumb, H.; Michikawa, T.; Mikoshiba, K.; Trewhella, J.; et al. Ligand-induced conformational changes via flexible linkers in the amino-terminal region of the inositol 1,4,5-trisphosphate receptor. J. Mol. Biol. 2007, 373, 1269–1280. [Google Scholar] [CrossRef]
- Lin, C.C.; Baek, K.; Lu, Z. Apo and InsP3-bound crystal structures of the ligand-binding domain of an InsP3 receptor. Nat. Struct. Mol. Biol. 2011, 18, 1172–1174. [Google Scholar] [CrossRef] [Green Version]
- Ludtke, S.J.; Tran, T.P.; Ngo, Q.T.; Moiseenkova-Bell, V.Y.; Chiu, W.; Serysheva, I.I. Flexible architecture of IP3R1 by Cryo-EM. Structure 2011, 19, 1192–1199. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Enomoto, M.; Rossi, A.M.; Seo, M.; Rahman, T.; Stathopulos, P.B.; Taylor, C.W.; Ikura, M.; Ames, J.B. CaBP1, a neuronal Ca2+ sensor protein, inhibits inositol trisphosphate receptors by clamping intersubunit interactions. Proc. Natl. Acad. Sci. USA 2013, 110, 8507–8512. [Google Scholar] [CrossRef] [Green Version]
- Des Georges, A.; Clarke, O.B.; Zalk, R.; Yuan, Q.; Condon, K.J.; Grassucci, R.A.; Hendrickson, W.A.; Marks, A.R.; Frank, J. Structural Basis for gating and activation of RyR1. Cell 2016, 167, 145–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, G.; Baker, M.R.; Wang, Z.; Seryshev, A.B.; Ludtke, S.J.; Baker, M.L.; Serysheva, I.I. Cryo-EM reveals ligand induced allostery underlying InsP3R channel gating. Cell Res. 2018, 28, 1158–1170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosanac, I.; Alattia, J.R.; Mal, T.K.; Chan, J.; Talarico, S.; Tong, F.K.; Tong, K.I.; Yoshikawa, F.; Furuichi, T.; Iwai, M.; et al. Structure of the inositol 1,4,5-trisphosphate receptor binding core in complex with its ligand. Nature 2002, 420, 696–700. [Google Scholar] [CrossRef]
- Uchida, K.; Miyauchi, H.; Furuichi, T.; Michikawa, T.; Mikoshiba, K. Critical regions for activation gating of the inositol 1,4,5-trisphosphate receptor. J. Biol. Chem. 2003, 278, 16551–16560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srikanth, S.; Wang, Z.; Tu, H.; Nair, S.; Mathew, M.K.; Hasan, G.; Bezprozvanny, I. Functional properties of the Drosophila melanogaster inositol 1,4,5-trisphosphate receptor mutants. Biophys. J. 2004, 86, 3634–3646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, J.; Yamazaki, H.; Ishiyama, N.; Seo, M.D.; Mal, T.K.; Michikawa, T.; Mikoshiba, K.; Ikura, M. Structural studies of inositol 1,4,5-trisphosphate receptor: Coupling ligand binding to channel gating. J. Biol. Chem. 2010, 285, 36092–36099. [Google Scholar] [CrossRef] [Green Version]
- Yuchi, Z.; Van Petegem, F. Common allosteric mechanisms between ryanodine and inositol-1,4,5-trisphosphate receptors. Channels 2011, 5, 120–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, A.M.; Riley, A.M.; Tovey, S.C.; Rahman, T.; Dellis, O.; Taylor, E.J.; Veresov, V.G.; Potter, B.V.; Taylor, C.W. Synthetic partial agonists reveal key steps in IP3 receptor activation. Nat. Chem. Biol. 2009, 5, 631–639. [Google Scholar] [CrossRef] [Green Version]
- Seo, M.D.; Enomoto, M.; Ishiyama, N.; Stathopulos, P.B.; Ikura, M. Structural insights into endoplasmic reticulum stored calcium regulation by inositol 1,4,5-trisphosphate and ryanodine receptors. Biochim. Biophys. Acta 2015, 1853, 1980–1991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhat, M.B.; Zhao, J.; Takeshima, H.; Ma, J. Functional calcium release channel formed by the carboxyl-terminal portion of ryanodine receptor. Biophys. J. 1997, 73, 1329–1336. [Google Scholar] [CrossRef] [Green Version]
- Rossi, D.; Sorrentino, V. Molecular genetics of ryanodine receptors Ca2+-release channels. Cell Calcium 2002, 32, 307–319. [Google Scholar] [CrossRef]
- Ponting, C.; Schultz, J.; Bork, P. SPRY domains in ryanodine receptors (Ca(2+)-release channels). Trends Biochem. Sci. 1997, 22, 193–194. [Google Scholar] [CrossRef]
- Sorrentino, V.; Barone, V.; Rossi, D. Intracellular Ca(2+) release channels in evolution. Curr. Opin. Genet. Dev. 2000, 10, 662–667. [Google Scholar] [CrossRef]
- Santulli, G.; Nakashima, R.; Yuan, Q.; Marks, A.R. Intracellular calcium release channels: An update. J. Physiol. 2017, 595, 3041–3051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikenoue, T.; Inoki, K.; Yang, Q.; Zhou, X.; Guan, K.L. Essential function of TORC2 in PKC and Akt turn motif phosphorylation, maturation and signalling. EMBO J. 2008, 27, 1919–1931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponting, C.P. Novel repeats in ryanodine and IP3 receptors and protein O-mannosyltransferases. Trends Biochem. Sci. 2000, 25, 48–50. [Google Scholar] [CrossRef]
- Woo, J.S.; Suh, H.Y.; Park, S.Y.; Oh, B.H. Structural basis for protein recognition by B30.2/SPRY domains. Mol. Cell. 2006, 24, 967–976. [Google Scholar] [CrossRef]
- Cui, Y.; Tae, H.S.; Norris, N.C.; Karunasekara, Y.; Pouliquin, P.; Board, P.G.; Dulhunty, A.F.; Casarotto, M.G. A dihydropyridine receptor α1s loop region critical for skeletal muscle contraction is intrinsically unstructured and binds to a SPRY domain of the type 1 ryanodine receptor. Int. J. Biochem. Cell Biol. 2009, 41, 677–686. [Google Scholar] [CrossRef]
- Callaway, C.; Seryshev, A.; Wang, J.P.; Slavik, K.J.; Needleman, D.H.; Cantu, C.; Wu, Y., 3rd; Jayaraman, T.; Marks, A.R.; Hamilton, S.L. Localization of the high and low affinity [3H]ryanodine binding sites on the skeletal muscle Ca2+ release channel. J. Biol. Chem. 1994, 269, 15876–15884. [Google Scholar] [CrossRef]
- Smith, J.S.; Rousseau, E.; Meissner, G. Calmodulin modulation of single sarcoplasmic reticulum Ca-release channels from cardiac and skeletal muscle. Circ. Res. 1989, 64, 352–359. [Google Scholar] [CrossRef] [Green Version]
- Tripathy, A.; Xu, L.; Mann, G.; Meissner, G. Calmodulin activation and inhibition of skeletal muscle Ca2+ release channel (ryanodine receptor). Biophys. J. 1995, 69, 106–119. [Google Scholar] [CrossRef] [Green Version]
- Wagenknecht, T.; Radermacher, M.; Grassucci, R.; Berkowitz, J.; Xin, H.B.; Fleischer, S. Locations of calmodulin and FK506-binding protein on the three-dimensional architecture of the skeletal muscle ryanodine receptor. J. Biol. Chem. 1997, 272, 32463–32471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balshaw, D.M.; Xu, L.; Yamaguchi, N.; Pasek, D.A.; Meissner, G. Calmodulin binding and inhibition of cardiac muscle calcium release channel (ryanodine receptor). J. Biol. Chem. 2001, 276, 20144–20153. [Google Scholar] [CrossRef] [Green Version]
- Sipma, H.; De Smet, P.; Sienaert, I.; Vanlingen, S.; Missiaen, L.; Parys, J.B.; De Smedt, H. Modulation of inositol 1,4,5-trisphosphate binding to the recombinant ligand-binding site of the type-1 inositol 1,4,5-trisphosphate receptor by Ca2+ and calmodulin. J. Biol. Chem. 1999, 274, 12157–12162. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.; Liu, C.; Qin, J.; Wang, J.; Dong, S.; Chen, W.; He, W.; Gao, Q.; You, M.; Yuchi, Z. Crystal structure of ryanodine receptor N-terminal domain from Plutella xylostella reveals two potential species-specific insecticide-targeting sites. Insect Biochem. Mol. Biol. 2018, 92, 73–83. [Google Scholar] [CrossRef]
- Xu, T.; Yuchi, Z. Crystal structure of diamondback moth ryanodine receptor Repeat34 domain reveals insect-specific phosphorylation sites. BMC Biol. 2019, 17, 77. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, D.; Lin, L.; You, M.; Yuchi, Z.; You, S. Crystal Structure of the ryanodine receptor SPRY2 domain from the diamondback moth provides insights into the development of novel insecticides. J. Agric. Food Chem. 2020, 68, 1731–1740. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Wang, W.; Salauddin, N.M.; Lin, L.; You, M.; You, S.; Yuchi, Z. Crystal structure of the N-terminal domain of ryanodine receptor from the honeybee, Apis mellifera. Insect Biochem. Mol. Biol. 2020, 125, 103454. [Google Scholar] [CrossRef]
- Lin, L.; Hao, Z.; Cao, P.; Yuchi, Z. Homology modeling and docking study of diamondback moth ryanodine receptor reveals the mechanisms for channel activation, insecticide binding and resistance. Pest Manag. Sci. 2020, 76, 1291–1303. [Google Scholar] [CrossRef]
- Zhao, M.; Li, P.; Li, X.; Zhang, L.; Winkfein, R.J.; Chen, S.R. Molecular identification of the ryanodine receptor pore-forming segment. J. Biol. Chem. 1999, 274, 25971–25974. [Google Scholar] [CrossRef] [Green Version]
- Troczka, B.J.; Williams, A.J.; Williamson, M.S.; Field, L.M.; Luemmen, P.; Davies, T.G. Stable expression and functional characterisation of the diamondback moth ryanodine receptor G4946E variant conferring resistance to diamide insecticides. Sci. Rep. 2015, 5, 14680. [Google Scholar] [CrossRef]
- Du, G.G.; Guo, X.; Khanna, V.K.; MacLennan, D.H. Functional characterization of mutants in the predicted pore region of the rabbit cardiac muscle Ca(2+) release channel (ryanodine receptor isoform 2). J. Biol. Chem. 2001, 276, 31760–31771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schug, Z.T.; da Fonseca, P.C.; Bhanumathy, C.D.; Wagner, L., 2nd; Zhang, X.; Bailey, B.; Morris, E.P.; Yule, D.I.; Joseph, S.K. Molecular characterization of the inositol 1,4,5-trisphosphate receptor pore-forming segment. J. Biol. Chem. 2008, 283, 2939–2948. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Balshaw, D.; Xu, L.; Tripathy, A.; Xin, C.; Meissner, G. Evidence for a role of the lumenal M3-M4 loop in skeletal muscle Ca(2+) release channel (ryanodine receptor) activity and conductance. Biophys. J. 2000, 79, 828–840. [Google Scholar] [CrossRef] [Green Version]
- Du, G.G.; MacLennan, D.H. Functional consequences of mutations of conserved, polar amino acids in transmembrane sequences of the Ca2+ release channel (ryanodine receptor) of rabbit skeletal muscle sarcoplasmic reticulum. J. Biol. Chem. 1998, 273, 31867–31872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.R.; Ebisawa, K.; Li, X.; Zhang, L. Molecular identification of the ryanodine receptor Ca2+ sensor. J. Biol. Chem. 1998, 273, 14675–14678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, S.; Casida, J.E. Species differences in chlorantraniliprole and flubendiamide insecticide binding sites in the ryanodine receptor. Pestic. Biochem. Physiol. 2013, 107, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Lümmen, P.; Nauen, R.; Casida, J.E. Diamide insecticide target site specificity in the Heliothis and Musca ryanodine receptors relative to toxicity. J. Agric. Food Chem. 2014, 62, 4077–4082. [Google Scholar] [CrossRef]
- Wang, R.; Bolstad, J.; Kong, H.; Zhang, L.; Brown, C.; Chen, S.R. The predicted TM10 transmembrane sequence of the cardiac Ca2+ release channel (ryanodine receptor) is crucial for channel activation and gating. J. Biol. Chem. 2004, 279, 3635–3642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorrentino, V.; Volpe, P. Ryanodine receptors: How many, where and why? Trends Pharmacol. Sci. 1993, 14, 98–103. [Google Scholar] [CrossRef]
- Xiong, H.; Feng, X.; Gao, L.; Xu, L.; Pasek, D.A.; Seok, J.H.; Meissner, G. Identification of a two EF-hand Ca2+ binding domain in lobster skeletal muscle ryanodine receptor/Ca2+ release channel. Biochemistry 1998, 37, 4804–4814. [Google Scholar] [CrossRef]
- Guo, W.; Sun, B.; Xiao, Z.; Liu, Y.; Wang, Y.; Zhang, L.; Wang, R.; Chen, S.R. The EF-hand Ca2+ binding domain is not required for cytosolic Ca2+ activation of the cardiac ryanodine receptor. J. Biol. Chem. 2016, 291, 2150–2160. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Gomez, A.C.; Pasek, D.A.; Meissner, G.; Yamaguchi, N. Two EF-hand motifs in ryanodine receptor calcium release channels contribute to isoform-specific regulation by calmodulin. Cell Calcium 2017, 66, 62–70. [Google Scholar] [CrossRef]
- Yamaguchi, N.; Xin, C.; Meissner, G. Identification of apocalmodulin and Ca2+-calmodulin regulatory domain in skeletal muscle Ca2+ release channel, ryanodine receptor. J. Biol. Chem. 2001, 276, 22579–22585. [Google Scholar] [CrossRef] [Green Version]
- Ladenburger, E.M.; Plattner, H. Calcium-release channels in paramecium. Genomic expansion, differential positioning and partial transcriptional elimination. PLoS ONE 2011, 6, e27111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiurillo, M.A.; Lander, N.; Vercesi, A.E.; Docampo, R. IP3 receptor-mediated Ca2+ release from acidocalcisomes regulates mitochondrial bioenergetics and prevents autophagy in Trypanosoma cruzi. Cell Calcium 2020, 92, 102284. [Google Scholar] [CrossRef] [PubMed]
- Futatsugi, A.; Kuwajima, G.; Mikoshiba, K. Tissue-specific and developmentally regulated alternative splicing in mouse skeletal muscle ryanodine receptor mRNA. Biochem. J. 1995, 305, 373–378. [Google Scholar] [CrossRef] [Green Version]
- George, C.H.; Rogers, S.A.; Bertrand, B.M.A.; Tunwell, R.E.A.; Thomas, N.L.; Steele, D.S.; Cox, E.V.; Pepper, C.; Hazeel, C.J.; Claycomb, W.C.; et al. Alternative splicing of ryanodine receptors modulates cardiomyocyte Ca2+ signaling and susceptibility to apoptosis. Circ. Res. 2007, 100, 874–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, T.; Lueck, J.D.; Harvey, P.J.; Pace, S.M.; Ikemoto, N.; Casarotto, M.G.; Dirksen, R.T.; Dulhunty, A.F. Alternative splicing of RyR1 alters the efficacy of skeletal EC coupling. Cell Calcium 2009, 45, 264–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takasawa, S.; Kuroki, M.; Nata, K.; Noguchi, N.; Ikeda, T.; Yamauchi, A.; Ota, H.; Itaya-Hironaka, A.; Sakuramoto-Tsuchida, S.; Takahashi, I.; et al. A novel ryanodine receptor expressed in pancreatic islets by alternative splicing from type 2 ryanodine receptor gene. Biochem. Biophys. Res. Commun. 2010, 397, 140–145. [Google Scholar] [CrossRef]
- Foskett, J.K.; White, C.; Cheung, K.H.; Mak, D.O. Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev. 2007, 87, 593–658. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Khakame, S.K.; Ye, C.; Yang, Y.; Wu, Y. Characterization of field evolved resistance to chlorantraniliprole in the diamondback moth, Plutella xylostella, from China. Pest Manag. Sci. 2013, 69, 661–665. [Google Scholar] [CrossRef]
- D’Cruz, A.A.; Kershaw, N.J.; Chiang, J.J.; Wang, M.K.; Nicola, N.A.; Babon, J.J.; Gack, M.U.; Nicholson, S.E. Crystal structure of the TRIM25 B30.2 (PRYSPRY) domain: A key component of antiviral signalling. Biochem. J. 2013, 456, 231–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nucifora, F.C., Jr.; Li, S.H.; Danoff, S.; Ullrich, A.; Ross, C.A. Molecular cloning of a cDNA for the human inositol 1,4,5-trisphosphate receptor type 1, and the identification of a third alternatively spliced variant. Brain Res. Mol. Brain Res. 1995, 32, 291–296. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. Mega X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Le, S.Q.; Gascuel, O. An improved general amino acid replacement matrix. Mol. Biol. Evol. 2008, 25, 1307–1320. [Google Scholar] [CrossRef] [Green Version]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783. [Google Scholar] [CrossRef]
- Meur, G.; Parker, A.K.; Gergely, F.V.; Taylor, C.W. Targeting and retention of type 1 ryanodine receptors to the endoplasmic reticulum. J. Biol. Chem. 2007, 282, 23096–23103. [Google Scholar] [CrossRef] [Green Version]
- Cárdenas, C.; Miller, R.A.; Smith, I.; Bui, T.; Molgó, J.; Müller, M.; Vais, H.; Cheung, K.H.; Yang, J.; Parker, I.; et al. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell 2010, 142, 270–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Csordás, G.; Weaver, D.; Hajnóczky, G. Endoplasmic reticulum-mitochondrial contactology: Structure and signaling functions. Trends Cell Biol. 2018, 28, 523–540. [Google Scholar] [CrossRef] [PubMed]
- López-Sanjurjo, C.I.; Tovey, S.C.; Prole, D.L.; Taylor, C.W. Lysosomes shape Ins(1,4,5)P3-evoked Ca2+ signals by selectively sequestering Ca2+ released from the endoplasmic reticulum. J. Cell Sci. 2013, 126 Pt 1, 289–300. [Google Scholar] [CrossRef] [Green Version]
- Garrity, A.G.; Wang, W.; Collier, C.M.; Levey, S.A.; Gao, Q.; Xu, H. The endoplasmic reticulum, not the pH gradient, drives calcium refilling of lysosomes. Elife 2016, 5, e15887. [Google Scholar] [CrossRef] [PubMed]
- Atakpa, P.; Thillaiappan, N.B.; Mataragka, S.; Prole, D.L.; Taylor, C.W. IP3 receptors preferentially associate with ER-lysosome contact sites and selectively deliver Ca2+ to lysosomes. Cell Rep. 2018, 25, 3180–3193.e7. [Google Scholar] [CrossRef] [Green Version]
- Meissner, G. The structural basis of ryanodine receptor ion channel function. J. Gen. Physiol. 2017, 149, 1065–1089. [Google Scholar] [CrossRef] [PubMed]
- Arruda, A.P.; Pers, B.M.; Parlakgul, G.; Güney, E.; Goh, T.; Cagampan, E.; Lee, G.Y.; Goncalves, R.L.; Hotamisligil, G.S. Defective STIM-mediated store operated Ca2+ entry in hepatocytes leads to metabolic dysfunction in obesity. Elife 2017, 6, e29968. [Google Scholar] [CrossRef]
- Parekh, A.B.; Putney, J.W., Jr. Store-operated calcium channels. Physiol. Rev. 2005, 85, 757–810. [Google Scholar] [CrossRef] [Green Version]
- Woodard, G.E.; López, J.J.; Jardín, I.; Salido, G.M.; Rosado, J.A. TRPC3 regulates agonist-stimulated Ca2+ mobilization by mediating the interaction between type I inositol 1,4,5-trisphosphate receptor, RACK1, and Orai1. J. Biol. Chem. 2010, 285, 8045–8053. [Google Scholar] [CrossRef] [Green Version]
- Béliveau, É.; Lessard, V.; Guillemette, G. STIM1 positively regulates the Ca2+ release activity of the inositol 1,4,5-trisphosphate receptor in bovine aortic endothelial cells. PLoS ONE 2014, 9, e114718. [Google Scholar] [CrossRef] [PubMed]
- Thillaiappan, N.B.; Chavda, A.P.; Tovey, S.C.; Prole, D.L.; Taylor, C.W. Ca2+ signals initiate at immobile IP3 receptors adjacent to ER-plasma membrane junctions. Nat. Commun. 2017, 8, 1505. [Google Scholar] [CrossRef] [PubMed]
- Sampieri, A.; Santoyo, K.; Asanov, A.; Vaca, L. Association of the IP3R to STIM1 provides a reduced intraluminal calcium microenvironment, resulting in enhanced store-operated calcium entry. Sci. Rep. 2018, 8, 13252. [Google Scholar] [CrossRef]
- Prakriya, M.; Lewis, R.S. Store-operated calcium channels. Physiol. Rev. 2015, 95, 1383–1436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Wu, G.; Yang, Y.; Fu, S.; Liu, X.; Kang, H.; Yang, X.; Su, X.C.; Shen, Y. Calmodulin dissociates the STIM1-Orai1 complex and STIM1 oligomers. Nat. Commun. 2017, 8, 1042. [Google Scholar] [CrossRef]
- Qazi, S.; Trimmer, B.A. The role of inositol 1,4,5-trisphosphate 5-phosphatase in inositol signaling in the CNS of larval Manduca sexta. Insect Biochem. Mol. Biol. 1999, 29, 161–175. [Google Scholar] [CrossRef]
- Sartain, C.V.; Wolfner, M.F. Calcium and egg activation in Drosophila. Cell Calcium 2013, 53, 10–15. [Google Scholar] [CrossRef] [Green Version]
- Berridge, M.J. The inositol trisphosphate/calcium signaling pathway in health and disease. Physiol. Rev. 2016, 96, 1261–1296. [Google Scholar] [CrossRef] [Green Version]
- Megha Hasan, G. Control of protein translation by IP3R-mediated Ca2+ release in Drosophila neuroendocrine cells. Fly 2017, 11. [Google Scholar] [CrossRef] [Green Version]
- Alzayady, K.J.; Wang, L.; Chandrasekhar, R.; Wagner, L.E.; Van Petegem, F.; Yule, D.I. Defining the stoichiometry of inositol 1,4,5-trisphosphate binding required to initiate Ca2+ release. Sci. Signal 2016, 9, ra35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandrasekhar, R.; Alzayady, K.J.; Wagner, L.E.; Yule, D.I. Unique regulatory properties of heterotetrameric inositol 1,4,5-trisphosphate receptors revealed by studying concatenated receptor constructs. J. Biol. Chem. 2016, 291, 4846–4860. [Google Scholar] [CrossRef] [Green Version]
- Marchant, J.S.; Taylor, C.W. Cooperative activation of IP3 receptors by sequential binding of IP3 and Ca2+ safeguards against spontaneous activity. Curr. Biol. 1997, 7, 510–518. [Google Scholar] [CrossRef] [Green Version]
- Adkins, C.E.; Taylor, C.W. Lateral inhibition of inositol 1,4,5-trisphosphate receptors by cytosolic Ca(2+). Curr. Biol. 1999, 9, 1115–1118. [Google Scholar] [CrossRef] [Green Version]
- Furutama, D.; Shimoda, K.; Yoshikawa, S.; Miyawaki, A.; Furuichi, T.; Mikoshiba, K. Functional expression of the type 1 inositol 1,4,5-trisphosphate receptor promoter-lacZ fusion genes in transgenic mice. J. Neurochem. 1996, 66, 1793–1801. [Google Scholar] [CrossRef]
- Parker, I.; Choi, J.; Yao, Y. Elementary events of InsP3-induced Ca2+ liberation in Xenopus oocytes: Hot spots, puffs and blips. Cell Calcium 1996, 20, 105–121. [Google Scholar] [CrossRef]
- Bootman, M.D.; Berridge, M.J.; Lipp, P. Cooking with calcium: The recipes for composing global signals from elementary events. Cell 1997, 91, 367–373. [Google Scholar] [CrossRef] [Green Version]
- Marchant, J.S.; Parker, I. Role of elementary Ca(2+) puffs in generating repetitive Ca(2+) oscillations. EMBO J. 2001, 20, 65–76. [Google Scholar] [CrossRef] [Green Version]
- Berridge, M.J. Inositol trisphosphate and calcium oscillations. Biochem. Soc. Symp. 2007, 74, 1–7. [Google Scholar] [CrossRef]
- Rapp, P.E.; Berridge, M.J. The control of transepithelial potential oscillations in the salivary gland of Calliphora erythrocephala. J. Exp. Biol. 1981, 93, 119–132. [Google Scholar] [CrossRef]
- Rosay, P.; Armstrong, J.D.; Wang, Z.; Kaiser, K. Synchronized neural activity in the Drosophila memory centers and its modulation by amnesiac. Neuron 2001, 30, 759–770. [Google Scholar] [CrossRef] [Green Version]
- Goldammer, J.; Mantziaris, C.; Büschges, A.; Schmidt, J. Calcium imaging of CPG-evoked activity in efferent neurons of the stick insect. PLoS ONE 2018, 13, e0202822. [Google Scholar] [CrossRef]
- Vanderheyden, V.; Devogelaere, B.; Missiaen, L.; De Smedt, H.; Bultynck, G.; Parys, J.B. Regulation of inositol 1,4,5-trisphosphate-induced Ca2+ release by reversible phosphorylation and dephosphorylation. Biochim. Biophys. Acta 2009, 1793, 959–970. [Google Scholar] [CrossRef] [Green Version]
- DeSouza, N.; Reiken, S.; Ondrias, K.; Yang, Y.M.; Matkovich, S.; Marks, A.R. Protein kinase A and two phosphatases are components of the inositol 1,4,5-trisphosphate receptor macromolecular signaling complex. J. Biol. Chem. 2002, 277, 39397–39400. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.T.; Wagner, L., 2nd; Yule, D.I.; Bhanumathy, C.; Joseph, S.K. Akt kinase phosphorylation of inositol 1,4,5-trisphosphate receptors. J. Biol. Chem. 2006, 281, 3731–3737. [Google Scholar] [CrossRef] [Green Version]
- Arguin, G.; Regimbald-Dumas, Y.; Fregeau, M.O.; Caron, A.Z.; Guillemette, G. Protein kinase C phosphorylates the inositol 1,4,5-trisphosphate receptor type 2 and decreases the mobilization of Ca2+ in pancreatoma AR4-2J cells. J. Endocrinol. 2007, 192, 659–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dean, D.M.; Maroja, L.S.; Cottrill, S.; Bomkamp, B.E.; Westervelt, K.A.; Deitcher, D.L. The wavy mutation maps to the inositol 1,4,5-trisphosphate 3-kinase 2 (IP3K2) gene of Drosophila and interacts with IP3R to affect wing development. G3 2016, 6, 299–310. [Google Scholar] [CrossRef] [Green Version]
- Adkins, C.E.; Morris, S.A.; De Smedt, H.; Sienaert, I.; Török, K.; Taylor, C.W. Ca2+-calmodulin inhibits Ca2+ release mediated by type-1, -2 and -3 inositol trisphosphate receptors. Biochem. J. 2000, 345, 357–363. [Google Scholar] [CrossRef]
- Kasri, N.N.; Török, K.; Galione, A.; Garnham, C.; Callewaert, G.; Missiaen, L.; Parys, J.B.; De Smedt, H. Endogenously bound calmodulin is essential for the function of the inositol 1,4,5-trisphosphate receptor. J. Biol. Chem. 2006, 281, 8332–8338. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Lin, Y.; Zhang, Z.; Tikunova, S.; Birnbaumer, L.; Zhu, M.X. Identification of common binding sites for calmodulin and inositol 1,4,5-trisphosphate receptors on the carboxyl termini of trp channels. J. Biol. Chem. 2001, 276, 21303–21310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meissner, G.; Rios, E.; Tripathy, A.; Pasek, D.A. Regulation of skeletal muscle Ca2+ release channel (ryanodine receptor) by Ca2+ and monovalent cations and anions. J. Biol. Chem. 1997, 272, 1628–1638. [Google Scholar] [CrossRef] [Green Version]
- Gutteridge, S.; Caspar, T.; Cordova, D.; Tao, Y.; Wu, L.; Smith, R.M. Nucleic Acids Encoding Ryanodine Receptors. U.S. Patent 7,205,147, 2003. [Google Scholar]
- Cordova, D.; Benner, E.A.; Sacher, M.D.; Rauh, J.J.; Sopa, J.S.; Lahm, G.P.; Selby, T.P.; Stevenson, T.M.; Flexner, L.; Gutteridge, S.; et al. Anthranilic diamides: A new class of insecticides with a novel mode of action, ryanodine receptor activation. Pestic. Biochem. Physiol. 2006, 84, 196–214. [Google Scholar] [CrossRef]
- Cordova, D.; Benner, E.A.; Sacher, M.D.; Rauh, J.J.; Sopa, J.S.; Lahm, G.P.; Selby, T.P.; Stevenson, T.M.; Flexner, L.; Gutteridge, S.; et al. The novel mode of action of anthranilic diamide insecticides: Ryanodine receptor activation. ACS Symp. Ser. 2007, 948, 223–234. [Google Scholar]
- Meissner, G. Ryanodine activation and inhibition of the Ca2+ release channel of sarcoplasmic reticulum. J. Biol. Chem. 1986, 261, 6300–6306. [Google Scholar] [CrossRef]
- Schmitt, M.; Turberg, A.; Londershausen, M.; Dorn, A. Binding sites for Ca2+ channel effectors and ryanodine in Periplaneta americana-possible targets for new insecticides. Pestic. Sci. 1996, 48, 375–385. [Google Scholar] [CrossRef]
- Ebbinghaus-Kintscher, U.; Luemmen, P.; Lobitz, N.; Schulte, T.; Funke, C.; Fischer, R.; Masaki, T.; Yasokawa, N.; Tohnishi, M. Phthalic acid diamides activate ryanodine-sensitive Ca2+ release channels in insects. Cell Calcium 2006, 39, 21–33. [Google Scholar] [CrossRef]
- Zimanyi, I.; Pessah, I.N. Pharmacological characterization of the specific binding of [3H]ryanodine to rat brain microsomal membranes. Brain Res. 1991, 561, 181–191. [Google Scholar] [CrossRef]
- Sharma, P.; Ishiyama, N.; Nair, U.; Li, W.; Dong, A.; Miyake, T.; Wilson, A.; Ryan, T.; MacLennan, D.H.; Kislinger, T.; et al. Structural determination of the phosphorylation domain of the ryanodine receptor. FEBS J. 2012, 279, 3952–3964. [Google Scholar] [CrossRef] [Green Version]
- Andersson, D.C.; Betzenhauser, M.J.; Reiken, S.; Umanskaya, A.; Shiomi, T.; Marks, A.R. Stress-induced increase in skeletal muscle force requires protein kinase A phosphorylation of the ryanodine receptor. J. Physiol. 2012, 590, 6381–6387. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.R.; Li, X.; Ebisawa, K.; Zhang, L. Functional characterization of the recombinant type 3 Ca2+ release channel (ryanodine receptor) expressed in HEK293 cells. J. Biol. Chem. 1997, 272, 24234–24246. [Google Scholar] [CrossRef] [Green Version]
- Fruen, B.R.; Bardy, J.M.; Byrem, T.M.; Strasburg, G.M.; Louis, C.F. Differential Ca(2+) sensitivity of skeletal and cardiac muscle ryanodine receptors in the presence of calmodulin. Am. J. Physiol. Cell Physiol. 2000, 279, C724–C733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, B.; Sullivan, K.M.; Beckingham, K. Drosophila calmodulin mutants with specific defects in the musculature or in the nervous system. Genetics 2003, 165, 1255–1268. [Google Scholar] [CrossRef] [PubMed]
- Arnon, A.; Cook, B.; Montell, C.; Selinger, Z.; Minke, B. Calmodulin regulation of calcium stores in phototransduction of Drosophila. Science 1997, 275, 1119–1121. [Google Scholar] [CrossRef] [PubMed]
- Arnon, A.; Cook, B.; Gillo, B.; Montell, C.; Selinger, Z.; Minke, B. Calmodulin regulation of light adaptation and store-operated dark current in Drosophila photoreceptors. Proc. Natl. Acad. Sci. USA 1997, 94, 5894–5899. [Google Scholar] [CrossRef] [Green Version]
- Scott, K.; Sun, Y.; Beckingham, K.; Zuker, C.S. Calmodulin regulation of Drosophila light-activated channels and receptor function mediates termination of the light response in vivo. Cell 1997, 91, 375–383. [Google Scholar] [CrossRef] [Green Version]
- Karagas, N.E.; Venkatachalam, K. Roles for the endoplasmic reticulum in regulation of neuronal calcium homeostasis. Cells 2019, 8, 1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inui, M.; Saito, A.; Fleischer, S. Isolation of the ryanodine receptor from cardiac sarcoplasmic reticulum and identity with the feet structures. J. Biol. Chem. 1987, 262, 15637–15642. [Google Scholar] [CrossRef]
- Zorzato, F.; Fujii, J.; Otsu, K.; Phillips, M.; Green, N.M.; Lai, F.A.; Meissner, G.; MacLennan, D.H. Molecular cloning of cDNA encoding human and rabbit forms of the Ca2+ release channel (ryanodine receptor) of skeletal muscle sarcoplasmic reticulum. J. Biol. Chem. 1990, 265, 2244–2256. [Google Scholar] [CrossRef]
- Giannini, G.; Conti, A.; Mammarella, S.; Scrobogna, M.; Sorrentino, V. The ryanodine receptor/calcium channel genes are widely and differentially expressed in murine brain and peripheral tissues. J. Cell Biol. 1995, 128, 893–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vázquez-Martínez, O.; Cañedo-Merino, R.; Díaz-Muñoz, M.; Riesgo-Escovar, J.R. Biochemical characterization, distribution and phylogenetic analysis of Drosophila melanogaster ryanodine and IP3 receptors, and thapsigargin-sensitive Ca2+ ATPase. J. Cell Sci. 2003, 116, 2483–2494. [Google Scholar] [CrossRef] [Green Version]
- Chintapalli, V.R.; Wang, J.; Dow, J.A. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat. Genet. 2007, 39, 715–720. [Google Scholar] [CrossRef] [PubMed]
- McQuilton, P.; St Pierre, S.E.; Thurmond, J. FlyBase Consortium. FlyBase 101--the basics of navigating FlyBase. Nucleic Acids Res. 2012, 40, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Chapman, R.F. The Insects: Structure and Function, 4th ed.; Cambridge University Press: New York, NY, USA, 1998. [Google Scholar]
- Miyatake, R.; Furukawa, A.; Matsushita, M.; Iwahashi, K.; Nakamura, K.; Ichikawa, Y.; Suwaki, H. Tissue-specific alternative splicing of mouse brain type ryanodine receptor/calcium release channel mRNA. FEBS Lett. 1996, 395, 123–126. [Google Scholar] [CrossRef] [Green Version]
- Jiang, D.; Xiao, B.; Li, X.; Chen, S.R. Smooth muscle tissues express a major dominant negative splice variant of the type 3 Ca2+ release channel (ryanodine receptor). J. Biol. Chem. 2003, 278, 4763–4769. [Google Scholar] [CrossRef] [Green Version]
- Verma, A.; Hirsch, D.J.; Snyder, S.H. Calcium pools mobilized by calcium or inositol 1,4,5-trisphosphate are differentially localized in rat heart and brain. Mol. Biol. Cell 1992, 3, 621–631. [Google Scholar] [CrossRef] [Green Version]
- Furuichi, T.; Simon-Chazottes, D.; Fujino, I.; Yamada, N.; Hasegawa, M.; Miyawaki, A.; Yoshikawa, S.; Guénet, J.L.; Mikoshiba, K. Widespread expression of inositol 1,4,5-trisphosphate receptor type 1 gene (Insp3r1) in the mouse central nervous system. Recept. Channels 1993, 1, 11–24. [Google Scholar]
- Gorza, L.; Schiaffino, S.; Volpe, P. Inositol 1,4,5-trisphosphate receptor in heart: Evidence for its concentration in Purkinje myocytes of the conduction system. J. Cell Biol. 1993, 121, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Ferreri-Jacobia, M.; Mak, D.O.; Foskett, J.K. Translational mobility of the type 3 inositol 1,4,5-trisphosphate receptor Ca2+ release channel in endoplasmic reticulum membrane. J. Biol. Chem. 2005, 280, 3824–3831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkatesh, K.; Siddhartha, G.; Joshi, R.; Patel, S.; Hasan, G. Interactions between the inositol 1,4,5-trisphosphate and cyclic AMP signaling pathways regulate larval molting in Drosophila. Genetics 2001, 158, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Raghu, P.; Hasan, G. The inositol 1,4,5-triphosphate receptor expression in Drosophila suggests a role for IP3 signalling in muscle development and adult chemosensory functions. Dev. Biol. 1995, 171, 564–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, D.O.; Beck, R.R. Role of calcium ion in hormone-stimulated lipolysis. Biochem. Pharmacol. 1986, 35, 767–772. [Google Scholar] [CrossRef]
- Shi, H.; Dirienzo, D.; Zemel, M.B. Effects of dietary calcium on adipocyte lipid metabolism and body weight regulation in energy-restricted aP2-agouti transgenic mice. FASEB J. 2001, 15, 291–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zemel, M.B. Regulation of adiposity and obesity risk by dietary calcium: Mechanisms and implications. J. Am. Coll Nutr. 2002, 21, 146S–151S. [Google Scholar] [CrossRef]
- Jacqmain, M.; Doucet, E.; Després, J.P.; Bouchard, C.; Tremblay, A. Calcium intake, body composition, and lipoprotein-lipid concentrations in adults. Am. J. Clin. Nutr. 2003, 77, 1448–1452. [Google Scholar] [CrossRef]
- Arruda, A.P.; Hotamisligil, G.S. Calcium homeostasis and organelle function in the pathogenesis of obesity and diabetes. Cell Metab. 2015, 22, 381–397. [Google Scholar] [CrossRef] [Green Version]
- Maus, M.; Cuk, M.; Patel, B.; Lian, J.; Ouimet, M.; Kaufmann, U.; Yang, J.; Horvath, R.; Hornig-Do, H.T.; Chrzanowska-Lightowlers, Z.M.; et al. Store-operated Ca2+ entry controls induction of lipolysis and the transcriptional reprogramming to lipid metabolism. Cell Metab. 2017, 25, 698–712. [Google Scholar] [CrossRef] [Green Version]
- Alomaim, H.; Griffin, P.; Swist, E.; Plouffe, L.J.; Vandeloo, M.; Demonty, I.; Kumar, A.; Bertinato, J. Dietary calcium affects body composition and lipid metabolism in rats. PLoS ONE 2019, 14, e0210760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toprak, U.; Hegedus, D.; Doğan, C.; Güney, G. A journey into the world of insect lipid metabolism. Arch. Insect Biochem. Physiol. 2020, 104, e21682. [Google Scholar] [CrossRef] [PubMed]
- Toprak, U.; Güz, N.; Gürkan, M.O.; Hegedus, D.D. Identification and coordinated expression of perilipin genes in the biological cycle of sunn pest, Eurygaster maura (Hemiptera: Scutelleridae): Implications for lipolysis and lipogenesis. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2014, 171, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Güney, G.; Toprak, U.; Hegedus, D.D.; Bayram, Ş.; Coutu, C.; Bekkaoui, D.; Baldwin, D.; Heckel, D.G.; Hänniger, S.; Cedden, D.; et al. A look into Colorado potato beetle lipid metabolism through the lens of lipid storage droplet proteins. Insect Biochem. Mol. Biol. 2021, 133, 103473. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, M.; Jayakumar, S.; Richhariya, S.; Hasan, G. Loss of IP3 receptor function in neuropeptide secreting neurons leads to obesity in adult Drosophila. BMC Neurosci. 2013, 14, 157. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, M.; Metya, S.K.; Sadaf, S.; Kumar, S.; Schwudke, D.; Hasan, G. Altered lipid homeostasis in Drosophila InsP3 receptor mutants leads to obesity and hyperphagia. Dis. Model Mech. 2013, 6, 734–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumbach, J.; Hummel, P.; Bickmeyer, I.; Kowalczyk, K.M.; Frank, M.; Knorr, K.; Hildebrandt, A.; Riedel, D.; Jäckle, H.; Kühnlein, R.P. A Drosophila in vivo screen identifies store-operated calcium entry as a key regulator of adiposity. Cell Metab. 2014, 19, 331–343. [Google Scholar] [CrossRef] [Green Version]
- Baumbach, J.; Xu, Y.; Hehlert, P.; Kühnlein, R.P. Gαq, Gγ1 and Plc21C control Drosophila body fat storage. J. Genet. Genom. 2014, 41, 283–292. [Google Scholar] [CrossRef] [Green Version]
- Bi, J.; Xiang, Y.; Chen, H.; Liu, Z.; Grönke, S.; Kühnlein, R.P.; Huang, X. Opposite and redundant roles of the two Drosophila perilipins in lipid mobilization. J. Cell Sci. 2012, 125, 3568–3577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bi, J.; Wang, W.; Liu, Z.; Huang, X.; Jiang, Q.; Liu, G.; Wang, Y.; Huang, X. Seipin promotes adipose tissue fat storage through the ER Ca2+-ATPase SERCA. Cell Metab. 2014, 19, 861–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Borcherding, A.F.; Heier, C.; Tian, G.; Roeder, T.; Kühnlein, R.P. Chronic dysfunction of stromal interaction molecule by pulsed RNAi induction in fat tissue impairs organismal energy homeostasis in Drosophila. Sci. Rep. 2019, 9, 6989. [Google Scholar] [CrossRef] [Green Version]
- Toprak, U. The role of peptide hormones in insect lipid metabolism. Front. Physiol. 2020, 11, 434. [Google Scholar] [CrossRef]
- Arrese, E.L.; Flowers, M.T.; Gazard, J.L.; Wells, M.A. Calcium and cAMP are second messengers in the adipokinetic hormone-induced lipolysis of triacylglycerols in Manduca sexta fat body. J. Lipid Res. 1999, 40, 556–564. [Google Scholar] [CrossRef]
- Venkiteswaran, G.; Hasan, G. Intracellular Ca2+ signaling and store-operated Ca2+ entry are required in Drosophila neurons for flight. Proc. Natl. Acad. Sci. USA 2009, 106, 10326–10331. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, N.; Venkiteswaran, G.; Sadaf, S.; Padmanabhan, N.; Banerjee, S.; Hasan, G. Inositol 1,4,5-trisphosphate receptor and dSTIM function in Drosophila insulin-producing neurons regulates systemic intracellular calcium homeostasis and flight. J. Neurosci. 2010, 30, 1301–1313. [Google Scholar] [CrossRef] [Green Version]
- Bednářová, A.; Kodrík, D.; Krishnan, N. Adipokinetic hormone exerts its anti-oxidative effects using a conserved signal-transduction mechanism involving both PKC and cAMP by mobilizing extra- and intracellular Ca2+ stores. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2013, 158, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Gáliková, M.; Diesner, M.; Klepsatel, P.; Hehlert, P.; Xu, Y.; Bickmeyer, I.; Predel, R.; Kühnlein, R.P. Energy homeostasis control in Drosophila adipokinetic hormone mutants. Genetics 2015, 201, 665–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, Y.; Bi, J.; Shui, G.; Liu, Z.; Xiang, Y.; Liu, Y.; Wenk, M.R.; Yang, H.; Huang, X. Tissue-autonomous function of Drosophila seipin in preventing ectopic lipid droplet formation. PLoS Genet. 2011, 7, e1001364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, S.; Yang, L.; Li, P.; Hofmann, O.; Dicker, L.; Hide, W.; Lin, X.; Watkins, S.M.; Ivanov, A.R.; Hotamisligil, G.S. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature 2011, 473, 528–531. [Google Scholar] [CrossRef] [Green Version]
- Starling, R.C.; Hammer, D.F.; Altschuld, R.A. Human myocardial ATP content and in vivo contractile function. Mol. Cell Biochem. 1998, 180, 171–177. [Google Scholar] [CrossRef]
- Cao, T.; Jin, J.P. Evolution of flight muscle contractility and energetic efficiency. Front. Physiol. 2020, 11, 1038. [Google Scholar] [CrossRef]
- Palade, P.; Györke, S. Excitation-contraction coupling in crustacea: Do studies on these primitive creatures offer insights about EC coupling more generally? J. Muscle Res. Cell Motil. 1993, 14, 283–287. [Google Scholar] [CrossRef]
- Maryon, E.B.; Coronado, R.; Anderson, P. unc-68 encodes a ryanodine receptor involved in regulating C. elegans body-wall muscle contraction. J. Cell Biol. 1996, 134, 885–893. [Google Scholar] [CrossRef]
- Devlin, C.L.; Amole, W.; Anderson, S.; Shea, K. Muscarinic acetylcholine receptor compounds alter net Ca2+ flux and contractility in an invertebrate smooth muscle. Invert. Neurosci. 2003, 5, 9–17. [Google Scholar] [CrossRef]
- Tamashiro, H.; Yoshino, M. Involvement of plasma membrane Ca2+ channels, IP3 receptors, and ryanodine receptors in the generation of spontaneous rhythmic contractions of the cricket lateral oviduct. J. Insect Physiol. 2014, 71, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Ellington, C.P. Power and efficiency of insect flight muscle. J. Exp. Biol. 1985, 115, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, H. Structure, function and evolution of insect flight muscle. Biophysics 2011, 7, 21–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamazawa, T.; Takeshima, H.; Shimuta, M.; Iino, M. A region of the ryanodine receptor critical for excitation-contraction coupling in skeletal muscle. J. Biol. Chem. 1997, 272, 8161–8164. [Google Scholar] [CrossRef] [Green Version]
- Calderón, J.C.; Bolaños, P.; Caputo, C. The excitation-contraction coupling mechanism in skeletal muscle. Biophys. Rev. 2014, 6, 133–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullivan, K.M.; Scott, K.; Zuker, C.S.; Rubin, G.M. The ryanodine receptor is essential for larval development in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2000, 97, 5942–5947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wegener, C.; Nässel, D.R. Peptide-induced Ca(2+) movements in a tonic insect muscle: Effects of proctolin and periviscerokinin-2. J. Neurophysiol. 2000, 84, 3056–3066. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, D.; Adebiyi, A.; Jaggar, J.H. Inositol trisphosphate receptors in smooth muscle cells. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, 2190–2210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bootman, M.D.; Collins, T.J.; Mackenzie, L.; Roderick, H.L.; Berridge, M.J.; Peppiatt, C.M. 2-aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J. 2002, 16, 1145–1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemonnier, L.; Prevarskaya, N.; Mazurier, J.; Shuba, Y.; Skryma, R. 2-APB inhibits volume-regulated anion channels independently from intracellular calcium signaling modulation. FEBS Lett. 2004, 556, 121–126. [Google Scholar] [CrossRef] [Green Version]
- Lange, A.B. Inositol phospholipid hydrolysis may mediate the action of proctolin on insect visceral muscle. Arch. Insect Biochem. Physiol. 1988, 9, 201–209. [Google Scholar] [CrossRef]
- Lange, A.B.; Nykamp, D.A. Signal transduction pathways regulating the contraction of an insect visceral muscle. Arch. Insect Biochem. Physiol. 1996, 33, 183–196. [Google Scholar] [CrossRef]
- Nykamp, D.A.; Lange, A.B. The effects of octopamine are mediated via a G protein in the oviducts of Locusta migratoria. Biog. Amines 1998, 14, 177. [Google Scholar]
- Nykamp, D.A.; Lange, A.B. Interaction between octopamine and proctolin on the oviducts of Locusta migratoria. J. Insect Physiol. 2000, 46, 809–816. [Google Scholar] [CrossRef]
- Lange, A.B. A review of the involvement of proctolin as a cotransmitter and local neurohormone in the oviduct of the locust, Locusta migratoria. Peptides 2002, 23, 2063–2070. [Google Scholar] [CrossRef]
- Hinton, J.M.; Nejad, M.; Issberner, J.P.; Hancock, J.T.; Osborne, R.H. Muscarinic acetylcholine and proctolin receptors in the foregut of the locust Schistocerca gregaria: Role of inositol phosphates, protein kinase C and calcium in second messenger effects. Insect Biochem. Mol. Biol. 1998, 28, 331–343. [Google Scholar] [CrossRef]
- Peron, S.; Zordan, M.A.; Magnabosco, A.; Reggiani, C.; Megighian, A. From action potential to contraction: Neural control and excitation-contraction coupling in larval muscles of Drosophila. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2009, 154, 173–183. [Google Scholar] [CrossRef]
- Banerjee, S.; Lee, J.; Venkatesh, K.; Wu, C.F.; Hasan, G. Loss of flight and associated neuronal rhythmicity in inositol 1,4,5-trisphosphate receptor mutants of Drosophila. J. Neurosci. 2004, 24, 7869–7878. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, T.; Sadaf, S.; Hasan, G.A. Genetic RNAi screen for IP3/Ca2+ coupled GPCRs in Drosophila identifies the PdfR as a regulator of insect flight. PLoS Genet. 2013, 9, e1003849. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, S.; Hasan, G. Spontaneous Ca2+ influx in Drosophila pupal neurons is modulated by IP3-receptor function and influences maturation of the flight circuit. Front. Mol. Neurosci. 2017, 10, 111. [Google Scholar] [CrossRef]
- Banerjee, S.; Hasan, G. The InsP3 receptor: Its role in neuronal physiology and neurodegeneration. Bioessays 2005, 27, 1035–1047. [Google Scholar] [CrossRef]
- Brembs, B.; Christiansen, F.; Pflüger, H.J.; Duch, C. Flight initiation and maintenance deficits in flies with genetically altered biogenic amine levels. J. Neurosci. 2007, 27, 11122–11131. [Google Scholar] [CrossRef]
- Sharma, A.; Hasan, G. Modulation of flight and feeding behaviours requires presynaptic IP3Rs in dopaminergic neurons. Elife 2020, 9, e62297. [Google Scholar] [CrossRef]
- Hoyle, G. Evidence that insect dorsal unpaired medican (DUM) neurons are octopaminergic. J. Exp. Zool. 1975, 193, 425–431. [Google Scholar] [CrossRef]
- Evans, P.D.; O’Shea, M. An octopaminergic neurone modulates neuromuscular transmission in the locust. Nature 1977, 270, 257–259. [Google Scholar] [CrossRef]
- Hoyle, G. Distributions of nerve and muscle fibre types in locust jumping muscle. J. Exp. Biol. 1978, 73, 205–233. [Google Scholar] [CrossRef]
- Evans, P.D.; Siegler, M.V. Octopamine mediated relaxation of maintained and catch tension in locust skeletal muscle. J. Physiol. 1982, 324, 93–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryglewski, S.; Pflueger, H.J.; Duch, C. Expanding the neuron’s calcium signaling repertoire: Intracellular calcium release via voltage-induced PLC and IP3R activation. PLoS Biol. 2007, 5, e66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baines, R.A.; Walther, C.; Hinton, J.M.; Osborne, R.H.; Konopińska, D. Selective activity of a proctolin analogue reveals the existence of two receptor subtypes. J. Neurophysiol. 1996, 75, 2647–2650. [Google Scholar] [CrossRef] [PubMed]
- Abbas, F.; Vinberg, F. Transduction and adaptation mechanisms in the cilium or microvilli of photoreceptors and olfactory receptors from ınsects to humans. Front. Cell Neurosci. 2021, 15, 662453. [Google Scholar] [CrossRef]
- Honkanen, A.; Immonen, E.V.; Salmela, I.; Heimonen, K.; Weckström, M. Insect photoreceptor adaptations to night vision. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160077. [Google Scholar] [CrossRef] [Green Version]
- Sokolinskaya, E.L.; Kolesov, D.V.; Lukyanov, K.A.; Bogdanov, A.M. Molecular principles of insect chemoreception. Acta Nat. 2020, 12, 81–91. [Google Scholar] [CrossRef]
- Paulsen, R.; Schwemer, J. Studies on the insect visual pigment sensitive to ultraviolet light: Retinal as the chromophoric group. Biochim. Biophys. Acta 1972, 283, 520–529. [Google Scholar] [CrossRef]
- Charlton-Perkins, M.; Cook, T.A. Building a fly eye: Terminal differentiation events of the retina, corneal lens, and pigmented epithelia. Curr. Top. Dev. Biol. 2010, 93, 129–173. [Google Scholar] [CrossRef] [Green Version]
- Scott, K.; Zuker, C. TRP, TRPL and trouble in photoreceptor cells. Curr. Opin. Neurobiol. 1998, 8, 383–388. [Google Scholar] [CrossRef]
- Henderson, S.R.; Reuss, H.; Hardie, R.C. Single photon responses in Drosophila photoreceptors and their regulation by Ca2+. J. Physiol. 2000, 524, 179–194. [Google Scholar] [CrossRef]
- Fain, G.L.; Hardie, R.; Laughlin, S.B. Phototransduction and the evolution of photoreceptors. Curr. Biol. 2010, 20, 114–124. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Liu, C.H.; Hughes, S.A.; Postma, M.; Schwiening, C.J.; Hardie, R.C. Activation of TRP channels by protons and phosphoinositide depletion in Drosophila photoreceptors. Curr. Biol. 2010, 20, 189–197. [Google Scholar] [CrossRef] [Green Version]
- Hardie, R.C.; Franze, K. Photomechanical responses in Drosophila photoreceptors. Science 2012, 338, 260–263. [Google Scholar] [CrossRef]
- Acharya, J.K.; Jalink, K.; Hardy, R.W.; Hartenstein, V.; Zuker, C.S. InsP3 receptor is essential for growth and differentiation but not for vision in Drosophila. Neuron 1997, 18, 881–887. [Google Scholar] [CrossRef] [Green Version]
- Raghu, P.; Colley, N.J.; Webel, R.; James, T.; Hasan, G.; Danin, M.; Selinger, Z.; Hardie, R.C. Normal phototransduction in Drosophila photoreceptors lacking an InsP(3) receptor gene. Mol. Cell Neurosci. 2000, 15, 429–445. [Google Scholar] [CrossRef]
- Hardie, R.C.; Raghu, P. Visual transduction in Drosophila. Nature 2001, 413, 186–193. [Google Scholar] [CrossRef]
- Kohn, E.; Katz, B.; Yasin, B.; Peters, M.; Rhodes, E.; Zaguri, R.; Weiss, S.; Minke, B. Functional cooperation between the IP3 receptor and phospholipase C secures the high sensitivity to light of Drosophila photoreceptors in vivo. J. Neurosci. 2015, 35, 2530–2546. [Google Scholar] [CrossRef] [Green Version]
- Bollepalli, M.K.; Kuipers, M.E.; Liu, C.H.; Asteriti, S.; Hardie, R.C. Phototransduction in Drosophila is compromised by Gal4 expression but not by InsP3 receptor knockdown or mutation. eNeuro 2017, 4. [Google Scholar] [CrossRef] [Green Version]
- Baumann, O. Distribution of ryanodine receptor Ca(2+) channels in insect photoreceptor cells. J. Comp. Neurol. 2000, 421, 347–361. [Google Scholar] [CrossRef]
- Menini, A. Calcium signalling and regulation in olfactory neurons. Curr. Opin. Neurobiol. 1999, 9, 419–426. [Google Scholar] [CrossRef]
- Matthews, H.R.; Reisert, J. Calcium, the two-faced messenger of olfactory transduction and adaptation. Curr. Opin. Neurobiol. 2003, 13, 469–475. [Google Scholar] [CrossRef]
- Pézier, A.; Acquistapace, A.; Renou, M.; Rospars, J.P.; Lucas, P. Ca2+ stabilizes the membrane potential of moth olfactory receptor neurons at rest and is essential for their fast repolarization. Chem. Senses 2007, 32, 305–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murmu, M.S.; Stinnakre, J.; Martin, J.R. Presynaptic Ca2+ stores contribute to odor-induced responses in Drosophila olfactory receptor neurons. J. Exp. Biol. 2010, 21, 4163–4173. [Google Scholar] [CrossRef] [Green Version]
- Clyne, P.J.; Certel, S.J.; de Bruyne, M.; Zaslavsky, L.; Johnson, W.A.; Carlson, J.R. The odor specificities of a subset of olfactory receptor neurons are governed by Acj6, a POU-domain transcription factor. Neuron 1999, 22, 339–347. [Google Scholar] [CrossRef] [Green Version]
- Gao, Q.; Chess, A. Identification of candidate Drosophila olfactory receptors from genomic DNA sequence. Genomics 1999, 60, 31–39. [Google Scholar] [CrossRef]
- Vosshall, L.B.; Amrein, H.; Morozov, P.S.; Rzhetsky, A.; Axel, R. A spatial map of olfactory receptor expression in the Drosophila antenna. Cell 1999, 96, 725–736. [Google Scholar] [CrossRef] [Green Version]
- Neuhaus, E.M.; Gisselmann, G.; Zhang, W.; Dooley, R.; Störtkuhl, K.; Hatt, H. Odorant receptor heterodimerization in the olfactory system of Drosophila melanogaster. Nat. Neurosci. 2005, 8, 15–17. [Google Scholar] [CrossRef]
- Sato, K.; Pellegrino, M.; Nakagawa, T.; Nakagawa, T.; Vosshall, L.B.; Touhara, K. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature 2008, 452, 1002–1006. [Google Scholar] [CrossRef]
- Wicher, D.; Schäfer, R.; Bauernfeind, R.; Stensmyr, M.C.; Heller, R.; Heinemann, S.H.; Hansson, B.S. Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature 2008, 452, 1007–1011. [Google Scholar] [CrossRef]
- Vosshall, L.B.; Hansson, B.S. A unified nomenclature system for the insect olfactory coreceptor. Chem. Senses 2011, 36, 497–498. [Google Scholar] [CrossRef] [Green Version]
- Benton, R.; Vannice, K.S.; Gomez-Diaz, C.; Vosshall, L.B. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell 2009, 136, 149–162. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Diaz, C.; Martin, F.; Garcia-Fernandez, J.M.; Alcorta, E. The two main olfactory receptor families in Drosophila, ORs and IRs: A comparative approach. Front. Cell Neurosci. 2018, 12, 253. [Google Scholar] [CrossRef] [PubMed]
- Boekhoff, I.; Seifert, E.; Göggerle, S.; Lindemann, M.; Krüger, B.W.; Breer, H. Pheromone-induced second-messenger signaling in insect antennae. Insect Biochem. Mol. Biol. 1993, 23, 757–762. [Google Scholar] [CrossRef]
- Kain, P.; Chakraborty, T.S.; Sundaram, S.; Siddiqi, O.; Rodrigues, V.; Hasan, G. Reduced odor responses from antennal neurons of G(q)alpha, phospholipase Cbeta, and rdgA mutants in Drosophila support a role for a phospholipid intermediate in insect olfactory transduction. J. Neurosci. 2008, 28, 4745–4755. [Google Scholar] [CrossRef] [Green Version]
- Smart, R.; Kiely, A.; Beale, M.; Vargas, E.; Carraher, C.; Kralicek, A.V.; Christie, D.L.; Chen, C.; Newcomb, R.D.; Warr, C.G. Drosophila odorant receptors are novel seven transmembrane domain proteins that can signal independently of heterotrimeric G proteins. Insect Biochem. Mol. Biol. 2008, 38, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Vosshall, L.B. Controversy and consensus: Noncanonical signaling mechanisms in the insect olfactory system. Curr. Opin. Neurobiol. 2009, 19, 284–292. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, A.; Roman, G.; Hardin, P.E. Go contributes to olfactory reception in Drosophila melanogaster. BMC Physiol. 2009, 9, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Y.; Zhang, W.; Farhat, K.; Oberland, S.; Gisselmann, G.; Neuhaus, E.M. The stimulatory Gα(s) protein is involved in olfactory signal transduction in Drosophila. PLoS ONE 2011, 6, e18605. [Google Scholar] [CrossRef] [Green Version]
- Sargsyan, V.; Getahun, M.N.; Llanos, S.L.; Olsson, S.B.; Hansson, B.S.; Wicher, D. Phosphorylation via PKC regulates the function of the Drosophila odorant co-receptor. Front. Cell Neurosci. 2011, 5, 5. [Google Scholar] [CrossRef] [Green Version]
- Miazzi, F.; Hansson, B.S.; Wicher, D. Odor-induced cAMP production in Drosophila melanogaster olfactory sensory neurons. J. Exp. Biol. 2016, 219, 1798–1803. [Google Scholar] [CrossRef] [Green Version]
- Murmu, M.S.; Martin, J.R. Interaction between cAMP and intracellular Ca(2+)-signaling pathways during odor-perception and adaptation in Drosophila. Biochim. Biophys. Acta. 2016, 1863, 2156–2174. [Google Scholar] [CrossRef]
- Fleischer, J.; Pregitzer, P.; Breer, H.; Krieger, J. Access to the odor world: Olfactory receptors and their role for signal transduction in insects. Cell Mol. Life Sci. 2018, 75, 485–508. [Google Scholar] [CrossRef]
- Murmu, M.S.; Stinnakre, J.; Réal, E.; Martin, J.R. Calcium-stores mediate adaptation in axon terminals of olfactory receptor neurons in Drosophila. BMC Neurosci. 2011, 12, 105. [Google Scholar] [CrossRef] [Green Version]
- Fadool, D.A.; Ache, B.W. Plasma membrane inositol 1,4,5-trisphosphate-activated channels mediate signal transduction in lobster olfactory receptor neurons. Neuron 1992, 9, 907–918. [Google Scholar] [CrossRef] [Green Version]
- Cunningham, A.M.; Ryugo, D.K.; Sharp, A.H.; Reed, R.R.; Snyder, S.H.; Ronnett, G.V. Neuronal inositol 1,4,5-trisphosphate receptor localized to the plasma membrane of olfactory cilia. Neuroscience 1993, 57, 339–352. [Google Scholar] [CrossRef]
- Schild, D.; Restrepo, D. Transduction mechanisms in vertebrate olfactory receptor cells. Physiol. Rev. 1998, 78, 429–466. [Google Scholar] [CrossRef]
- Deshpande, M.; Venkatesh, K.; Rodrigues, V.; Hasan, G. The inositol 1,4,5-trisphosphate receptor is required for maintenance of olfactory adaptation in Drosophila antennae. J. Neurobiol. 2000, 43, 282–288. [Google Scholar] [CrossRef]
- Kurahashi, T.; Menini, A. Mechanism of odorant adaptation in the olfactory receptor cell. Nature 1997, 385, 725–729. [Google Scholar] [CrossRef]
- Devaud, J.M.; Acebes, A.; Ferrús, A. Odor exposure causes central adaptation and morphological changes in selected olfactory glomeruli in Drosophila. J. Neurosci. 2001, 21, 6274–6282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez-Diaz, C.; Martin, F.; Alcorta, E. The cAMP transduction cascade mediates olfactory reception in Drosophila melanogaster. Behav. Genet. 2004, 34, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Stengl, M. Pheromone transduction in moths. Front. Cell Neurosci. 2010, 4, 133. [Google Scholar] [CrossRef] [Green Version]
- Sklar, P.B.; Anholt, R.R.; Snyder, S.H. The odorant-sensitive adenylate cyclase of olfactory receptor cells. Differential stimulation by distinct classes of odorants. J. Biol. Chem. 1986, 261, 15538–15543. [Google Scholar] [CrossRef]
- Venkatesh, K.; Hasan, G. Disruption of the IP3 receptor gene of Drosophila affects larval metamorphosis and ecdysone release. Curr. Biol. 1997, 7, 500–509. [Google Scholar] [CrossRef] [Green Version]
- Lane, M.E.; Kalderon, D. Genetic investigation of cAMP-dependent protein kinase function in Drosophila development. Genes. Dev. 1993, 7, 1229–1243. [Google Scholar] [CrossRef]
- Liu, P.C.; Wang, J.X.; Song, Q.S.; Zhao, X.F. The participation of calponin in the cross talk between 20-hydroxyecdysone and juvenile hormone signaling pathways by phosphorylation variation. PLoS ONE 2011, 6, e19776. [Google Scholar] [CrossRef] [Green Version]
- Jing, Y.P.; Liu, W.; Wang, J.X.; Zhao, X.F. The steroid hormone 20-hydroxyecdysone via nongenomic pathway activates Ca2+/calmodulin-dependent protein kinase II to regulate gene expression. J. Biol. Chem. 2015, 290, 8469–8481. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Pei, X.Y.; Zhao, W.L.; Zhao, X.F. Steroid hormone 20-hydroxyecdysone promotes higher calcium mobilization to induce apoptosis. Cell Calcium 2016, 60, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.B.; Pei, X.Y.; Wang, D.; Chen, C.H.; Cai, M.J.; Wang, J.X.; Zhao, X.F. The steroid hormone 20-hydroxyecdysone upregulates calcium release-activated calcium channel modulator 1 expression to induce apoptosis in the midgut of Helicoverpa armigera. Cell Calcium 2017, 68, 24–33. [Google Scholar] [CrossRef]
- Jayakumar, S.; Richhariya, S.; Reddy, O.V.; Texada, M.J.; Hasan, G. Drosophila larval to pupal switch under nutrient stress requires IP3R/Ca(2+) signalling in glutamatergic interneurons. Elife 2016, 5, e17495. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.; Venkatesh, K.; Srinivas, R.; Nair, S.; Hasan, G. Genetic dissection of itpr gene function reveals a vital requirement in aminergic cells of Drosophila larvae. Genetics 2004, 166, 225–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vermassen, E.; Parys, J.B.; Mauger, J.P. Subcellular distribution of the inositol 1,4,5-trisphosphate receptors: Functional relevance and molecular determinants. Biol. Cell 2004, 96, 3–17. [Google Scholar] [CrossRef]
- Restrepo, S.; Basler, K. Drosophila wing imaginal discs respond to mechanical injury via slow InsP3R-mediated intercellular calcium waves. Nat. Commun. 2016, 7, 12450. [Google Scholar] [CrossRef] [Green Version]
- Sass, M. Autophagy research on insects. Autophagy 2008, 4, 265–267. [Google Scholar] [CrossRef] [Green Version]
- Tettamanti, G.; Saló, E.; González-Estévez, C.; Felix, D.A.; Grimaldi, A.; de Eguileor, M. Autophagy in invertebrates: Insights into development, regeneration and body remodeling. Curr. Pharm. Des. 2008, 14, 116–125. [Google Scholar] [CrossRef]
- Li, Y.B.; Li, X.R.; Yang, T.; Wang, J.X.; Zhao, X.F. The steroid hormone 20-hydroxyecdysone promotes switching from autophagy to apoptosis by increasing intracellular calcium levels. Insect Biochem. Mol. Biol. 2016, 79, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Hall, L.M.; Ren, D.; Feng, G.; Eberl, D.F.; Dubald, M.; Yang, M.; Hannan, F.; Kousky, C.T.; Zheng, W. Calcium channel as a new potential target for insecticides. In Molecular Action of Insecticides on Ion Channels; Clark, J.M., Ed.; ACS Symposium Series; ACS Publications: Washington, DC, USA, 1995; pp. 162–172. [Google Scholar]
- Bloomquist, J.R. Ion channels as targets for insecticides. Annu. Rev. Entomol. 1996, 41, 163–190. [Google Scholar] [CrossRef] [PubMed]
- Lümmen, P. Calcium channels as molecular target sites of novel ınsecticides. Adv. Insect Physiol. 2013, 44, 287–347. [Google Scholar] [CrossRef]
- Ffrench-Constant, R.H.; Williamson, M.S.; Davies, T.G.; Bass, C. Ion channels as insecticide targets. J. Neurogenet. 2016, 30, 163–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nauen, R. Insecticide mode of action: Return of the ryanodine receptor. Pest Manag. Sci. 2006, 62, 690–692. [Google Scholar] [CrossRef]
- Jenden, D.J.; Fairhurst, A.S. The pharmacology of ryanodine. Pharmacol. Rev. 1969, 21, 1–25. [Google Scholar]
- Folkers, K.; Rogers, E.; Heal, R.E. Ryania Insecticides. U.S. Patent 2,400,295, 1946. [Google Scholar]
- Rogers, E.F.; Konıuszy, F.R. Plant insecticides; ryanodine, a new alkaloid from Ryania speciosa Vahl. J. Am. Chem. Soc. 1948, 70, 3086–3088. [Google Scholar] [CrossRef]
- Sparks, T.C.; Nauen, R. IRAC: Mode of action classification and insecticide resistance management. Pestic. Biochem. Physiol. 2015, 121, 122–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lahm, G.P.; Selby, T.P.; Freudenberger, J.H.; Stevenson, T.M.; Myers, B.J.; Seburyamo, G.; Smith, B.K.; Flexner, L.; Clark, C.E.; Cordova, D. Anthranilic diamides: A new class of potent ryanodine receptor activators. Bioorg. Med. Chem. Lett. 2005, 15, 4898–4906. [Google Scholar] [CrossRef] [PubMed]
- Sattelle, D.B.; Cordova, D.; Cheek, T.R. Insect ryanodine receptors: Molecular targets for novel pest control chemicals. Invert. Neurosci. 2008, 8, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Jeanguenat, A. The story of a new insecticidal chemistry class: The diamides. Pest Manag. Sci. 2013, 69, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Tohnishi, M.; Nakao, H.; Furuya, T.; Seo, A.; Kodama, H.; Tsubata, K.; Fujioka, S.; Kodama, H.; Hirooka, T.; Nishimatsu, T. Flubendiamide, a novel insecticide highly active against lepidopterous insect pests. J. Pestic. Sci. 2005, 30, 354–360. [Google Scholar] [CrossRef] [Green Version]
- Masaki, T.; Yasokawa, N.; Tohnishi, M.; Nishimatsu, T.; Tsubata, K.; Inoue, K.; Motoba, K.; Hirooka, T. Flubendiamide, a novel Ca2+ channel modulator, reveals evidence for functional cooperation between Ca2+ pumps and Ca2+ release. Mol. Pharmacol. 2006, 69, 1733–1739. [Google Scholar] [CrossRef]
- Hirooka, T.; Nishimatsu, T.; Kodama, H.; Reckmann, U.; Nauen, R. The biological profile of flubendiamide, a new benzenedicarboxamide insecticide. Pflanzenschutz Nachr. Bayer 2007, 60, 183–202. [Google Scholar]
- Lahm, G.P.; Stevenson, T.M.; Selby, T.P.; Freudenberger, J.H.; Dubas, C.M.; Smith, B.K.; Cordova, D.; Flexner, L.; Clark, C.E.; Bellin, C.A.; et al. RynaxypyrTM: A new anthranilic diamide insecticide acting at the ryanodine receptor. In Pesticide Chemistry: Crop Protection, Public Health, Environmental Safety; Ohkawa, H., Miyagawa, H., Lee, P.W., Eds.; Wiley-VCH: Weinheim, Germany, 2007; pp. 111–120. [Google Scholar] [CrossRef]
- Lahm, G.P.; Cordova, D.; Barry, J.D. New and selective ryanodine receptor activators for insect control. Bioorg. Med. Chem. 2009, 17, 4127–4133. [Google Scholar] [CrossRef]
- Cordova, D.; Benner, E.A.; Clark, D.A.; Bolgunas, S.P.; Lahm, G.P.; Gutteridge, S.; Rhoades, D.F.; Wu, L.; Sopa, J.S.; Rauh, J.J.; et al. Pyrrole-2 carboxamides—A novel class of insect ryanodine receptor activators. Pestic. Biochem. Physiol. 2021, 174, 104798. [Google Scholar] [CrossRef]
- Cameron, R.A.; Williams, C.J.; Portillo, H.E.; Marçon, P.C.; Teixeira, L.A. Systemic application of chlorantraniliprole to cabbage transplants for control of foliar-feeding lepidopteran pests. Crop Prot. 2015, 67, 13–19. [Google Scholar] [CrossRef]
- Foster, S.P.; Denholm, I.; Rison, J.L.; Portillo, H.E.; Margaritopoulis, J.; Slater, R. Susceptibility of standard clones and European field populations of the green peach aphid, Myzus persicae, and the cotton aphid, Aphis gossypii (Hemiptera: Aphididae), to the novel anthranilic diamide insecticide cyantraniliprole. Pest Manag. Sci. 2012, 68, 629–633. [Google Scholar] [CrossRef]
- Selby, T.P.; Lahm, G.P.; Stevenson, T.M.; Hughes, K.A.; Cordova, D.; Annan, I.B.; Barry, J.D.; Benner, E.A.; Currie, M.J.; Pahutski, T.F. Discovery of cyantraniliprole, a potent and selective anthranilic diamide ryanodine receptor activator with cross-spectrum insecticidal activity. Bioorg. Med. Chem. Lett. 2013, 23, 6341–6345. [Google Scholar] [CrossRef] [PubMed]
- Barry, J.D.; Portillo, H.E.; Annan, I.B.; Cameron, R.A.; Clagg, D.G.; Dietrich, R.F.; Watson, L.J.; Leighty, R.M.; Ryan, D.L.; McMillan, J.A.; et al. Movement of cyantraniliprole in plants after foliar applications and its impact on the control of sucking and chewing insects. Pest Manag. Sci. 2015, 71, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Grávalos, C.; Fernández, E.; Belando, A.; Moreno, I.; Ros, C.; Bielza, P. Cross-resistance and baseline susceptibility of Mediterranean strains of Bemisia tabaci to cyantraniliprole. Pest Manag. Sci. 2015, 71, 1030–1036. [Google Scholar] [CrossRef]
- Sparks, T.C.; Crossthwaite, A.J.; Nauen, R.; Banba, S.; Cordova, D.; Earley, F.; Ebbinghaus-Kintscher, U.; Fujioka, S.; Hirao, A.; Karmon, D. Insecticides, biologics and nematicides: Updates to IRAC’s mode of action classification—A tool for resistance management. Pestic. Biochem. Physiol. 2020, 167, 104587. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, L.; Qi, S.; Zhang, H.; Liu, K.; Liu, R.; Liang, P.; Casida, J.E.; Liu, S. Fluorescent probes for insect ryanodine receptors: Candidate anthranilic diamides. Molecules 2014, 19, 4105–4114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samurkas, A.; Fan, X.; Ma, D.; Sundarraj, R.; Lin, L.; Yao, L.; Ma, R.; Jiang, H.; Cao, P.; Gao, Q.; et al. Discovery of potential species-specific green insecticides targeting the lepidopteran ryanodine receptor. J. Agric. Food Chem. 2020, 68, 4528–4537. [Google Scholar] [CrossRef] [PubMed]
- Kadala, A.; Charretona, M.; Colleta, C. Flubendiamide, the first phthalic acid diamide insecticide, impairs neuronal calcium signalling in the honey bee’s antennae. J. Insect Physiol. 2020, 125, 104086. [Google Scholar] [CrossRef]
- Masaki, T.; Yasokawa, N.; Ebbinghaus-Kintscher, U.; Luemmen, P. Flubendiamide stimulates Ca2+ pump activity coupled to RyR-mediated calcium release in lepidopterous insects. In Pesticide Chemistry; Ohkawa, H., Miyagawa, H., Lee, P.W., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007. [Google Scholar] [CrossRef]
- Tao, Y.; Gutteridge, S.; Benner, E.A.; Wu, L.; Rhoades, D.F.; Sacher, M.D.; Rivera, M.A.; Desaeger, J.; Cordova, D. Identification of a critical region in the Drosophila ryanodine receptor that confers sensitivity to diamide insecticides. Insect Biochem. Mol. Biol. 2013, 43, 820–828. [Google Scholar] [CrossRef]
- Chen, S.R.; Li, P.; Zhao, M.; Li, X.; Zhang, L. Role of the proposed pore-forming segment of the Ca2+ release channel (ryanodine receptor) in ryanodine interaction. Biophys. J. 2002, 82, 2436–2447. [Google Scholar] [CrossRef] [Green Version]
- Steinbach, D.; Gutbrod, O.; Lümmen, P.; Matthiesen, S.; Schorn, C.; Nauen, R. Geographic spread, genetics and functional characteristics of ryanodine receptor based target-site resistance to diamide insecticides in diamondback moth, Plutella xylostella. Insect Biochem. Mol. Biol. 2015, 63, 14–22. [Google Scholar] [CrossRef]
- Casida, J.E. Radioligand recognition of insecticide targets. J. Agric. Food. Chem. 2018, 66, 3277–3290. [Google Scholar] [CrossRef]
- Isaacs, A.K.; Qi, S.; Sarpong, R.; Casida, J.E. Insect ryanodine receptor: Distinct but coupled insecticide binding sites for [N-C(3)H(3)]chlorantraniliprole, flubendiamide, and [(3)H]ryanodine. Chem. Res. Toxicol. 2012, 25, 1571–1573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, R.; Haji-ghassemi, O.; Ma, D.; Jiang, H.; Lin, L.; Yao, L.; Samurkas, A.; Li, Y.; Wang, Y.; Cao, P.; et al. Structural basis for diamide modulation of ryanodine receptor. Nat. Chem. Biol. 2020, 16, 1246–1254. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Wang, Y.; Zhou, X.; Li, Z.; Liu, S.; Pei, L.; Gao, X. Functional analysis of a point mutation in the ryanodine receptor of Plutella xylostella (L.) associated with resistance to chlorantraniliprole. Pest Manag. Sci 2014, 70, 1083–1089. [Google Scholar] [CrossRef] [PubMed]
- Nauen, R.; Steinbach, D. Resistance to Diamide Insecticides in Lepidopteran Pests. In Advances in Insect Control and Resistance Management; Horowitz, A.R., Ishaaya, I., Eds.; Springer International Publishing: Cham, Switzerland, 2016; p. 219. [Google Scholar] [CrossRef]
- Richardson, E.B.; Troczka, B.J.; Gutbrod, O.; Emyr Davies, T.G.; Nauen, R. Diamide resistance: 10 years of lessons from lepidopteran pests. J. Pest Sci. 2020, 93, 911–928. [Google Scholar] [CrossRef] [Green Version]
- Uchiyama, T.; Ozawa, A. Rapid development of resistance to diamide insecticides in the smaller tea tortrix, Adoxophyes honmai (Lepidoptera: Tortricidae), in the tea fields of Shizuoka Prefecture, Japan. Appl. Entomol. Zool. 2014, 49, 529–534. [Google Scholar] [CrossRef]
- Sial, A.A.; Brunner, J.F.; Doerr, M.D. Susceptibility of Choristoneura rosaceana (Lepidoptera: Tortricidae) to two new reduced-risk insecticides. J. Econ. Entomol. 2010, 103, 140–146. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Shen, A.; Wu, Y. Baseline susceptibility of the diamondback moth (Lepidoptera: Plutellidae) to chlorantraniliprole in China. J. Econ. Entomol. 2010, 103, 843–848. [Google Scholar] [CrossRef]
- Su, J.; Lai, T.; Li, J. Susceptibility of field populations of Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae) in China to chlorantraniliprole and the activities of detoxification enzymes. Crop Prot. 2012, 42, 217–222. [Google Scholar] [CrossRef]
- Lai, T.C.; Li, J.; Su, J.Y. Monitoring of beet armyworm Spodoptera exigua (Lepidoptera: Noctuidae) resistance to chlorantraniliprole in China. Pestic. Biochem. Physiol. 2011, 101, 198–205. [Google Scholar] [CrossRef]
- Che, W.; Shi, T.; Wu, Y.; Yang, Y. Insecticide resistance status of field populations of Spodoptera exigua (Lepidoptera: Noctuidae) from China. J. Econ. Entomol. 2013, 106, 1855–1862. [Google Scholar] [CrossRef]
- Li, X.; Degain, B.A.; Harpold, V.S.; Marçon, P.G.; Nichols, R.L.; Fournier, A.J.; Naranjo, S.E.; Palumbo, J.C.; Ellsworth, P.C. Baseline susceptibilities of B- and Q-biotype Bemisia tabaci to anthranilic diamides in Arizona. Pest Manag. Sci. 2012, 68, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Sukonthabhirom, S.; Dumrongsak, D.; Jumroon, S.; Saroch, T.; Chaweng, A.; Tanaka, T. Update on DBM diamide resistance from Thailand: Causal factors and learnings. In Proceedings of the Sixth International Workshop on Management of the Diamondback Moth and Other Crucifer Insect Pests, Nakhon Pathom, Thailand, 21–25 March 2011; Srinivasan, R., Shelton, A.M., Collins, H.L., Eds.; AVRDC-The World Vegetable Center: Tailem, Taiwan, 2011; pp. 202–212. [Google Scholar]
- Guo, L.; Liang, P.; Zhou, X.; Gao, X. Novel mutations and mutation combinations of ryanodine receptor in a chlorantraniliprole resistant population of Plutella xylostella (L.). Sci. Rep. 2014, 4, 6924. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wu, Y. High levels of resistance to chlorantraniliprole evolved in field populations of Plutella xylostella. J. Econ. Entomol. 2012, 105, 1019–1023. [Google Scholar] [CrossRef]
- Su, J.; Zhang, Z.; Wu, M.; Gao, C. Geographic susceptibility of Chilo suppressalis Walker (Lepidoptera: Crambidae), to chlorantraniliprole in China. Pest Manag. Sci. 2014, 70, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Troczka, B.; Zimmer, C.T.; Elias, J.; Schorn, C.; Bass, C.; Davies, T.G.E.; Field, L.M.; Williamson, M.S.; Slater, R.; Nauen, R. Resistance to diamide insecticides in diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae) is associated with a mutation in the membrane-spanning domain of the ryanodine receptor. Insect Biochem. Mol. Biol. 2012, 42, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Yao, R.; Zhang, Z.; Wu, M.; Zhang, S.; Su, J. Susceptibility baseline and chlorantraniliprole resistance monitoring in Chilo suppressalis (Lepidoptera: Pyralidae). J. Econ. Entomol. 2013, 106, 2190–2194. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Yan, H.H.; Gao, L.; Guo, Y.Y.; Xue, C.B. Chlorantraniliprole resistance in the diamondback moth (Lepidoptera: Plutellidae). J. Econ. Entomol. 2014, 107, 806–814. [Google Scholar] [CrossRef]
- He, Y.; Zhang, J.; Chen, J. Effect of synergists on susceptibility to chlorantraniliprole in field populations of Chilo suppressalis (Lepidoptera: Pyralidae). J. Econ. Entomol. 2014, 107, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, L.M.; Wanderley-Teixeira, V.; Ferreira, H.N.; Teixeira, A.A.; Siqueira, H.A. Fitness costs associated with field-evolved resistance to chlorantraniliprole in Plutella xylostella (Lepidoptera: Plutellidae). Bull. Entomol. Res. 2014, 104, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.H.; Xue, C.B.; Li, G.Y.; Zhao, X.L.; Che, X.Z.; Wang, L.L. Flubendiamide resistance and Bi-PASA detection of ryanodine receptor G4946E mutation in the diamondback moth (Plutella xylostella L.). Pestic. Biochem. Physiol. 2014, 115, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zhang, S.; Yao, R.; Wu, S.; Su, J.; Gao, C. Susceptibility of the rice stem borer, Chilo suppressalis (Lepidoptera: Crambidae), to flubendiamide in China. J. Econ. Entomol. 2014, 107, 1250–1255. [Google Scholar] [CrossRef] [PubMed]
- Roditakis, E.; Vasakis, E.; Grispou, M.; Stavrakaki, M.; Nauen, R.; Gravouil, M.; Bassi, A. First report of Tuta absoluta resistance to diamide insecticides. J. Pest Sci. 2015, 88, 9–16. [Google Scholar] [CrossRef]
- Sang, S.; Shu, B.; Yi, X.; Liu, J.; Hu, M.; Zhong, G. Cross-resistance and baseline susceptibility of Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae) to cyantraniliprole in the south of China. Pest Manag. Sci. 2016, 72, 922–928. [Google Scholar] [CrossRef]
- Silva, J.E.; Assis, C.P.; Ribeiro, L.M.; Siqueira, H.A. Field-Evolved Resistance and Cross-resistance of Brazilian Tuta absoluta (Lepidoptera: Gelechiidae) populations to diamide insecticides. J. Econ. Entomol. 2016, 109, 2190–2195. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, G.; Zhong, L.; Zhang, F.; Bai, Q.; Zheng, X.; Lu, Z. Resistance monitoring of Chilo suppressalis (Walker) (Lepidoptera: Crambidae) to chlorantraniliprole in eight field populations from East and Central China. Crop Prot. 2017, 100, 196–202. [Google Scholar] [CrossRef]
- Ribeiro, L.M.S.; Siqueira, H.A.A.; Wanderley-Teixeira, V.; Ferreira, H.N.; Silva, W.M.; Silva, J.E.; Teixeira, A.A.C. Field resistance of Brazilian Plutella xylostella to diamides is not metabolism-mediated. Crop Prot. 2017, 93, 82–88. [Google Scholar] [CrossRef]
- Roditakis, E.; Mavridis, K.; Riga, M.; Vasakis, E.; Morou, E.; Rison, J.L.; Vontas, J. Identification and detection of indoxacarb resistance mutations in the para sodium channel of the tomato leafminer, Tuta absoluta. Pest Manag. Sci. 2017, 73, 1679–1688. [Google Scholar] [CrossRef]
- Yao, R.; Zhao, D.D.; Zhang, S.; Zhou, L.Q.; Wang, X.; Gao, C.F.; Wu, S.F. Monitoring and mechanisms of insecticide resistance in Chilo suppressalis (Lepidoptera: Crambidae), with special reference to diamides. Pest Manag. Sci. 2017, 73, 1169–1178. [Google Scholar] [CrossRef]
- Cho, S.R.; Kyung, Y.; Shin, S.; Kang, W.J.; Jung, D.H.; Lee, S.J.; Park, G.H.; Kim, S.I.I.; Cho, S.W.; Kim, H.K.; et al. Susceptibility of field populations of Plutella xylostella and Spodoptera exigua to four diamide insecticides. J. Appl. Entomol. 2018, 57, 43–50. [Google Scholar] [CrossRef]
- Gutiérrez-Moreno, R.; Mota-Sanchez, D.; Blanco, C.A.; Whalon, M.E.; Terán-Santofimio, H.; Rodriguez-Maciel, J.C.; DiFonzo, C. Field-evolved resistance of the fall armyworm (Lepidoptera: Noctuidae) to synthetic insecticides in Puerto Rico and Mexico. J. Econ. Entomol. 2019, 112, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, J.; Che, W.; Sun, Y.; Li, W.; Luo, C. Characterization of field-evolved resistance to cyantraniliprole in Bemisia tabaci MED from China. J. Integr. Agric. 2019, 18, 2571–2578. [Google Scholar] [CrossRef]
- Zuo, Y.Y.; Ma, H.H.; Lu, W.J.; Wang, X.L.; Wu, S.W.; Nauen, R.; Wu, Y.D.; Yang, Y.H. Identification of the ryanodine receptor mutation I4743M and its contribution to diamide insecticide resistance in Spodoptera exigua (Lepidoptera: Noctuidae). Insect Sci. 2020, 4, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.M.; Zhao, Y.X.; Sun, H.; Ni, H.; Liu, C.; Wang, X.; Gao, C.F.; Wu, S.F. Monitoring and mechanisms of insecticide resistance in Spodoptera exigua (Lepidoptera: Noctuidae), with special reference to diamides. Pestic. Biochem. Physiol. 2021, 174, 104831. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Nam, H.Y.; Kwon, M.; Choi, J.H.; Cho, S.R.; Kim, G.H. Development of a diamide resistance diagnostic method using LAMP based on a resistance-specific indel in ryanodine receptors for Spodoptera exigua (Lepidoptera: Noctuidae). bioRxiv 2020. [Google Scholar] [CrossRef]
- Wang, P.; Yang, F.; Wang, Y.; Zhou, L.L.; Luo, H.B.; Zhang, S.; Si, S.Y. Monitoring the resistance of the beet armyworm (Lepidoptera: Noctuidae) to four insecticides in Southern China from 2014 to 2018. J. Econ. Entomol. 2021, 114, 332–338. [Google Scholar] [CrossRef]
- Zhang, S.K.; Ren, X.B.; Wang, Y.C.; Su, J. Resistance in Cnaphalocrocis medinalis (Lepidoptera: Pyralidae) to new chemistry insecticides. J. Econ. Entomol. 2014, 107, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Owen, L.N.; Catchot, A.L.; Musser, F.R.; Gore, J.; Cook, D.C.; Jackson, R. Susceptibility of Chrysodeixis includens (Lepidoptera: Noctuidae) to reduced-risk insecticides. Fla. Entomol. 2013, 96, 554–559. [Google Scholar] [CrossRef]
- Zhang, R.; He, S.; Chen, J. Monitoring of Bactrocera dorsalis (Diptera: Tephritidae) resistance to cyantraniliprole in the south of China. J. Econ. Entomol. 2014, 107, 1233–1238. [Google Scholar] [CrossRef]
- Liu, X.; Ning, Y.; Wang, H.; Wang, K. Cross-resistance, mode of inheritance, synergism, and fitness effects of cyantraniliprole resistance in Plutella xylostella. Entomol. Exp. Appl. 2015, 157, 271–278. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X.; Shen, J.; Mao, K.; You, H.; Li, J. Susceptibility of field populations of the diamondback moth, Plutella xylostella, to a selection of insecticides in Central China. Pestic. Biochem. Physiol. 2016, 132, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.Y.; Wang, H.; Xu, Y.; Huang, J.; Wu, S.; Wu, Y.; Yang, Y. CRISPR/Cas9 mediated G4946E substitution in the ryanodine receptor of Spodoptera exigua confers high levels of resistance to diamide insecticides. Insect Biochem. Mol. Biol. 2017, 89, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Bolzan, A.; Padovez, F.E.; Nascimento, A.R.; Kaiser, I.S.; Lira, E.C.; Amaral, F.S.; Kanno, R.H.; Malaquias, J.B.; Omoto, C. Selection and characterization of the inheritance of resistance of Spodoptera frugiperda (Lepidoptera: Noctuidae) to chlorantraniliprole and cross-resistance to other diamide insecticides. Pest Manag. Sci. 2019, 75, 2682–2689. [Google Scholar] [CrossRef]
- Teixeira, L.A.; Andaloro, J.T. Diamide insecticides: Global efforts to address insect resistance stewardship challenges. Pestic. Biochem. Physiol. 2013, 106, 76–78. [Google Scholar] [CrossRef]
- Douris, V.; Papapostolou, K.M.; Ilias, A.; Roditakis, E.; Kounadi, S.; Riga, M.; Nauen, R.; Vontas, J. Investigation of the contribution of RyR target-site mutations in diamide resistance by CRISPR/Cas9 genome modification in Drosophila. Insect Biochem. Mol. Biol. 2017, 87, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Douris, V.; Denecke, S.; Van Leeuwen, T.; Bass, C.; Nauen, R.; Vontas, J. Using CRISPR/Cas9 genome modification to understand the genetic basis of insecticide resistance: Drosophila and beyond. Pestic. Biochem. Physiol. 2020, 167, 104595. [Google Scholar] [CrossRef]
- Jiang, W.H.; Lu, W.P.; Guo, W.C.; Xia, Z.H.; Fu, W.J.; Li, G.Q. Chlorantraniliprole susceptibility in Leptinotarsa decemlineata in the north Xinjiang Uygur autonomous region in China. J. Econ. Entomol. 2012, 105, 549–554. [Google Scholar] [CrossRef] [Green Version]
- Sial, A.A.; Brunner, J.F.; Garczynski, S.F. Biochemical characterization of chlorantraniliprole and spinetoram resistance in laboratory-selected obliquebanded leafroller, Choristoneura rosaceana (Harris) (Lepidoptera: Tortricidae). Pestic. Biochem. Physiol. 2011, 99, 274–279. [Google Scholar] [CrossRef]
- Liu, X.; Wang, H.Y.; Ning, Y.B.; Qiao, K.; Wang, K.Y. Resistance selection and characterization of chlorantraniliprole resistance in Plutella xylostella (Lepidoptera: Plutellidae). J. Econ. Entomol. 2015, 108, 1978–1985. [Google Scholar] [CrossRef]
- Lin, Q.; Jin, F.; Hu, Z.; Chen, H.; Yin, F.; Li, Z.; Dong, X.; Zhang, D.; Ren, S.; Feng, X. Transcriptome analysis of chlorantraniliprole resistance development in the diamondback moth Plutella xylostella. PLoS ONE 2013, 8, e72314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Z.; Lin, Q.; Chen, H.; Li, Z.; Yin, F.; Feng, X. Identification of a novel cytochrome P450 gene, CYP321E1 from the diamondback moth, Plutella xylostella (L.) and RNA interference to evaluate its role in chlorantraniliprole resistance. Bull. Entomol. Res. 2014, 104, 716–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Li, R.; Zhu, B.; Gao, X.; Liang, P. Overexpression of cytochrome P450 CYP6BG1 may contribute to chlorantraniliprole resistance in Plutella xylostella (L.). Pest Manag. Sci. 2018, 74, 1386–1393. [Google Scholar] [CrossRef]
- Wang, J.D.; Chen, L.F.; Wang, Y.R.; Fu, H.Y.; Ali, A.; Xiao, D.; Wang, R.; Gao, S.J. Silence of ryanodine receptor gene decreases susceptibility to chlorantraniliprole in the oriental armyworm, Mythimna separata Walker. Pestic. Biochem. Physiol. 2018, 148, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.Y.; Huang, J.L.; Wang, J.; Feng, Y.; Han, T.T.; Wu, Y.D.; Yang, Y.H. Knockout of a P-glycoprotein gene increases susceptibility to abamectin and emamectin benzoate in Spodoptera exigua. Insect Mol. Biol. 2018, 27, 36–45. [Google Scholar] [CrossRef]
- Meng, X.; Yang, X.; Wu, Z.; Shen, Q.; Miao, L.; Zheng, Y.; Qian, K.; Wang, J. Identification and transcriptional response of ATP-binding cassette transporters to chlorantraniliprole in the rice striped stem borer, Chilo suppressalis. Pest Manag. Sci. 2020, 76, 3626–3635. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, L.; Zhou, X.; Gao, X.; Liang, P. miRNAs regulated overexpression of ryanodine receptor is involved in chlorantraniliprole resistance in Plutella xylostella (L.). Sci. Rep. 2015, 5, 14095. [Google Scholar] [CrossRef] [Green Version]
- Ma, S.; Liu, J.; Lu, X.; Zhang, X.; Ma, Z. Effect of wilforine on the calcium signaling pathway in Mythimna separata Walker myocytes using the calcium imaging technique. J. Agric. Food Chem. 2019, 67, 13751–13757. [Google Scholar] [CrossRef]
- Ma, Z.; Li, Y.; Wu, L.; Zhang, X. Isolation and insecticidal activity of sesquiterpenes alkaloids from Tripterygium wilfordii Hook f. Ind. Crops Prod. 2014, 52, 642–648. [Google Scholar] [CrossRef]
- Li, Y.; Lian, X.; Wan, Y.; Wang, D.; Chen, W.; Di, F.; Wu, W.; Li, Z. Modulation of the Ca(2+) signaling pathway by celangulin I in the central neurons of Spodoptera exigua. Pestic. Biochem. Physiol. 2016, 127, 76–81. [Google Scholar] [CrossRef]
- Lapied, B.; Pennetier, C.; Apaire-Marchais, V.; Licznar, P.; Corbel, V. Innovative applications for insect viruses: Towards insecticide sensitization. Trends Biotechnol. 2009, 27, 190–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Licznar, P.; List, O.; Goven, D.; Nna, R.N.; Lapied, B.; Apaire-Marchais, V. A novel method using Autographa californica multiple nucleopolyhedrovirus for increasing the sensitivity of insecticide through calcium influx in insect cell line. J. Virol. Methods. 2014, 195, 72–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abd-Ella, A.; Stankiewicz, M.; Mikulska, K.; Nowak, W.; Pennetier, C.; Goulu, M.; Fruchart-Gaillard, C.; Licznar, P.; Apaire-Marchais, V.; List, O.; et al. The Repellent DEET potentiates carbamate effects via insect muscarinic receptor ınteractions: An alternative strategy to control insect vector-borne diseases. PLoS ONE 2015, 10, e0126406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apaire-Marchais, V.; Ogliastro, M.; Chandre, F.; Pennetier, C.; Raymond, V.; Lapied, B. Virus and calcium: An unexpected tandem to optimize insecticide efficacy. Environ. Microbiol. Rep. 2016, 8, 168–178. [Google Scholar] [CrossRef] [Green Version]
- Deshayes, C.; Moreau, E.; Pitti-Caballero, J.; Froger, J.A.; Apaire-Marchais, V.; Lapied, B. Synergistic agent and intracellular calcium, a successful partnership in the optimization of insecticide efficacy. Curr. Opin. Insect Sci. 2018, 30, 52–58. [Google Scholar] [CrossRef]
- Monette, R.; Potvin, L.; Baines, D.; Laprade, R.; Schwartz, J.L. Interaction between calcium ions and Bacillus thuringiensis toxin activity against Sf9 cells (Spodoptera frugiperda, Lepidoptera). Appl. Environ. Microbiol. 1997, 63, 440–447. [Google Scholar] [CrossRef] [Green Version]
- Toprak, U.; Bayram, S.; Gürkan, O.M. Comparative biological activities of a plaque-purified variant and a Turkish native isolate of SpliNPV-B against Spodoptera littoralis (Lepidoptera: Noctuidae). Pest Manag. Sci. 2006, 62, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Prabu, S.; Shabbir, M.Z.; Wang, Z.; He, K. Analysis of Cry1Ah toxin-binding reliability to midgut membrane proteins of the Asian corn borer. Toxins 2020, 12, 418. [Google Scholar] [CrossRef]
- Erlandson, M.; Toprak, U.; Hegedus, D.D. Role of the peritrophic matrix in insect-pathogen interactions. J. Insect Physiol. 2019, 117, 103894. [Google Scholar] [CrossRef]
- Güney, G.; Cedden, D.; Hänniger, S.; Heckel, D.G.; Coutu, C.; Hegedus, D.D.; Amutkan Mutlu, D.; Suludere, Z.; Sezen, K.; Güney, E.; et al. Silencing of an ABC transporter, but not a cadherin, decreases the susceptibility of Colorado potato beetle larvae to Bacillus thuringiensis ssp. tenebrionis Cry3Aa toxin. Arch. Insect Biochem. Physiol. 2021, in press. [Google Scholar] [CrossRef]
- Toprak, U.; Baldwin, D.; Erlandson, M.; Gillott, C.; Harris, S.; Hegedus, D.D. In vitro and in vivo application of RNA interference for targeting genes involved in peritrophic matrix synthesis in a lepidopteran system. Insect Sci. 2013, 20, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wan, P.; Lai, F.; Zhu, T.; Fu, Q. Double-stranded RNA targeting calmodulin reveals a potential target for pest management of Nilaparvata lugens. Pest Manag. Sci. 2018, 74, 1711–1719. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, S.J.; Reeves, P.T.; Hoang, B.T.; Mitter, N. A perspective on RNAi-based biopesticides. Front. Plant Sci. 2020, 11, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Receptor | Mammalians | Insects | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| # of Genes | Basic Structure | Primary Exp. Site | Phosphoryl. Status | CaM Binding | Alternative Splicing | Function | # of Genes | Basic Structure | Primary Exp. Site | Phosphory Status | CaM Binding | Alternative Splicing | Function | |
| RyRs | 3 | N-terminal domain including the A, B, and C subdomains, MIR, RIH, RIHA. SPRY and RyR domains, C-terminal regions with transmembrane domains and EF-hands. |
|
| + | + |
| 1 | N-terminal region including MIR, RIH, three SPRY, RyR repeat, RIHA domains, and a carboxy-terminal region including transmembrane domains and calcium-binding EF-hand domains. |
|
| Putative binding sites are present. | + |
|
| IP3Rs | 3 | N-terminal domain including the suppressor (inhibitory) domain (SD) and IP3-binding core β (IBC-β), α (IBC-α) with MIR domain; central modulatory domain including RIH and RIHA domains, C-terminal region with transmembrane domains. |
|
| + | + |
| 1 | N-terminal domain including MIR domains, a regulatory and transducing region with RIH and RIHA domains, and a carboxy-terminal region including transmembrane domains. |
|
| ? | + |
|
| Insecticide | LC50 (95%) mg/L or μg/mL | RR# | Year | Pest | Population | Country | Reference |
|---|---|---|---|---|---|---|---|
| Flubendiamide | 0.16 (0.04–0.8) | 1 | 2009 | Plutella xylostella | Tub Berg (field susceptible) | Thailand | [372] |
| 770.8 (123.3–26,336.8) | 4817 | 2011 | Plutella xylostella | Tha Muang | Thailand | [372] | |
| 10.6 (3.8–22.8) | 66 | 2010 | Plutella xylostella | Sai Noi | Thailand | [372] | |
| 65.1 (2.7–157.4) | 407 | 2011 | Plutella xylostella | Sai Noi | Thailand | [372] | |
| 4256.6 (2690.1–9373.2) | 26,603 | 2011 | Plutella xylostella | Lat Lum Kaew | Thailand | [372] | |
| 0.08 (0.06–0.11) | 1 | 2011 | Plutella xylostella | Chiang Mai (field susceptible) | Thailand | [376] | |
| >60 | >750 | 2011 | Plutella xylostella | Bang Bua Thong | Thailand | [376] | |
| >200 | >1300 | 2011 | Plutella xylostella | Sudlon, Cebu Island | Phillippines | [376] | |
| 0.11 (0.08–0.16) | 1 | 2011 | Plutella xylostella | Roth (lab susceptible) | China | [130] | |
| 1.68 (1.14–2.35) | 15 | 2011 | Plutella xylostella | Panyu, Guangdong F3 | China | [130] | |
| 1.92 (1.19–2.78) | 17 | 2011 | Plutella xylostella | Zhuhai, Guangdong | China | [130] | |
| 88.5 (66.1–115) | 805 | 2011 | Plutella xylostella | Zengcheng, Guangdong | China | [130] | |
| 0.9 (0.4–1.4) *** | 1 | 2007 | Plutella xylostella | Susceptible strain | China | [381] | |
| 22.2 (9.3–35.4) *** | 24 | Plutella xylostella | BY, BaiYun Int. Airport, Guangdong | China | [381] | ||
| 1639 (1016–2227) *** | 1779 | Plutella xylostella | ZC, ZengChengi Guangdong | China | [381] | ||
| 0.029 (0.026–0.033) | 1 | 2011 | Plutella xylostella | BCS-S (lab susceptible) | Phillippines | [358] | |
| >1000 | >10,000 | 2011 | Plutella xylostella | Sudlon, Cebu Island | Phillippines | [358] | |
| 0.05 (0.03–0.10) | 1 | 2017 | Plutella xylostella | Susceptible strain | Korea | [390] | |
| 9.6 (2.8–19.4) | 192 | 2017 | Plutella xylostella | PC, Pyeongchang | Korea | [390] | |
| 1.3 (0.6–2.9) | 27 | 2017 | Plutella xylostella | GN, Gangneung | Korea | [390] | |
| 0.008 (0.005–0.011) | 1 | 1998 | Plutella xylostella | RCF-Lab, Recife | Brazil | [387] | |
| 23.0 (7.2–270.1) | 2893 | 2011 | Plutella xylostella | BNV1, Boas Novas I | Brazil | [387] | |
| 86.1 (23.4–189.7) | 1843 | 2011 | Plutella xylostella | SPC, Sapucarana | Brazil | [387] | |
| 280.6 (12.9–1038.7) | 35,316 | 2012 | Plutella xylostella | CGD, Cha Grande | Brazil | [387] | |
| 4111 (2211–8780) | 519,157 | 2012 | Plutella xylostella | BZR, Bezerros | Brazil | [387] | |
| 0.09 (0.06–0.13) | 1 | 2011/12 | Chilo suppressalis | Pooled susceptible strains | China | [382] | |
| 1.09 (0.6–2.11) | 12 | 2012 | Chilo suppressalis | JH12, Jinhua, Zhejiang | China | [389] | |
| 1.08 (0.63–2.11) | 12 | 2013 | Chilo suppressalis | XS13, Xiangshan, Zhejiang | China | [389] | |
| 1.3 (0.76–2.87) | 14 | 2014 | Chilo suppressalis | XS14, Xiangshan, Zhejiang | China | [389] | |
| 3.92 (3.02–5.07) | 43 | 2014 | Chilo suppressalis | YY14, Yuyao, Zhejiang | China | [389] | |
| 0.98 (0.63–1.73) | 11 | 2014 | Chilo suppressalis | HG14, Huanggang, Hubei | China | [389] | |
| 0.98 (0.64–1.64) | 11 | 2013 | Chilo suppressalis | SG13, Shanggao, Jiangxi | China | [389] | |
| 0.038 (0.017–0.056) | 1 | 2010 | Tuta absoluta | GBN, Guaraciaba do Norte-CE | Brazil | [385] | |
| 0.41 (0.34–0.51) | 11 | 2015 | Tuta absoluta | BZR, Bezerros-PE | Brazil | [385] | |
| 202.8 (153.2–259.9) | 5405 | 2014 | Tuta absoluta | JDR1 João Dourado I-BA | Brazil | [385] | |
| 221.48 (146.6–312.2) | 5901 | 2014 | Tuta absoluta | JDR2, João Dourado II-BA | Brazil | [385] | |
| 673.4 (391.3–989.0) | 17,943 | 2014 | Tuta absoluta | LGD, Lagoa Grande-PE | Brazil | [385] | |
| 1045 (698–1525) | 27,854 | 2014 | Tuta absoluta | GML2 Gameleira II-BA | Brazil | [385] | |
| 1398 (773–2215) | 37,254 | 2014 | Tuta absoluta | PSQ Pesqueira-PE | Brazil | [385] | |
| 2178 (1422–3179) | 58,044 | 2014 | Tuta absoluta | AMD América Dourada-BA | Brazil | [385] | |
| 3018 (2226–3964) | 80,413 | 2014 | Tuta absoluta | GML1 Gameleira I-BA | Brazil | [385]] | |
| 0.79 (0.3–1.5) | 1 | 2014 | Tuta absoluta | Lab | [383] | ||
| 993 (384–1649) | 1257 | 2014 | Tuta absoluta | IT-PACH-14-1 Siracusa, Pachino | Italy | [383] | |
| 1376 (792–2772) | 1742 | 2014 | Tuta absoluta | IT-PACH-14-2 Siracusa, Pachino | Italy | [383] | |
| 1019 (500–2130) | 1290 | 2014 | Tuta absoluta | IT-GELA-14-1 Caltanissetta, Gela | Italy | [383] | |
| 8.4 (3.6–17.0) | 11 | 2014 | Tuta absoluta | GR-IER-14-3 Ierapetra, Mpountoules | Greece | [383] | |
| 1.75 (1.36–2.23) | 1 | 2007 | Adoxophyes honmai | Kanaya (susceptible strain) | Japan | [365] | |
| 55.5 (49.1–63.7) | 32 | 2008 | Adoxophyes honmai | Shimada-Yui | Japan | [365] | |
| 35.2 (30.1–42.0) | 20 | 2009 | Adoxophyes honmai | Shimada-Yui | Japan | [365] | |
| 1174 (454 > 10,000) | 671 | 2011 June | Adoxophyes honmai | Shimada-Yui | Japan | [365] | |
| 196 (175–221) | 112 | 2011 Aug | Adoxophyes honmai | Shimada-Yui | Japan | [365] | |
| 1.54 (1.03–1.97) | 1 | 2007 | Adoxophyes honmai | Kanaya (susceptible strain) | Japan | [365] | |
| 16.2 (12.9–20.6) | 10 | 2007 | Adoxophyes honmai | Shimada-Yui | Japan | [365] | |
| 41.8 (37.1–47.2) | 27 | 2008 | Adoxophyes honmai | Shimada-Yui | Japan | [365] | |
| 24.4 (21.4–28.0) | 16 | 2009 | Adoxophyes honmai | Shimada-Yui | Japan | [365] | |
| 110 (80.8–173) | 71 | 2010 | Adoxophyes honmai | Shimada-Yui | Japan | [365] | |
| 141 (119–176) | 91 | 2011 June | Adoxophyes honmai | Shimada-Yui | Japan | [365] | |
| 161 (144–181) | 105 | 2011 Aug | Adoxophyes honmai | Shimada-Yui | Japan | [365] | |
| 0.001 (0.0002–0.003) | 1 | 2017 | Spodoptera exigua | Susceptible strain | Korea | [390] | |
| >100 | >100,000 | 2017 | Spodoptera exigua | CJ, Cheongju | Korea | [390] | |
| >100 | >100,000 | 2017 | Spodoptera exigua | JD, Jindo | Korea | [390] | |
| 9.6 (0.8–27.2) | 9560 | 2017 | Spodoptera exigua | YG, Yeonggwang | Korea | [390] | |
| 0.66 (0.006–6.51) | 660 | 2017 | Spodoptera exigua | MR, Miryang | Korea | [390] | |
| 6.5 (5–8.2) | 6500 | 2017 | Spodoptera exigua | GC, Geochang | Korea | [390] | |
| 0.0007 | 1 | Spodoptera exigua | Susceptible strain | Korea | [395] | ||
| 0.3 (0.2–0.5) | 428 | 2019 | Spodoptera exigua | Anseong | Korea | [395] | |
| 10.5 (7.0–14.4) | 14,957 | 2019 | Spodoptera exigua | Cheongju | Korea | [395] | |
| 210.1 (71.7–295.1) | 300,143 | 2019 | Spodoptera exigua | Gangneung | Korea | [395] | |
| 52.31 (32.1–70.0) | 74,729 | 2019 | Spodoptera exigua | Icheon | Korea | [395] | |
| 27.9 (24.1–32.2) | 39,929 | 2019 | Spodoptera exigua | Jindo | Korea | [395] | |
| 90.4 (67.8–132.0) | 129,186 | 2019 | Spodoptera exigua | Yeoju | Korea | [395] | |
| 0.003 (0.003–0.005) ** | 1 | Spodoptera frugiperda | SUS, Monsanto Company | USA | [391] | ||
| 0.03 (0.02–1.5) ** | 10 | 2015 | Spodoptera frugiperda | SIN2015, Sinaloa—Los Mochis | Mexico | [391] | |
| 1.5 (0.8–5.2) ** | 500 | 2016 | Spodoptera frugiperda | PR2016, Ponce | Puerto Rico | [391] | |
| Clorantraniliprole | 0.225 (0.0535–0.587) | 1 | 2009 | Plutella xylostella | Tub Berg (field susceptible) | Thailand | [372] |
| 8 (4.1–13.7) | 35 | 2010 | Plutella xylostella | Sai Noi | Thailand | [372] | |
| 34.4 (12.1–60.6) | 152 | 2011 | Plutella xylostella | Sai Noi | Thailand | [372] | |
| 19.7 (7.3–92.4) | 87 | 2011 | Plutella xylostella | Tha Muang | Thailand | [372] | |
| 174.4 (137.1–219.8) | 775 | 2011 | Plutella xylostella | Lat Lum Kaew | Thailand | [372] | |
| 0.13 (0.01–0.18) | 1 | 2010 | Plutella xylostella | Roth (lab susceptible) | China | [374] | |
| 2.4 (1.8–3.7) | 18 | 2010 | Plutella xylostella | Shenzhen, Guangdong | China | [374] | |
| 10.7 (6.6–26.6) | 81 | 2011 | Plutella xylostella | Panyu, Guangdong | China | [374] | |
| 265 (184–444) | 2000 | 2011 | Plutella xylostella | Zengcheng, Guangdong | China | [374] | |
| 18.7 (10.9–28.62) | 140 | 2011 | Plutella xylostella | Zhuhai, Guangdong | China | [374] | |
| 0.13 (0.09–0.19) | 1 | 2011 | Plutella xylostella | Roth (lab susceptible) | China | [130] | |
| 2.3 (1.6–3.3) | 18 | 2011 | Plutella xylostella | Panyu, Guangdong F3 | China | [130] | |
| 4 (2.8–5.5) | 30 | 2011 | Plutella xylostella | Zhuhai, Guangdong | China | [130] | |
| 150 (105–240) | 800 | 2011 | Plutella xylostella | Zengcheng, Guangdong | China | [130] | |
| 0.30 (0.25–0.38) | 1 | 2011 | Plutella xylostella | Chiang Mai (field susceptible) | Thailand | [376] | |
| >60 | >200 | 2011 | Plutella xylostella | Bang Bua Thong | Thailand | [376] | |
| >200 | >4,100 | 2011 | Plutella xylostella | Sudlon, Cebu Island | Phillippines | [376] | |
| 0.007 (0.004–0.012) | 1 | 2011 | Plutella xylostella | BCS-S (lab susceptible) | Brazil | [380] | |
| 204 (176.9–236.4) | 27,793 | 2011 | Plutella xylostella | Camocim | Brazil | [380] | |
| 0.006 (0.004–0.008) | 1 | 1998 | Plutella xylostella | RCF-Lab, Recife | Brazil | [387] | |
| 43.3 (29.7–59.2) | 7492 | 2012 | Plutella xylostella | BNV2, Boas Novas II | Brazil | [387] | |
| 77.2 (63.6–93.6) | 13,365 | 2012 | Plutella xylostella | CGD, Cha Grande | Brazil | [387] | |
| 89.6 (75.3–105.9) | 15,507 | 2011 | Plutella xylostella | SPC, Sapucarana | Brazil | [387] | |
| 112.4 (96.4–130.9) | 19,474 | 2011 | Plutella xylostella | CSF1, Camocim I | Brazil | [387] | |
| 115.2 (96.3–137.8) | 19,944 | 2011 | Plutella xylostella | BNV1, Boas Novas I | Brazil | [387] | |
| 123.9 (97–157.3) | 21,440 | 2011 | Plutella xylostella | JPI, Jupi | Brazil | [387] | |
| 149.1 (113.4–197.7) | 25,798 | 2011 | Plutella xylostella | CSF2, Camocim II | Brazil | [387] | |
| 162.6 (137.3–193.4) | 28,125 | 2012 | Plutella xylostella | BZR, Bezerros | Brazil | [387] | |
| 0.011 (0.005–0.018) | 1 | 2010 | Plutella xylostella | JA (lab susceptible) | Japan | [373] | |
| 23.4 (18.3–31.3) | 2128 | 2010 | Plutella xylostella | Tonghai city, Yunnan | China | [373] | |
| 0.020 (0.013–0.031) | 1 | 2011 | Plutella xylostella | BCS-S (lab susceptible) | Phillippines | [358] | |
| >1000 | >10,000 | Plutella xylostella | Sudlon, Cebu Island | Phillippines | [358] | ||
| 0.03 (0.02–0.05) | 1 | 2017 | Plutella xylostella | Susceptible strain | Korea | [390] | |
| 35.9 (21.1–57.4) | 1196 | 2017 | Plutella xylostella | PC, Pyeongchang | Korea | [390] | |
| 1.2 (0.4–3) | 40 | 2017 | Plutella xylostella | GN, Gangneung | Korea | [390] | |
| 0.49 (0.33–0.72) | 16 | 2017 | Plutella xylostella | SJ, Seongju | Korea | [390] | |
| 0.9 (0.2–1.5) *** | 1 | 2007 | Plutella xylostella | Susceptible strain | China | [378] | |
| 17.6 (12.5–22.9) *** | 20 | Plutella xylostella | BY, BaiYun Int. Airport, Guangdong | China | [378] | ||
| 1954 (1415–2437) *** | 2246 | Plutella xylostella | ZC, ZengChengi Guangdong | China | [378] | ||
| 0.82 (0.36–1.5) | 1 | 2011 | Chilo suppressalis | Fushun11, Fushun, Sichuan (Field Sus.) | China | [375] | |
| 8.4 (5.7–12.2) | 10 | 2010 | Chilo suppressalis | Yizheng10, Yizheng, Jiangsu | China | [375] | |
| 8.9 (6–14.5) | 11 | 2011 | Chilo suppressalis | Xiangshan11,Xiangshan, Zhejiang | China | [375] | |
| 10.4 (6.8–15.7) | 13 | 2010 | Chilo suppressalis | Lujiang10, Lujiang, Anhui | China | [375] | |
| 11.2 (6–20.5) | 14 | 2010 | Chilo suppressalis | Longyou10, Longyou, Zhejiang | China | [375] | |
| 10.4 (5–23.7) | 17 | 2011 | Chilo suppressalis | Dong-An11, Dong-An, Hunan | China | [375] | |
| 17.7 (10.6–31.8) | 22 | 2010 | Chilo suppressalis | Wuxue10, Wuxue, Hubei | China | [375] | |
| 3 (1.4–4.5) **** | 1 | 2012 | Chilo suppressalis | RA12, Ruian, Zhejiang (Sus. Strain) | China | [379] | |
| 47 (28.4–103) **** | 16 | 2012 | Chilo suppressalis | ZJ12, Zhuji, Zhejiang | China | [379] | |
| 43.2 (20.1–107.6) **** | 14 | 2013 | Chilo suppressalis | ZJ13, Zhuji, Zhejiang | China | [379] | |
| 1.4 (1.1–1.7) | 1 | 2011–2012 | Chilo suppressalis | Pooled susceptible strains | China | [377] | |
| 16.2 (11–27.2) | 11 | 2014 | Chilo suppressalis | XS14, Xiangshan, Zhejiang | China | [389] | |
| 108.1 (79.5–178.5) | 78 | 2014 | Chilo suppressalis | YY14, Yuyao, Zhejiang | China | [389] | |
| 0.43 (0.37–0.5) | 1 | 2016 | Chilo suppressalis | CAAS (lab susceptible) | China | [44] | |
| 108.5 (86.2–136.4) | 250 | 2016 | Chilo suppressalis | Tong Nan, Nanchang, Jiangxi | China | [44] | |
| 1.4 (1.1–1.7) | 1 | 2011/12 | Chilo suppressalis | Pooled susceptible strains | China | [377] | |
| 114.5 (71.7–162.1) | 82 | 2016 | Chilo suppressalis | XS, Xiaoshan, Zhejiang | China | [386] | |
| 199.9 (173.5–229.9) | 143 | 2016 | Chilo suppressalis | JH, Jinhua, Zhejiang | China | [386] | |
| 147.3 (62.8–280.8) | 106 | 2016 | Chilo suppressalis | QZ, Quzhou, Zhejiang | China | [386] | |
| 154.8 (103.8–222.1) | 111 | 2016 | Chilo suppressalis | LY, Longyou, Zhejiang | China | [386] | |
| 195.3 (164.2–232) | 140 | 2016 | Chilo suppressalis | YQ, Yueqing, Zhejiang | China | [386] | |
| 214 (183.2–250.8) | 154 | 2016 | Chilo suppressalis | WL, Wenling, Zhejiang | China | [386] | |
| 89.2 (73.9–107) | 64 | 2016 | Chilo suppressalis | HY, Hengyang, Hu’nan | China | [386] | |
| 109.6 (91.4–131.9) | 79 | 2016 | Chilo suppressalis | XY, Xinyang, He’nan | China | [386] | |
| 0.18 (0.13–0.30) | 1 | 2014 | Tuta absoluta | Lab (susceptible strain) | [383] | ||
| 47.6 (30.8–77.1) | 264 | 2014 | Tuta absoluta | IT-PACH-14-1 Siracusa, Pachino | Italy | [383] | |
| 63.7 (42.1–128) | 354 | 2014 | Tuta absoluta | IT-PACH-14-2 Siracusa, Pachino | Italy | [383] | |
| 435 (165–1193) | 2417 | 2014 | Tuta absoluta | IT-ACAT-14-1 Ragusa, Acate | Italy | [383] | |
| 225 (135–343) | 1250 | 2014 | Tuta absoluta | IT-GELA-14-1 Caltanissetta, Gela | Italy | [383] | |
| 2.4 (1.2–17.0) | 14 | 2014 | Tuta absoluta | GR-IER-14-1 Ierapetra, Kentri | Greece | [383] | |
| 0.0044 (0.0024–0.0068) | 1 | 2014 | Tuta absoluta | BSL, Brasília-DF | Brazil | [385] | |
| 0.19 (0.12-0.28) | 45 | 2015 | Tuta absoluta | BZR, Bezerros-PE | Brazil | [385] | |
| 1.5 (1.2–2) | 356 | 2014 | Tuta absoluta | LGD, Lagoa Grande-PE | Brazil | [385] | |
| 2.3 (1.4–3.4) | 525 | 2014 | Tuta absoluta | JDR2, João Dourado II-BA | Brazil | [385] | |
| 2.9 (1.9–4.4) | 658 | 2014 | Tuta absoluta | JDR1 João Dourado I-BA | Brazil | [385] | |
| 4.6 (3.2–7) | 1064 | 2014 | Tuta absoluta | GML2 Gameleira II-BA | Brazil | [385] | |
| 92.4 (60–129.9) | 21,155 | 2014 | Tuta absoluta | GML1 Gameleira I-BA | Brazil | [385] | |
| 646 (423–917) | 147,928 | 2014 | Tuta absoluta | PSQ Pesqueira-PE | Brazil | [385] | |
| 1263 (946–1673) | 288,995 | 2014 | Tuta absoluta | AMD América Dourada-BA | Brazil | [385] | |
| 0.3 (0.22–0.45) | 1 | 2010 | Tuta absoluta | Gr-Lab, Peloponnesus | Greece | [388] | |
| 161 (44.2–596) | 519 | 2015 | Tuta absoluta | GR-IndR, Ierapetra | Greece | [388] | |
| 17 (8.7–42) | 55 | 2015 | Tuta absoluta | GR-IER-15-2 | Greece | [47] | |
| 56 (14–120) | 180 | 2014 | Tuta absoluta | IT-GELA-14-1, Sicily, Gela | Italy | [47] | |
| 0.21 (0.15–0.29) | 1 | 2005 | Tuta absoluta | BCS-TA-S, Paulinia, SP | Brazil | [47] | |
| 92 (60–130) | 438 | 2014 | Tuta absoluta | BR-GML1, Gameleira, BA | Brazil | [47] | |
| 650 (420–920) | 3095 | 2014 | Tuta absoluta | BR-PSQ, Pesqueira, PE | Brazil | [47] | |
| 1.6 (1.4–1.8) | 1 | 2010 | Adoxophyes honmai | Kanaya (susceptible strain) | Japan | [365] | |
| 26.3 (21.2–33.8) | 17 | 2010 | Adoxophyes honmai | Shimada-Yui | Japan | [365] | |
| 64.6 (55.4–78.0) | 41 | 2011 June | Adoxophyes honmai | Shimada-Yui | Japan | [365] | |
| 114 (101–132) | 73 | 2011 Aug | Adoxophyes honmai | Shimada-Yui | Japan | [365] | |
| 1.3 (1.1–1.5) | 1 | 2010 | Adoxophyes honmai | Kanaya (susceptible strain) | Japan | [365] | |
| 25.3 (20.7–31.9) | 20 | 2010 | Adoxophyes honmai | Shimada-Yui | Japan | [365] | |
| 50.0 (43.2–59.0) | 39 | 2011 June | Adoxophyes honmai | Shimada-Yui | Japan | [365] | |
| 98.8 (86.7–114) | 77 | 2011 Aug | Adoxophyes honmai | Shimada-Yui | Japan | [365] | |
| 0.014 (0.010–0.017) | 1 | Spodoptera exigua | Lab-Sus (susceptible strain) | China | [369] | ||
| 0.15 (0.13–0.18) | 11 | 2008 | Spodoptera exigua | SH08 Minhang, Shanghai | China | [369] | |
| 0.14 (0.11–0.17) | 10 | 2010 | Spodoptera exigua | SH10 Minhang, Shanghai | China | [369] | |
| 0.14 (0.12–0.16) | 10 | 2008 | Spodoptera exigua | TA08 Tai’an, Shandong | China | [369] | |
| 0.16 (0.14–0.18) | 12 | 2010 | Spodoptera exigua | HF10 Hefei, Anhui | China | [369] | |
| 0.21 (0.18–0.25) | 15 | 2010 | Spodoptera exigua | SZ10 Shengzhen, Guangdong | China | [369] | |
| 0.24 (0.2–0.28) | 17 | 2010 | Spodoptera exigua | DG10 Dongguang, Guangdong | China | [369] | |
| 0.21 (0.18–0.25) | 15 | 2010 | Spodoptera exigua | HZ10 Huizhou, Guangdong | China | [369] | |
| 0.16 (0.14–0.19) | 12 | 2010 | Spodoptera exigua | ZZ10 Zhangzhou, Fujian | China | [369] | |
| 0.37 (0.26–0.52) | 1 | Spodoptera exigua | WH-S (Lab. susceptible) | China | [370] | ||
| 12.2 (5.8–35.4) | 33 | 2010 | Spodoptera exigua | JN, Jinning, Yunnan | China | [370] | |
| 4.7 (2.2–7.9) | 13 | 2009 | Spodoptera exigua | YL-1, Yanliang, Shanxi | China | [370] | |
| 16.5 (12.6–22) | 44 | 2009 | Spodoptera exigua | YX, Yongxiu, Jiangxi | China | [370] | |
| 5.3 (1.6–13.9) | 14 | 2009 | Spodoptera exigua | LG, Longhai, Fujian | China | [370] | |
| 7.5 (3–15.8) | 20 | 2009 | Spodoptera exigua | HA, Huaian, Jiangsu | China | [370] | |
| 4 (2.6–5.7) | 11 | 2009 | Spodoptera exigua | LH-1, Luhe, Jiangsu | China | [370] | |
| 3.6 (2.3–6) | 10 | 2010 | Spodoptera exigua | LH-2, Luhe, Jiangsu | China | [370] | |
| 12.7 (5.1–27.4) | 34 | 2009 | Spodoptera exigua | FX-1, Fengxian, Shanghai | China | [370] | |
| 6 (3.1–10.8) | 16 | 2010 | Spodoptera exigua | FX-2, Fengxian, Shanghai | China | [370] | |
| 5.1 (2.4–8.2) | 14 | 2011 | Spodoptera exigua | FX-3, Fengxian, Shanghai | China | [370] | |
| 0.08 (0.06–0.1) | 1 | Spodoptera exigua | WH-S (Lab. susceptible) | China | [393 | ||
| 2.2 (1.7–2.9) | 27 | 2014 | Spodoptera exigua | Baiyun, Guangzhou | China | [396] | |
| 60 (46.1–79.8) | 750 | 2015 | Spodoptera exigua | Baiyun, Guangzhou | China | [396] | |
| 64 (43.5–87) | 800 | 2016 | Spodoptera exigua | Baiyun, Guangzhou | China | [396] | |
| 54.5 (41.6–72.3) | 682 | 2017 | Spodoptera exigua | Baiyun, Guangzhou | China | [396] | |
| 140.7 (106.7–179.1) | 1759 | 2018 | Spodoptera exigua | Baiyun, Guangzhou | China | [396] | |
| 1.3 (0.97–1.74) | 16 | 2014 | Spodoptera exigua | Fengxian, Shanghai | China | [396] | |
| 1.9 (1.3–2.6) | 24 | 2015 | Spodoptera exigua | Fengxian, Shanghai | China | [396] | |
| 45.6 (35–60.7) | 571 | 2016 | Spodoptera exigua | Fengxian, Shanghai | China | [396] | |
| 159.6 (120.9–210.8) | 1995 | 2017 | Spodoptera exigua | Fengxian, Shanghai | China | [396] | |
| 207.8 (162.3–267.4) | 2597 | 2018 | Spodoptera exigua | Fengxian, Shanghai | China | [396] | |
| 0.97 (0.6–1.7) | 12 | 2015 | Spodoptera exigua | Huangpi, Wuhan | China | [396] | |
| 3.7 (2.6–4.9) | 46 | 2016 | Spodoptera exigua | Huangpi, Wuhan | China | [396] | |
| 10.3 (7.7–13.6) | 129 | 2017 | Spodoptera exigua | Huangpi, Wuhan | China | [396] | |
| 17.6 (13.8–22.2) | 221 | 2018 | Spodoptera exigua | Huangpi, Wuhan | China | [396] | |
| 0.01 (0.0002–0.07) | 1 | 2017 | Spodoptera exigua | Susceptible strain | Korea | [390] | |
| >25 | >2500 | 2017 | Spodoptera exigua | CJ, Cheongju | Korea | [390] | |
| >25 | >2500 | 2017 | Spodoptera exigua | JD, Jindo | Korea | [390] | |
| >25 | >2500 | 2017 | Spodoptera exigua | YG, Yeonggwang | Korea | [390] | |
| 1.8 (0.8–4.2) | 177 | 2017 | Spodoptera exigua | MR, Miryang | Korea | [390] | |
| 10.1 (6.5–16.3) | 1006 | 2017 | Spodoptera exigua | GC, Geochang | Korea | [390] | |
| 0.002 | 1 | Spodoptera exigua | Susceptible strain | Korea | [395] | ||
| 8 (5.3–12.5) | 4000 | 2019 | Spodoptera exigua | Anseong | Korea | [395] | |
| 1.2 (0.3–2.7) | 600 | 2019 | Spodoptera exigua | Cheongju | Korea | [395] | |
| 6.6 (5.3–8.2) | 3300 | 2019 | Spodoptera exigua | Gangneung | Korea | [395] | |
| 4.6 (2.3–7.0) | 2300 | 2019 | Spodoptera exigua | Icheon | Korea | [395] | |
| 13.4 (7.6–25.3) | 6700 | 2019 | Spodoptera exigua | Jindo | Korea | [395] | |
| 21.2 (9.9–498.0) | 12,500 | 2019 | Spodoptera exigua | Yeoju | Korea | [395] | |
| 0.032 * (0.025–0.041) | 1 | Spodoptera exigua | WH-S strain, Hubei (Susceptible Str.) | China | [393] | ||
| 4.9 * (3.9–6.6) | 154 | 2018 | Spodoptera exigua | WF strain, Weifang, Shandong | China | [393] | |
| 0.055 (0.040–0.072) | 1 | Spodoptera exigua | SS | China | [394] | ||
| 9.9 (4.9–19) | 180 | 2018 | Spodoptera exigua | CL18, Changle, Shandong | China | [394] | |
| 4.1 (1.4–12.4) | 74 | 2019 | Spodoptera exigua | CL19, Changle, Shandong | China | [394] | |
| 1.5 (1.2–2) | 28 | 2018 | Spodoptera exigua | AQ18, Anqiu, Shandong | China | [394] | |
| 5.5 (1.8–11.6) | 100 | 2018 | Spodoptera exigua | NY18, Nanyang, Henan | China | [394] | |
| 4.6 (3.2–6.4) | 83 | 2019 | Spodoptera exigua | NY19, Nanyang, Henan | China | [394] | |
| 29.3 (17.6–50) | 534 | 2019 | Spodoptera exigua | AY19, Anyang, Henan | China | [394] | |
| 16.7 (10.6–31.3) | 304 | 2018 | Spodoptera exigua | XZ18, Xuzhou, Jiangsu | China | [394] | |
| 16.5 (8.7–31.8) | 301 | 2018 | Spodoptera exigua | XA18, Xian, Shanxi | China | [394] | |
| 136.3 (83.2–229.3) | 2477 | 2019 | Spodoptera exigua | JX19, Jiaxing, Zhejiang | China | [394] | |
| 4.20 (3.51–4.95) | 1 | Spodoptera litura | XW-Sus (Susceptible Str.) | China | [368] | ||
| 47.2 (40.7–53.9) | 11 | 2010 | Spodoptera litura | SH10, Minhang, Shanghai | China | [368] | |
| 71.6 (54.4–94.9) | 17 | 2008 | Spodoptera litura | HF08, Hefei, Anhui | China | [368] | |
| 75.5 (61.7–89.8) | 18 | 2010 | Spodoptera litura | HF10, Hefei, Anhui | China | [368] | |
| 100.3 (84.3–119.3) | 24 | 2009 | Spodoptera litura | HX09, Hexian, Anhui | China | [368] | |
| 78.9 (64.3–93.5) | 19 | 2010 | Spodoptera litura | ZZ10, Zhangzhou, Fujian | China | [368] | |
| 102.5 (84–121) | 24 | 2010 | Spodoptera litura | SZ10, Shenzheng, Guangdong | China | [368] | |
| 80.4 (63.5–96.8) | 19 | 2010 | Spodoptera litura | HZ10, Huizhou, Guangdong | China | [368] | |
| 98.8 (79.5–118) | 23 | 2010 | Spodoptera litura | DG10, Dongguang, Guangdong | China | [368] | |
| 0.083 (0.066–0.106) | 1 | Spodoptera litura | SS (Lab. susceptible) | China | [384] | ||
| 0.83 (0.65–1.06) | 10 | 2013 | Spodoptera litura | HZ13, Huizhou, Guangdong | China | [384] | |
| 1.2 (0.9–1.7) | 15 | 2014 | Spodoptera litura | ZC14, Zengcheng, Guangdong | China | [384] | |
| 0.9 (0.7–1.24) | 11 | 2014 | Spodoptera litura | ND14, Ningde, Fujian | China | [384] | |
| 1.2 (0.8–1.9) | 14 | 2014 | Spodoptera litura | HK14, Haikou, Hainan | China | [384] | |
| 1.3 (0.9–1.9) | 16 | 2014 | Spodoptera litura | GL14, Guilin, Guangxi | China | [384] | |
| 0.001 (0.0007–0.002) ** | 1 | Spodoptera frugiperda | SUS, Monsanto Company | USA | [391] | ||
| 0.16 (0.06–0.32) ** | 160 | 2016 | Spodoptera frugiperda | PR2016, Ponce | Puerto Rico | [391] | |
| Cyantraniliprole | 0.0068 (0.0039–0.012) | 1 | 2011 | Plutella xylostella | BCS-S (susceptible strain) | Phillippines | [358] |
| 18 (5.1–66) | 2647 | 2011 | Plutella xylostella | Sudlon, Cebu Island | Phillippines | [358] | |
| 0.009 (0.003–0.03) | 1 | 2017 | Plutella xylostella | Susceptible strain | Korea | [390] | |
| 0.95 (0.34–2.06) | 106 | 2017 | Plutella xylostella | PC, Pyeongchang | Korea | [390] | |
| 0.88 (0.35–1.85) | 98 | 2017 | Plutella xylostella | GN, Gangneung | Korea | [390] | |
| 0.43 (0.24–0.65) | 48 | 2017 | Plutella xylostella | SJ, Seongju | Korea | [390] | |
| 0.029 (0.025–0.034) | 1 | 1998 | Plutella xylostella | RCF-Lab, Recife | Brazil | [387] | |
| 0.43 (0.14–0.92) | 13 | 2012 | Plutella xylostella | BZR, Bezerros | Brazil | [387] | |
| 0.55 (0.25–1.00) | 16 | 2012 | Plutella xylostella | BNV2, Boas Novas II | Brazil | [387] | |
| 1.3 (0.7–2.2) | 39 | 2011 | Plutella xylostella | BNV1, Boas Novas I | Brazil | [387] | |
| 10.6 (5.8–18.8) | 308 | 2011 | Plutella xylostella | SPC, Sapucarana | Brazil | [387] | |
| 33.1 (20.9–56.5) | 962 | 2011 | Plutella xylostella | CSF2, Camocim II | Brazil | [387] | |
| 37 (31.2–44) | 1075 | 2012 | Plutella xylostella | CGD, Cha Grande | Brazil | [387] | |
| 64 (43.8–91.9) | 1943 | 2011 | Plutella xylostella | CSF1, Camocim I | Brazil | [387] | |
| 69.7 (55.4–87.4) | 2024 | 2011 | Plutella xylostella | JPI, Jupi | Brazil | [387] | |
| 0.08 (0.04–0.13) | 1 | 2017 | Spodoptera exigua | Susceptible strain | Korea | [390] | |
| 1.8 (1.7–2.2) | 23 | 2017 | Spodoptera exigua | CJ, Cheongju | Korea | [390] | |
| >25 | >312 | 2017 | Spodoptera exigua | JD, Jindo | Korea | [390] | |
| 1.7 (0.01–6.3) | 21 | 2017 | Spodoptera exigua | YG, Yeonggwang | Korea | [390] | |
| 0.015 (0.011–0.020) | 1 | 2014 | Tuta absoluta | BSL, Brasília-DF | Brazil | [385] | |
| 1.2 (0.9–1.5) | 78 | 2015 | Tuta absoluta | BZR, Bezerros-PE | Brazil | [385] | |
| 1.7 (1.2–2.2) | 109 | 2014 | Tuta absoluta | JDR1 João Dourado I-BA | Brazil | [385] | |
| 2.2 (1.6–3) | 147 | 2014 | Tuta absoluta | GML2 Gameleira II-BA | Brazil | [385] | |
| 8.5 (6.2–11.4) | 556 | 2014 | Tuta absoluta | JDR2, João Dourado II-BA | Brazil | [385] | |
| 28.9 (17.3–41.9) | 1895 | 2014 | Tuta absoluta | LGD, Lagoa Grande-PE | Brazil | [385] | |
| 90.6 (63.3–121.4) | 5932 | 2014 | Tuta absoluta | GML1 Gameleira I-BA | Brazil | [385] | |
| 152.9 (96.2–224.3) | 10,010 | 2014 | Tuta absoluta | PSQ Pesqueira-PE | Brazil | [385] | |
| 281.3 (190.8–405) | 18,423 | 2014 | Tuta absoluta | AMD América Dourada-BA | Brazil | [385] | |
| 0.17 (0.11–0.26) | 1 | Aphis gossypii | 171B (Sus. Strain) | Spain | [347] | ||
| 2.5 (1.5–3.9)c | 14 | 2010 | Aphis gossypii | Spain 1, Blanca, Murcia | Spain | [347] | |
| 1.7 (1.4–1.9) | 1 | 2009 | Bemisia tabaci | MED-S (Sus. Strain) | China | [392] | |
| 43.8 (37.4–51.3) | 26 | 2016 | Bemisia tabaci | SX, Shanxi | China | [392] | |
| Cyclaniliprole | 0.002 (0.00009–0.02) | 1 | 2017 | Spodoptera exigua | Susceptible strain | Korea | [390] |
| >22.5 | >11,250 | 2017 | Spodoptera exigua | CJ, Cheongju | Korea | [390] | |
| >22.5 | >11,250 | 2017 | Spodoptera exigua | JD, Jindo | Korea | [390] | |
| >22.5 | >11,250 | 2017 | Spodoptera exigua | YG, Yeonggwang | Korea | [390] | |
| 10.7 (4.8–21.2) | 5350 | 2017 | Spodoptera exigua | MR, Miryang | Korea | [390] | |
| 6.3 (4.9–8.1) | 3150 | 2017 | Spodoptera exigua | GC, Geochang | Korea | [390] | |
| Tetraniliprole | 0.04 (0.03–0.07) | 1 | Spodoptera exigua | SS | China | [394] | |
| 1.4 (1–1.9) | 33 | 2018 | Spodoptera exigua | XA18, Xian, Shanxi | China | [394] | |
| 0.5 (0.3–0.7) | 12 | 2018 | Spodoptera exigua | NY18, Nanyang, Henan | China | [394] | |
| 5.5 (4.1–7.8) | 128 | 2019 | Spodoptera exigua | AY19, Anyang, Henan | China | [394] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toprak, U.; Doğan, C.; Hegedus, D. A Comparative Perspective on Functionally-Related, Intracellular Calcium Channels: The Insect Ryanodine and Inositol 1,4,5-Trisphosphate Receptors. Biomolecules 2021, 11, 1031. https://doi.org/10.3390/biom11071031
Toprak U, Doğan C, Hegedus D. A Comparative Perspective on Functionally-Related, Intracellular Calcium Channels: The Insect Ryanodine and Inositol 1,4,5-Trisphosphate Receptors. Biomolecules. 2021; 11(7):1031. https://doi.org/10.3390/biom11071031
Chicago/Turabian StyleToprak, Umut, Cansu Doğan, and Dwayne Hegedus. 2021. "A Comparative Perspective on Functionally-Related, Intracellular Calcium Channels: The Insect Ryanodine and Inositol 1,4,5-Trisphosphate Receptors" Biomolecules 11, no. 7: 1031. https://doi.org/10.3390/biom11071031
APA StyleToprak, U., Doğan, C., & Hegedus, D. (2021). A Comparative Perspective on Functionally-Related, Intracellular Calcium Channels: The Insect Ryanodine and Inositol 1,4,5-Trisphosphate Receptors. Biomolecules, 11(7), 1031. https://doi.org/10.3390/biom11071031