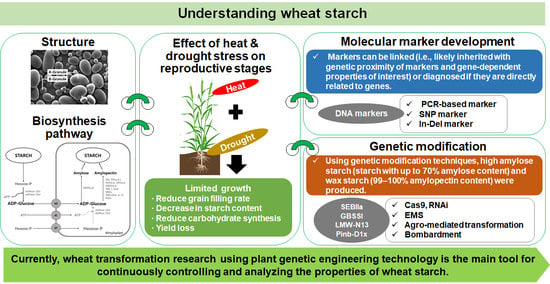
Understanding Wheat Starch Metabolism in Properties, Environmental Stress Condition, and Molecular Approaches for Value-Added Utilization
Abstract
:1. Introduction
2. Characterization of Wheat Starch
2.1. Starch Structure
2.2. Starch Biosynthesis Mechanism
2.3. Resistant Starch Wheat
3. Wheat Starch under Heat/Drought Stress
3.1. Grain Filling Stage under Heat/Drought Stress
3.2. Starch & Drought and Heat Stress during Anthesis and Grain Filling Stage
3.3. Starch & High Night Temperature during Anthesis and Grain Filling Stage
3.4. Sucrose and Starch Biosynthetic Pathway Carbohydrate Metabolism under Stress
3.5. Carbohydrate Metabolism under Stress
3.6. Regulation of Starch Metabolism under Stresses
3.7. Starch Synthetic Metabolism under Stresses
3.8. Starch and Other Stresses during Anthesis and Grain Filling Stage
4. Molecular Marker Development and Application for Wheat Starch
5. Genetic Modification of Starch Composition in Wheat
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OECD/FAO. OECD-FAO Agricultural Outlook 2011–2020, OECD Publishing and FAO. 2011. Available online: https://www.oecd-ilibrary.org/agriculture-and-food/oecd-fao-agricultural-outlook_19991142 (accessed on 17 June 2011).
- Shewry, P.R.; Halford, N.G.; Belton, P.S.; Tatham, A.S. The structure and properties of gluten: An elastic protein from wheat grain. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2002, 357, 133–142. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N.; Bajaj, R.; Kaur, A. Wheat starch production, structure, functionality and applications—A review. Int. J. Food Sci. Technol. 2017, 52, 38–58. [Google Scholar] [CrossRef]
- Lim, S.T.; Jane, J.L.; Rajagopalan, S.; Seib, P.A. Effects of starch granule size on physical properties of starch-filled polyethylene film. Biotechnol. Prog. 1992, 8, 51–55. [Google Scholar] [CrossRef]
- Shewry, P.R.; Hazard, B.; Lovegrove, A.; Uauy, C. Improving starch and fibre in wheat grain for human health. Biochemist 2020, 42, 40–45. [Google Scholar] [CrossRef]
- Hurkman, W.; McCue, K.F.; Altenbach, S.; Korn, A.M.; Tanaka, C.; Kothari, K.; Johnson, D.; Bechtel, D.; Wilson, J.D.; Anderson, O. Effect of temperature on expression of genes encoding enzymes for starch biosynthesis in developing wheat endosperm. Plant Sci. 2003, 164, 873–881. [Google Scholar] [CrossRef]
- Tester, R.F.; Karkalas, J.; Qi, X. Starch-composition, fine structure and architecture. J. Cereal Sci. 2004, 39, 151–165. [Google Scholar] [CrossRef]
- Blennow, A.; Bay-Smidt, A.M.; Wischmann, B.; Olsen, C.E.; Moller, B.L. The degree of starch phosphorylation is related to the chain length distribution of the neutral and phosphorylated chains of amylopectin. Carbohydr. Res. 1998, 307, 45–54. [Google Scholar] [CrossRef]
- Blennow, A.; Nielsen, T.H.; Baunsgaard, L.; Mikkelsen, R.; Englelsen, S.B. Tarch phosphorylation: A new front line in starch research. Trends Plant Sci. 2002, 7, 445–450. [Google Scholar] [CrossRef]
- Hung, P.V.; Maeda, T.; Morita, N. Waxy and high-amylose wheat starches and flours-characteristics, functionality and application. Trends Food Sci. Technol. 2006, 17, 448–456. [Google Scholar] [CrossRef]
- White, P.J. Properties of corn starch. In Specialty Corns; Hallauer, A., Ed.; CRC Press: Boca Raton, FL, USA, 2000; pp. 29–54. [Google Scholar]
- Whistler, R.L.; BeMiller, J.N. Starch. In Carbohydrate Chemistry for Food Scientists, 3rd ed.; Whistler, R.L., BeMiller, J.N., Eds.; Eagan Press: St Paul, MN, USA, 2018; pp. 117–151. [Google Scholar]
- Reddy, I.; Seib, P.A. Modified waxy wheat starch compared to modified waxy corn starch. J. Cereal Sci. 2006, 17, 448–456. [Google Scholar] [CrossRef]
- French, D. Fine structure of starch and its relationship to then organisation of starch granules. J. Jpn. Soc. Starch Sci. 1972, 19, 8–25. [Google Scholar] [CrossRef] [Green Version]
- Lineback, D.R. The starch granule: Organisation and properties. Bakers Digest. 1984, 58, 16–21. [Google Scholar]
- Swinkels, J.J.M. Composition and properties of commercial native starches. Starch Starke 1985, 37, 1–5. [Google Scholar] [CrossRef]
- Buléon, A.; Colonna, P.; Planchot, V.; Ball, S. Starch granules: Structure and biosynthesis. Int. J. Biol. Macromol. 1998, 23, 85–112. [Google Scholar] [CrossRef] [Green Version]
- Mua, J.P.; Jackson, D.S. Fine structure of corn amylose and amylopectin fractions with various molecular weights. J. Agric. Food Chem. 1997, 45, 3840–3847. [Google Scholar] [CrossRef]
- Wang, X.; Cai, J.; Liu, F.; Jin, M.; Yu, H.; Jiang, D.; Wollenweber, B.; Dai, T.; Cao, W. Pre-anthesis high temperature acclimation alleviates the negative effects of post-anthesis heat stress on stem stored carbohydrates remobilization and grain starch accumulation in wheat. J. Cereal Sci. 2012, 55, 331–336. [Google Scholar] [CrossRef]
- Zhang, C.; Jiang, D.; Liu, F.; Cai, J.; Dai, T.; Cao, W. Starch granules size distribution in superior and inferior grains of wheat is related to enzyme activities and their gene expressions during grain filling. J. Cereal Sci. 2010, 51, 226–233. [Google Scholar] [CrossRef]
- Halford, N.G.; Curtis, T.Y.; Chen, Z.; Huang, J. Effects of abiotic stress and crop management on cereal grain composition: Implications for food quality and safety. J. Environ. Exp. Bot. 2015, 66, 1145–1156. [Google Scholar] [CrossRef] [Green Version]
- Begcy, K.; Sandhu, J.; Walia, H. Transient heat stress during early seed development primes germination and seedling establishment in rice. Front. Plant Sci. 2018, 9, 1768. [Google Scholar] [CrossRef]
- Greenberg, E.; Preiss, J. The occurrence of adenosine diphosphate glucose: Glycogen transglucosylase in bacteria. J. Biol. Chem. 1964, 239, 4314–4315. [Google Scholar] [CrossRef]
- Recondo, E.; Leloir, L. Adenosine diphosphate glucose and starch biosynthesis. Biochem. Biophys. Res. Commun. 1961, 6, 85–88. [Google Scholar] [CrossRef]
- Tetlow, I.J. Starch biosynthesis in developing seeds. Seed Sci. Res. 2011, 21, 5–23. [Google Scholar] [CrossRef] [Green Version]
- Thitisaksakul, M.; Jiménez, R.C.; Arias, M.C.; Beckles, D.M. Effects of environmental factors on cereal starch biosynthesis and composition. J. Cereal Sci. 2012, 56, 67–80. [Google Scholar] [CrossRef]
- Preiss, J.; Sivak, M. Starch synthesis in sinks and sources. In Photoassimilate Distribution in Plants and Crops; Zamski, E., Schaffter, A.A., Eds.; Marcel Dekker: New York, NY, USA, 1996; pp. 63–69. [Google Scholar]
- Villand, P.; Aalen, R.; Olsen, O.-A.; Lonneborg, A.; Lüthi, E.; Kleczkowski, L.A. PCR-amplification and sequence of cDNA clones for the small and large subunits of ADP-glucose pyrophosphorylase from barley tissues. Plant Mol. Biol. 1992, 19, 381–389. [Google Scholar] [CrossRef]
- Olive, M.R.; Ellis, R.J.; Schuch, W.W. Isolation and nucleotide sequences of cDNA clones encoding ADP glucose pyrophosphorylase polypeptides from wheat leaf and endosperm. Plant Mol. Biol. 1989, 12, 525–538. [Google Scholar] [CrossRef]
- Lee, S.-K.; Hwang, S.-K.; Han, M.; Eom, J.-S.; Kang, H.-G.; Han, Y.; Choi, S.-B.; Cho, M.-H.; Bhoo, S.H.; An, G.; et al. Identification of the ADP-glucose pyrophosphorylase isoforms essential for starch synthesis in the leaf and seed endosperm of rice (Oryza sativa L.). Plant Mol. Biol. 2007, 65, 531–546. [Google Scholar] [CrossRef]
- Müller-Rober, B.; Kossmann, J.; Hannah, L.C.; Willmitzer, L.; Sonnewald, U. Only one of two different ADP glucose pyrophosphorylase genes from potato responds strongly to elevated levels of sucrose. Mol. Gen. Genet. 1990, 224, 136–146. [Google Scholar] [CrossRef]
- Duwenig, E.; Steup, M.; Kossmann, J. Induction of genes encoding plastidic phosphorylase from spinach (Spinacia oleracea L.) and potato (Solanum tuberosum L.) by exogenously supplied carbohydrates in excised leaf discs. Planta 1997, 203, 111–120. [Google Scholar]
- Tharanathan, M.; Tharanathan, R.N. Resistant starch in wheat-based products: Isolation and characterisation. J. Cereal Sci. 2001, 34, 73–84. [Google Scholar] [CrossRef]
- Vetrani, C.; Sestili, F.; Vitale, M.; Botticella, E.; Giacco, R.; Griffo, E.; Costabile, G.; Cipriano, P.; Tura, A.; Pacini, G.; et al. Metabolic response to amylose-rich wheat-based rusks in overweight individuals. Eur. J. Clin. Nutr. 2018, 72, 904–912. [Google Scholar] [CrossRef]
- Wong, J.M.; de Souza, R.; Kendall, C.W.; Eman, A.; Jenkins, D.J. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Englyst, H.N.; Cummings, J.H. Digestion of the polysaccharides of some cereal foods in the human small intestine. Am. J. Clin. Nutr. 1985, 42, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Englyst, H.N.; Cummings, J.H. Digestion of the carbohydrates of banana (Musaparadisiaca sapientwn) in the human small intestine. Am. J. Clin. Nutr. 1986, 44, 42–50. [Google Scholar] [CrossRef]
- Englyst, H.N.; Cummings, J.H. Digestion of the polysaccharides of potato in the small intestine of man. Am. J. Clin. Nutr. 1987, 45, 423–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Englyst, H.N.; Wiggins, H.S.; Cummings, J.H. Determination of the non-starch polysaccharides in plant foods by gas-liquid chromatography of constituent sugars as alditol acetates. Analyst 1982, 107, 307–318. [Google Scholar] [CrossRef]
- Englyst, H.N.; Kingman, S.M.; Hudson, G.J.; Cummings, J.H. Measurement of resistant starch in vitro and in vivo. Br. J. Nutr. 1996, 75, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Brouns, F.; Kettlitz, B.; Arrigon, E. Resistant starch and “the butyrate revolution”. Trends Food Sci. Technol. 2002, 13, 251–261. [Google Scholar] [CrossRef]
- Nugent, A.P. Health properties of resistant starch. Nutr. Bull. 2005, 30, 27–54. [Google Scholar] [CrossRef]
- Birt, D.F.; Boylston, T.; Hendrich, S.; Jane, J.-L.; Hollis, J.; Li, L.; McClelland, J.; Moore, S.; Phillips, G.J.; Rowling, M. Resistant starch: Promise for improving human health. Adv. Nutr. 2013, 4, 587–601. [Google Scholar] [CrossRef] [Green Version]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46, S33–S50. [Google Scholar]
- Woo, K.S.; Seib, P.A. Cross-linked resistant starch: Preparation and properties. Cereal Chem. 2002, 79, 819–825. [Google Scholar] [CrossRef]
- Han, J.-A.; BeMiller, J.N. Preparation and physical characteristics of slowly digesting modified food starches. Carbohydr. Polym. 2007, 67, 366–374. [Google Scholar] [CrossRef]
- Seneviratne, H.D.; Biliaderis, C.G. Action of α-amylases on amylose-lipid complex superstructures. J. Cereal Sci. 1991, 13, 129–143. [Google Scholar] [CrossRef]
- Hasjim, J.; Lee, S.-O.; Hendrich, S.; Setiawan, S.; Ai, Y.; Jane, J. Characterization of a novel resistant-starch and its effects on postprandial plasma-glucose and insulin responses. Cereal Chem. 2010, 87, 257–262. [Google Scholar] [CrossRef]
- Hasjim, J.; Ai, Y.; Jane, J. Novel applications of amylose-lipid complex as resistant starch type 5. In Resistant Starch: Sources, Applications and Health Benefits, 1st ed.; Shi, Y.C., Maningat, C.C., Eds.; John Wiley and Sons: Hoboken, NJ, USA, 2013; pp. 79–94. [Google Scholar]
- Kim, T.; Kay, D. Starch synthesis in cereal grains. Adv. Bot. Res. 2003, 40, 1–61. [Google Scholar]
- Abhinandan, K.; Skori, L.; Stanic, M.; Hickerson, N.M.N.; Jamshed, M.; Samuel, M.A. Abiotic stress signaling in wheat—An inclusive overview of hormonal interactions during abiotic stress responses in wheat. Front. Plant Sci. 2018, 9, 734. [Google Scholar] [CrossRef]
- Singh, S.; Singh, G.; Singh, P.; Singh, N. Effect of water stress at different stages of grain development on the characteristics of starch and protein of different wheat varieties. Food Chem. 2008, 108, 130–139. [Google Scholar] [CrossRef]
- Wahid, A.; Close, T. Expression of dehydrins under heat stress and their relationship with water relations of sugarcane leaves. Biol. Plant. 2007, 51, 104–109. [Google Scholar] [CrossRef]
- Easterling, D.R.; Horton, B.; Jones, P.D.; Peterson, T.C.; Karl, T.R.; Parker, D.E.; Salinger, M.J.; Razuvayev, V.; Plummer, N.; Jamason, P. Maximum and minimum temperature trends for the globe. Science 1997, 277, 364–367. [Google Scholar] [CrossRef] [Green Version]
- Pittock, A.B. Climate Change: An Australian Guide to the Science and Potential Impacts; Australian Greenhouse Office: Canberra, Australia, 2003; Volume 54, pp. 87–88. [Google Scholar]
- Hoegh-Guldberg, O.; Jacob, D.; Taylor, M. Chapter 3: Impacts of 1.5 °C global warming on natural and human systems. In Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Preindustrial Levels and Related Global Greenhouse Gas Emission Pathways; An IPCC Special Report; Marengo, J.A., Pereira, J., Sherstyukov, B., Eds.; IPCC: Geneva, Switzerland, 2018; pp. 175–311. [Google Scholar]
- Barnabás, B.; Jäger, K.; Fehér, A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2008, 31, 11–38. [Google Scholar] [CrossRef] [PubMed]
- Stone, P.; Nicolas, M. A survey of the effects of high temperature during grain filling on yield and quality of 75 wheat cultivars. Aust. J. Agric. Res. 1995, 46, 475–492. [Google Scholar] [CrossRef]
- Fischer, R.; Byerlee, D. Trends of Wheat Production in Warmer Areas: Issues and Economic Consideration. Wheat for Non-Traditional, Warm Areas; CIMMYT: Mexico City, Mexico, 1991; 322p. [Google Scholar]
- Jaleel, C.A.; Manivannan, P.; Wahid, A.; Farooq, M.; Al-Juburi, H.J.; Somasundaram, R.; Panneerselvam, R. Drought stress in plants: A review on morphological characteristics and pigments composition. Int. J. Agric. Biol. 2009, 11, 100–105. [Google Scholar]
- Yoon, J.S.; Kim, J.Y.; Kim, D.Y.; Seo, Y.W. A novel wheat ASR gene, TaASR2D, enhances drought tolerance in brachypodium distachyon. Plant Physiol. Biochem. 2020, 159, 400–414. [Google Scholar] [CrossRef]
- Faghani, E.; Gharechahi, J.; Komatsu, S.; Mirzaei, M.; Khavarinejad, R.A.; Najafi, F.; Farsad, L.K.; Salekdeh, G.H. Comparative physiology and proteomic analysis of two wheat genotypes contrasting in drought tolerance. J. Proteome 2015, 114, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.S.; Kim, J.B.; Hong, M.J.; Kim, K.H.; Seo, Y.W. Transcript analysis of wheat WAS-2 gene family under high temperature stress during ripening period. Plant Breed. Biotechnol. 2018, 6, 363–380. [Google Scholar] [CrossRef]
- Farooq, M.; Bramley, H.; Palta, J.A.; Siddique, K.H. Heat stress in wheat during reproductive and grain-filling phases. CRC. Crit. Rev. Plant Sci. 2011, 30, 491–507. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Khanna-Chopra, R. Effect of heat stress on grain starch content in diploid, tetraploid and hexaploid wheat species. J. Agron. Crop Sci. 2003, 189, 242–249. [Google Scholar] [CrossRef]
- Matsuki, J.; Yasui, T.; Kohyama, K.; Sasaki, T. Effects of environmental temperature on structure and gelatinization properties of wheat starch. Cereal Chem. 2003, 80, 476–480. [Google Scholar] [CrossRef]
- Lu, D.; Shen, X.; Cai, X.; Yan, F.; Lu, W.; Shi, Y.-C. Effects of heat stress during grain filling on the structure and thermal properties of waxy maize starch. Food Chem. 2014, 143, 313–318. [Google Scholar] [CrossRef]
- Denyer, K.; Hylton, C.; Smith, A. The effect of high temperature on starch synthesis and the activity of starch synthase. Funct. Plant Biol. 1994, 21, 783–789. [Google Scholar] [CrossRef]
- Jenner, C. Starch synthesis in the kernel of wheat under high temperature conditions. Funct. Plant Biol. 1994, 21, 791–806. [Google Scholar] [CrossRef]
- Ciaffi, M.; Margiotta, B.; Colaprico, G.; Stefanis, E.; Sgrulletta, D.; Lafiandra, D. Effect of high temperatures during grain filling on the amount of insoluble proteins in durum wheat [Apulia, Latium, Lombardy, Sicily]. J. Genet. Breed. 1995, 49, 285–296. [Google Scholar]
- Zhang, H.; Liang, W.; Yang, X.; Luo, X.; Jiang, N.; Ma, H.; Zhang, D. Carbon starved anther encodes a MYB domain protein that regulates sugar partitioning required for rice pollen development. Plant Cell 2010, 22, 672–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Xu, C.; He, Y.; Zong, J.; Yang, X.; Si, H.; Sun, Z.; Hu, J.; Liang, W.; Zhang, D. Mutation in CSA creates a new photoperiod-sensitive genic male sterile line applicable for hybrid rice seed production. Proc. Natl. Acad. Sci. USA 2013, 110, 76–81. [Google Scholar] [CrossRef] [Green Version]
- Yi, B.; Zhou, Y.F.; Gao, M.; Zhang, Z.; Han, Y.; Yang, G.; Wenjuan, X.; Huang, R. Effect of drought stress during flowering stage on starch accumulation and starch synthesis enzymes in sorghum grains. J. Integr. Agric. 2014, 13, 2399–2406. [Google Scholar] [CrossRef]
- Ahmed, I.M.; Cao, F.; Han, Y.; Nadira, U.A.; Zhang, G.; Wu, F. Differential changes in grain ultrastructure, amylase, protein and amino acid profiles between Tibetan wild and cultivated barleys under drought and salinity alone and combined stress. Food Chem. 2013, 141, 2743–2750. [Google Scholar] [CrossRef]
- Lu, H.; Wang, C.; Guo, T.; Xie, Y.; Feng, W.; Li, S. Starch composition and its granules distribution in wheat grains in relation to post-anthesis high temperature and drought stress treatments. Starch Stärke 2014, 66, 419–428. [Google Scholar] [CrossRef]
- Prasad, P.V.; Pisipati, S.; Ristic, Z.; Bukovnik, U.; Fritz, A. Impact of nighttime temperature on physiology and growth of spring wheat. Crop Sci. 2008, 48, 2372–2380. [Google Scholar] [CrossRef]
- Song, W.F.; Zhao, L.J.; Zhang, X.M.; Zhang, Y.M.; Li, J.L.; Zhang, L.L.; Song, Q.J.; Zhao, H.B.; Zhang, Y.B.; Zhang, C.L. Effect of timing of heat stress during grain filling in two wheat varieties under moderate and very high temperature. Indian J. Genet. 2015, 75, 121–124. [Google Scholar] [CrossRef]
- Dias, A.S.; Bagulho, A.S.; Lidon, F.C. Ultrastructure and biochemical traits of bread and durum wheat grains under heat stress. Braz. J. Plant Physiol. 2008, 20, 323–333. [Google Scholar] [CrossRef] [Green Version]
- Impa, S.M.; Vennapusa, A.R.; Bheemanahalli, R.; Sabela, D.; Boyle, D.; Walia, H.; Jagadish, S.K. High night temperature induced changes in grain starch metabolism alters starch, protein, and lipid accumulation in winter wheat. Plant Cell Environ. 2020, 43, 431–447. [Google Scholar] [CrossRef]
- Wang, Y.; Frei, M. Stressed food—The impact of abiotic environmental stresses on crop quality. Agric. Ecosyst. Environ. 2011, 141, 271–286. [Google Scholar] [CrossRef]
- Wang, S.; Li, T.; Miao, Y.; Zhang, Y.; He, Z.; Wang, S. Effects of heat stress and cultivar on the functional properties of starch in Chinese wheat. Cereal Chem. 2017, 94, 443–450. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, J.; Wang, Z.; Xu, G.; Zhu, Q. Activities of key enzymes in sucrose-to-starch conversion in wheat grains subjected to water deficit during grain filling. Plant Physiol. 2004, 135, 1621–1629. [Google Scholar] [CrossRef] [Green Version]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. J. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Huber, S.C.; Huber, J.L. Role and regulation of sucrose-phosphate synthase in higher plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1996, 47, 431–444. [Google Scholar] [CrossRef] [PubMed]
- Sato, A.; Okubo, H.; Saitou, K. Increase in the expression of an alpha-amylase gene and sugar accumulation induced during cold period reflects shoot elongation in hyacinth bulbs. J. Am. Soc. Hortic. Sci. 2006, 131, 185–191. [Google Scholar] [CrossRef]
- Liu, X.; Huang, B. Heat stress injury in relation to membrane lipid peroxidation in creeping bentgrass. Crop Sci. 2000, 40, 503–510. [Google Scholar] [CrossRef]
- Stobrawa, K.; Lorenc-Plucińska, G. Changes in carbohydrate metabolism in fine roots of the native European black poplar (Populus nigra L.) in a heavy-metal-polluted environment. Sci. Total Environ. 2007, 373, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.F.; Sartorelli, E.F.; Castilho, A.C.S.; Satrapa, R.A.; Puelker, R.Z.; Razza, E.M.; Ticianelli, H.P.; Loureiro, B.; Barros, C.M. Effects of heat stress on development, quality and survival of Bos indicus and Bos taurus embryos produced in vitro. Theriogenology 2013, 79, 351–357. [Google Scholar] [CrossRef] [Green Version]
- Nishanth, M.; Sheshadri, S.; Rathore, S.S.; Srinidhi, S.; Simon, B. Expression analysis of cell wall invertase under abiotic stress conditions influencing specialized metabolism in Catharanthus roseus. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basu, S.; Ramegowda, V.; Kumar, A.; Pereira, A. Plant adaptation to drought stress. F1000Research 2016, 5, 1–10. [Google Scholar] [CrossRef]
- Wilhelm, E.P.; Mullen, R.E.; Keeling, P.; Singletary, G. Heat stress during grain filling in maize: Effects on kernel growth and metabolism. Crop Sci. 1999, 39, 1733–1741. [Google Scholar] [CrossRef]
- Lu, H.; Hu, Y.; Wang, C.; Liu, W.; Ma, G.; Han, Q.; Ma, D. Effects of high temperature and drought stress on the expression of gene encoding enzymes and the activity of key enzymes involved in starch biosynthesis in wheat grains. Front. Plant Sci. 2019, 10, 1414. [Google Scholar] [CrossRef] [PubMed]
- Preiss, J. Biochemistry and molecular biology of starch biosynthesis and its regulation. Oxf. Surv. Plant Mol. Cell Biol. 1991, 7, 59–114. [Google Scholar]
- Duke, E.R.; Doehlert, D.C. Effects of heat stress on enzyme activities and transcript levels in developing maize kernels grown in culture. J. Environ. Exp. Bot. 1996, 36, 199–208. [Google Scholar] [CrossRef]
- Sowokinos, J.R. Pyrophosphorylases in Solanum tuberosum: I. Changes in ADP-glucose and UDP-glucose pyrophosphorylase activities associated with starch biosynthesis during tuberization, maturation, and storage of potatoes. Plant Physiol. 1976, 57, 63–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feliciano, P. Hexokinase 2 required in tumors. Nat. Genet. 2013, 45, 969. [Google Scholar] [CrossRef]
- Xiao, W.; Sheen, J.; Jang, J.C. The role of hexokinase in plant sugar signal transduction and growth and development. Plant Mol. Biol. 2000, 44, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Hayano-Kanashiro, C.; Calderón-Vázquez, C.; Ibarra-Laclette, E.; Herrera-Estrella, L.; Simpson, J. Analysis of gene expression and physiological responses in three Mexican maize landraces under drought stress and recovery irrigation. PLoS ONE 2009, 4, e7531. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Jing, L.; Yan, F.; Lu, D.; Wang, G.; Lu, W. Effects of heat stress during grain filling on sugar accumulation and enzyme activity associated with sucrose metabolism in sweet corn. Acta Agron. Sin. 2013, 39, 1644–1651. [Google Scholar] [CrossRef]
- Ahmed, N.; Tetlow, I.J.; Nawaz, S.; Iqbal, A.; Mubin, M.; Nawaz ul Rehman, M.S.N.; Butt, A.; Lighfoot, D.A.; Maekawa, M. Effect of high temperature on grain filling period, yield, amylose content and activity of starch biosynthesis enzymes in endosperm of basmati rice. J. Sci. Food Agric. 2015, 95, 2237–2243. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Gu, X.; Ding, M.; Lu, W.; Lu, D. Heat stress during grain filling affects activities of enzymes involved in grain protein and starch synthesis in waxy maize. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.R.; Sharma, S.K.; Goswami, S.; Verma, P.; Singh, K.; Dixit, N.; Pathak, H.; Viswanathan, C.; Rai, R.D. Salicylic acid alleviates the heat stress-induced oxidative damage of starch biosynthesis pathway by modulating the expression of heat-stable genes and proteins in wheat (Triticum aestivum). Acta Physiol. Plant 2015, 37, 1–12. [Google Scholar] [CrossRef]
- Sumesh, K.; Sharma-Natu, P.; Ghildiyal, M. Starch synthase activity and heat shock protein in relation to thermal tolerance of developing wheat grains. Biol. Plant 2008, 52, 749–753. [Google Scholar] [CrossRef]
- Sharma-Natu, P.; Sumesh, K.; Ghildiyal, M. Heat shock protein in developing grains in relation to thermotolerance for grain growth in wheat. J. Agron. Crop Sci. 2010, 196, 76–80. [Google Scholar] [CrossRef]
- Yang, X.; Westcott, S.; Gong, X.; Evans, E.; Zhang, X.-Q.; Lance, R.C.; Li, C. Amino acid substitutions of the limit dextrinase gene in barley are associated with enzyme thermostability. Mol. Breed. 2009, 23, 61–74. [Google Scholar] [CrossRef]
- Gomes, I.; Gomes, J.; Steiner, W. Highly thermostable amylase and pullulanase of the extreme thermophilic eubacterium Rhodothermus marinus: Production and partial characterization. Bioresour. Technol. 2003, 90, 207–214. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Cai, S.; Ye, L.; Zhou, M.; Chen, Z.; Chen, Z.; Zhkang, G.; Dai, F. Genetic diversity and QTL mapping of thermostability of limit dextrinase in barley. J. Agric. Food Chem. 2015, 63, 3778–3783. [Google Scholar] [CrossRef]
- Ye, H.; Zhang, G. The influence of drought stress on malt quality traits of the wild and cultivated barleys. J. Integr. Agric. 2020, 19, 2009–2015. [Google Scholar]
- Yu, X.; Li, B.; Wang, L.; Chen, X.; Wang, W.; Gu, Y.; Wang, Z.; Xiong, F. Effect of drought stress on the development of endosperm starch granules and the composition and physicochemical properties of starches from soft and hard wheat. J. Sci. Food Agric. 2016, 96, 2746–2754. [Google Scholar] [CrossRef]
- Wu, X.; Tang, Y.; Li, C.; McHugh, A.D.; Li, Z.; Wu, C. Individual and combined effects of soil waterlogging and compaction on physiological characteristics of wheat in southwestern China. Field Crops Res. 2018, 215, 163–172. [Google Scholar] [CrossRef]
- Yang, H.; Zhai, S.; Li, Y.; Zhou, J.; He, R.; Liu, J.; Xue, Y.; Meng, Y. Waterlogging reduction and wheat yield increase through long-term ditch-buried straw return in a rice—Wheat rotation system. Field Crops Res. 2017, 209, 189–197. [Google Scholar] [CrossRef]
- Araki, H.; Hamada, A.; Hossain, M.A.; Takahashi, T. Waterlogging at jointing and/or after anthesis in wheat induces early leaf senescence and impairs grain filling. Field Crops Res. 2012, 137, 27–36. [Google Scholar] [CrossRef]
- Xie, Z.; Jiang, D.; Cao, W.; Dai, T. Effects of post-anthesis soil water status on grain starch and protein accumulation in specialty wheat varieties. Plant Growth Regul. 2003, 29, 309–316. [Google Scholar]
- Ahmadi, A.; Baker, D. The effect of water stress on the activities of key regulatory enzymes of the sucrose to starch pathway in wheat. Plant Growth Regul. 2001, 35, 81–91. [Google Scholar] [CrossRef]
- Zhou, Q.; Huang, M.; Huang, X.; Liu, J.; Wang, X.; Cai, J.; Dai, T.; Cao, W.; Jiang, D. Effect of post-anthesis waterlogging on biosynthesis and granule size distribution of starch in wheat grains. Plant Physiol. Biochem. 2018, 132, 222–228. [Google Scholar] [CrossRef]
- Zhou, Q.; Wu, X.; Xin, L.; Jiang, H.; Wang, X.; Cai, J.; Jiang, D. Waterlogging and simulated acid rain after anthesis deteriorate starch quality in wheat grain. Plant Growth Regul. 2018, 85, 257–265. [Google Scholar] [CrossRef]
- Bewley, J.D.; Black, M.; Halmer, P. The Encyclopedia of Seeds: Science, Technology and Uses; CABI: London, UK, 2006; 828p. [Google Scholar]
- Vetch, J.M.; Stougaard, R.N.; Martin, J.M.; Giroux, M.J. Revealing the genetic mechanisms of pre-harvest sprouting in hexaploid wheat (Triticum aestivum L.). Plant Sci. 2019, 281, 180–185. [Google Scholar] [CrossRef]
- Hagberg, S. A rapid method for determining alpha-amylase activity. Cereal Chem. 1960, 37, 218–222. [Google Scholar]
- Wahl, T.I.; O’Rourke, A.D. The economics of sprout damage in wheat. Agribusiness 1994, 10, 27–41. [Google Scholar] [CrossRef]
- Rasul, G.; Humphreys, D.G.; Brûlé-Babel, A.; McCartney, C.A.; Knox, R.E.; DePauw, R.M.; Somers, D.J. Mapping QTLs for pre-harvest sprouting traits in the spring wheat cross ‘RL4452/AC Domain’. Euphytica 2009, 168, 363–378. [Google Scholar] [CrossRef]
- Kamal, A.H.M.; Kim, K.H.; Shin, D.H.; Seo, H.S.; Shin, K.H.; Park, C.S.; Heo, H.Y.; Woo, S.H. Proteomics profile of pre-harvest sprouting wheat by using MALDI-TOF mass spectrometry. Plant Omics 2009, 2, 110. [Google Scholar]
- Chen, H.J.; Chen, J.Y.; Wang, S.J. Molecular regulation of starch accumulation in rice seedling leaves in response to salt stress. Acta Physiol. Plant 2008, 30, 135–142. [Google Scholar] [CrossRef]
- Theerawitaya, C.; Boriboonkaset, T.; Cha-Um, S.; Supaibulwatana, K.; Kirdmanee, C. Transcriptional regulations of the genes of starch metabolism and physiological changes in response to salt stress rice (Oryza sativa L.) seedlings. Physiol. Mol. Biol. Plants 2012, 18, 197–208. [Google Scholar] [CrossRef] [Green Version]
- He, J.F.; Goyal, R.; Laroche, A.; Zhao, M.L.; Lu, Z.X. Effects of salinity stress on starch morphology, composition and thermal properties during grain development in triticale. Can. J. Plant Sci. 2013, 93, 765–771. [Google Scholar] [CrossRef] [Green Version]
- Gale, K.R. Diagostic DNA markers for quality traits in wheat. J. Cereal Sci. 2005, 41, 181–192. [Google Scholar] [CrossRef]
- Liu, L.; Ikeda, T.M.; Branlard, G.; Peña, R.J.; Rogers, W.J.; Lerner, S.E.; Kolman, M.A.; Xia, X.; Wang, L.; Ma, W.; et al. Comparison of low molecular weight glutenin subunits identified by SDS-PAGE, 2-DE, MALDI-TOF-MS and PCR in common wheat. BMC Plant Biol. 2010, 10, 124. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Li, G.; Peña, R.J.; Xia, X.; He, Z. Development of STS markers and establishment of multiplex PCR for Glu-A3 alleles in common wheat (Triticum aestivum L.). J. Cereal Sci. 2010, 51, 305–312. [Google Scholar] [CrossRef]
- Liu, S.; Chao, S.; Anderson, J.A. New DNA markers for high molecular weight glutenin subunits in wheat. Theor. Appl. Genet. 2008, 118, 177–183. [Google Scholar] [CrossRef]
- Ma, W.; Zhang, W.; Gale, K.R. Multiplex-PCR typing of high molecular weight glutenin alleles in wheat. Euphytica 2003, 134, 51–60. [Google Scholar] [CrossRef]
- Ragupathy, R.; Naeem, H.A.; Reimer, E.; Lukow, O.M.; Sapirstein, H.D.; Cloutier, S. Evolutionary origin of the segmental duplication encompassing the wheat GLU-B1 locus encoding the overexpressed Bx7 (Bx7OE) high molecular weight glutenin subunit. Theor. Appl. Genet. 2008, 116, 283–296. [Google Scholar] [CrossRef]
- Xu, Q.; Xu, J.; Liu, C.L.; Chang, C.; Wang, C.P.; You, M.S.; Li, B.Y.; Liu, G.T. PCR-based markers for identification of HMW-GS at Glu-B1x loci in common wheat. J. Cereal Sci. 2008, 47, 394–398. [Google Scholar] [CrossRef]
- Lei, Z.S.; Gale, K.R.; He, Z.H.; Gianibelli, C.; Larroque, O.; Xia, X.C.; Butow, B.J.; Ma, W. Y-type gene specific markers for enhanced discrimination of high-molecular weight glutenin alleles at the Glu-B1 locus in hexaploid wheat. J. Cereal Sci. 2006, 43, 94–101. [Google Scholar] [CrossRef]
- Wang, J.; He, X.; He, Z.; Wang, H.; Xia, X. Cloning and phylogenetic analysis of phytoene synthase 1 (Psy1) genes in common wheat and related species. Hereditas 2009, 146, 208–256. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhang, F.Y.; Xia, X.C.; Dong, Z.D.; Cui, D.Q. Distribution of puroindoline alleles in bread wheat cultivars of the Yellow and Huai valley of China and discovery of a novel puroindoline a allele without PINA protein. Mol. Breed. 2012, 29, 371–378. [Google Scholar] [CrossRef]
- Giroux, M.J.; Morris, C.F. A glycine to serine change in puroindoline b is associated with wheat grain hardness and low levels of starch-surface friabilin. Theor. Appl. Genet. 1997, 95, 857–864. [Google Scholar] [CrossRef]
- Li, G.; He, Z.; Lillemo, M.; Sun, Q.; Xia, X. Molecular characterization of allelic variations at Pina and Pinb loci in Shandong wheat landraces, historical and current cultivars. J. Cereal Sci. 2008, 47, 510–517. [Google Scholar] [CrossRef]
- Chen, F.; Beecher, B.; Morris, C. Physical mapping and a new variant of Puroindoline b-2 genes in wheat. Theor. Appl. Genet. 2010, 120, 745–751. [Google Scholar] [CrossRef]
- Nakamura, T.; Vrinten, P.; Saito, M.; Konda, M. Rapid classification of partial waxy wheats using PCR-based markers. Genome 2002, 45, 1150–1156. [Google Scholar] [CrossRef] [PubMed]
- Furtado, A.; Bundock, P.C.; Banks, P.M.; Fox, G.; Yin, X.; Henry, R.J. A novel highly differentially expressed gene in wheat endosperm associated with bread quality. Sci. Rep. 2015, 5, 10446. [Google Scholar] [CrossRef]
- Rasheed, A.; Wen, W.; Gao, F.; Zhai, S.; Jin, H.; Liu, J.; Guo, Q.; Zhang, Y.; Dreisigacker, S.; Xia, X.; et al. Development and validation of KASP assays for genes underpinning key economic traits in bread wheat. Theor. Appl. Genet. 2016, 129, 1843–1860. [Google Scholar] [CrossRef]
- He, Z.; Rasheed, A.; Xia, X.; Ma, W. Molecular marker development and application for improving qualities in bread wheat. In Wheat Quality for Improving Processing and Human Health; Igrejas, G., Ikeda, T.M., Guzmán, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 323–345. [Google Scholar]
- Jin, H.; Yan, J.; Peña, R.; Xia, X.; Morgounov, A.; Han, L.; Zhang, Y.; He, Z.H. Molecular detection of high- and low-molecular-weight glutenin subunit genes in common wheat cultivars from 20 countries using allele-specific markers. Crop Pasture Sci. 2011, 62, 746–754. [Google Scholar] [CrossRef]
- Yan, L.; Helguera, M.; Kato, K.; Fukuyama, S.; Sherman, J.; Dubcovsky, J. Allelic variation at the VRN-1 promoter region in polyploid wheat. Theor. Appl. Genet. 2004, 109, 1677–1686. [Google Scholar] [CrossRef] [Green Version]
- Murai, J.; Taira, T.; Ohta, D. Isolation and characterization of the three Waxy genes encoding the granule-bound starch synthase in hexaploid wheat. Gene 1999, 234, 71–79. [Google Scholar] [CrossRef]
- Vrinten, P.; Nakamura, T.; Yamamori, M. Molecular characterization of waxy mutations in wheat. Mol. Gen. Genet. 1999, 261, 463–471. [Google Scholar] [CrossRef]
- Saito, M.; Konda, M.; Vrinte, P.; Nakamura, K.; Nakamura, T. Molecular comparison of waxy null alleles in common wheat and identification of a unique null allele. Theor. Appl. Genet. 2004, 108, 1205–1211. [Google Scholar] [CrossRef]
- Schofield, J.D.; Greenwell, P. Wheat starch granule proteins and their technological significance. Cereals Eur. Context 1988, 8, 407–420. [Google Scholar]
- Shure, M.; Wessler, S.; Fedoroff, N. Molecular identification of the waxy locus in maize. Cell 1983, 35, 235–242. [Google Scholar] [CrossRef]
- Wickramasinghe, H.A.M.; Miura, H. Gene dosage effects of the wheat Wx alleles and their interaction on amylose synthesis in the endosperm. Euphytica 2003, 132, 303–310. [Google Scholar] [CrossRef]
- Zeng, M.; Morris, C.F.; Batey, I.L.; Wrigley, C.W. Sources of variation for starch gelatinisation, pasting and gelation properties in wheat. Cereal Chem. 1997, 74, 63–71. [Google Scholar] [CrossRef]
- Zhao, X.C.; Sharp, P.J. Production of all eight genotypes of null alleles at ‘waxy’ loci in bread wheat Triticum aestivum L. Plant Breed. 1998, 117, 488–490. [Google Scholar] [CrossRef]
- Crosbie, G.B. The relationship between starch swelling properties, paste viscosity and boiled noodle quality in wheat flours. J. Cereal Sci. 1991, 13, 145–150. [Google Scholar] [CrossRef]
- Crosbie, G.B.; Lambe, W.J.; Tsutsui, H.; Gilmour, R.F. Further evaluation of the flour swelling volume test for identifying wheats potentially suitable for Japanese noodles. J. Cereal Sci. 1992, 15, 271–280. [Google Scholar] [CrossRef]
- Sharma, R.; Sissons, M.J.; Rathjen, A.J.; Jenner, C.F. The null-4A allele at the waxy locus in durum wheat affects pasta cooking quality. J. Cereal Sci. 2002, 35, 287–297. [Google Scholar] [CrossRef]
- Vrinten, P.; Nakamura, T. Wheat granule-bound starch synthase I and II are encoded by separate genes that are expressed in different tissues. Plant Physiol. 2000, 122, 255–263. [Google Scholar] [CrossRef] [Green Version]
- Echt, C.S.; Schwartz, D. Evidence for the inclusion of controlling elements within the structural gene at the waxy locus in maize. Genetics 1981, 99, 275–284. [Google Scholar] [CrossRef]
- Nakamura, T.; Yamamori, M.; Hirano, H.; Hidaka, S.; Nagamine, T. Production of waxy (amylose-free) wheats. Mol. Gen. Genet. 1995, 248, 253–259. [Google Scholar] [CrossRef]
- Zhao, X.C.; Sharp, P.J. An improved 1D SDS-PAGE method for the identification of three bread wheat ‘waxy’ proteins. J. Cereal Sci. 1996, 23, 191–193. [Google Scholar] [CrossRef]
- Briney, A.; Wilson, R.; Potter, R.H.; Barclay, I.; Crosbie, G.; Appels, R.; Jones, M.G.K. A PCR-based marker for selection of starch and potential noodle quality in wheat. Mol. Breed. 1998, 4, 427–433. [Google Scholar] [CrossRef]
- Bhat, J.A.; Yu, D. High-throughput NGS-based genotyping and phenotyping: Role in genomics-assisted breeding for soybean improvement. Legume Sci. 2021, 3, e81. [Google Scholar] [CrossRef]
- Panahabadi, R.; Ahmadikhah, A.; McKee, L.S.; Ingvarsson, P.K.; Farrokhi, N. Genome-wide association mapping of mixed linkage (1,3;1,4)-β-glucan and starch contents in rice whole grain. Front. Plant Sci. 2021, 12, 665745. [Google Scholar] [CrossRef]
- Biselli, C.; Volante, A.; Desiderio, F.; Tondelli, A.; Gianinetti, A.; Finocchiaro, F.; Taddei, F.; Gazza, L.; Sgrulletta, D.; Cattivelli, L.; et al. GWAS for starch-related parameters in japonica rice (Oryza sativa L.). Plant. 2019, 8, 292. [Google Scholar] [CrossRef] [Green Version]
- Muqaddasi, Q.H.; Brassac, J.; Ebmeyer, E.; Kollers, S.; Korzun, V.; Argiller, O.; Stiewe, G.; Plieske, J.; Ganal, M.W.; Rode, M. Prospects of GWAS and predictive breeding for European winter wheat’s grain protein content, grain starch content, and grain hardness. Sci Rep. 2020, 10, 12541. [Google Scholar] [CrossRef]
- Hucl, P.; Chibbar, R.N. Variation for starch concentration in spring wheat and its repeatability relative to protein concentration. Cereal Chem. 1996, 73, 756–758. [Google Scholar]
- Miura, H.; Tanii, S. Endosperm starch properties in several wheat cultivars preferred for Japanese noodles. Euphytica 1994, 71, 171–175. [Google Scholar] [CrossRef]
- Båga, M.; Repellin, A.; Demeke, T.; Caswell, K.; Leung, N.; Abdel-Aal, E.S.; Hucl, P.; Chibbar, R.N. Wheat starch modification through biotechnology. Starch Stärke 1999, 51, 111–116. [Google Scholar] [CrossRef]
- Denyer, K.; Hylton, C.M.; Jenner, C.F.; Smith, A.M. Identification of multiple isoforms of soluble and granule-bound starch synthase in developing wheat endosperm. Planta 1995, 196, 256–265. [Google Scholar] [CrossRef]
- Hylton, C.M.; Denyer, K.; Keeling, P.L.; Chang, M.T.; Smith, A.M. The effect of waxy mutations on the granule-bound starch synthases of barley and maize endosperms. Planta 1996, 198, 230–237. [Google Scholar] [CrossRef]
- Davis, J.P.; Supatcharee, N.; Khandelwal, R.L.; Chibbar, R.N. Synthesis of novel starches in planta: Opportunities and challenges. Starch Stärke 2003, 55, 107–120. [Google Scholar] [CrossRef]
- Kaur, B.; Ariffin, F.; Bhar, R.; Karim, A.A. Progress in starch modification in the last decade. Food Hydrocoll. 2012, 26, 398–404. [Google Scholar] [CrossRef]
- Regina, A.; Bird, A.; Topping, D.; Bowden, S.; Freeman, J.; Barsby, T.; Kosar-Hashemi, B.; Li, Z.; Rahman, S.; Morell, M. High-amylose wheat generated by RNA interference improves indices of large-bowel health in rats. Proc. Natl. Acad. Sci. USA 2006, 103, 3546–3551. [Google Scholar] [CrossRef] [Green Version]
- Mishra, A.; Singh, A.; Sharma, M.; Kumar, P.; Roy, J. Development of EMS-induced mutation population for amylose and resistant starch variation in bread wheat (Triticum aestivum) and identification of candidate genes responsible for amylose variation. BMC Plant Biol. 2016, 16, 217. [Google Scholar] [CrossRef]
- Liu, H.; Wang, K.; Jia, Z.; Gong, Q.; Lin, Z.; Du, L.; Pei, X.; Ye, X. Efficient induction of haploid plants in wheat by editing of TaMTL using an optimized Agrobacterium-mediated CRISPR system. J. Environ. Exp. Bot. 2020, 71, 1337–1349. [Google Scholar] [CrossRef]
- Du, X.; Wei, J.; Luo, X.; Liu, Z.; Qian, Y.; Zhu, B.; Weng, Q.; Tang, H. Low-molecular-weight glutenin subunit LMW-N13 improves dough quality of transgenic wheat. Food Chem. 2020, 327, 127048. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, J.; Wang, X.; Li, T.; Majeed, U.; Hao, C.; Zhang, X. The NAC transcription factor NAC019-A1 is a negative regulator of starch synthesis in wheat developing endosperm. J. Environ. Exp. Bot. 2020, 71, 5794–5807. [Google Scholar] [CrossRef]
- Li, J.; Jiao, G.; Sun, Y.; Chen, J.; Zhong, Y.; Yan, L.; Jiang, D.; Ma, Y.; Xia, L. Modification of starch composition, structure and properties through editing of TaSBEIIa in both winter and spring wheat varieties by CRISPR/Cas9. Plant Biotechnol. J. 2020, 19, 937–951. [Google Scholar] [CrossRef]
- Guo, D.; Hou, Q.; Zhang, R.; Lou, H.; Li, Y.; Zhang, Y.; You, M.; Xie, C.; Liang, R.; Li, B. Over-Expressing TaSPA-B Reduces Prolamin and Starch Accumulation in Wheat (Triticum aestivum L.) Grains. Int. J. Mol. Sci. 2020, 21, 3257. [Google Scholar] [CrossRef]
- Ma, X.; Xue, H.; Sun, J.; Sajjad, M.; Wang, J.; Yang, W.; Li, X.; Zhang, A.; Liu, D. Transformation of Pinb-D1x to soft wheat produces hard wheat kernel texture. J. Cereal Sci. 2020, 91, 102889. [Google Scholar] [CrossRef]
- Song, Y.; Luo, G.; Shen, L.; Yu, K.; Yang, W.; Li, X.; Sun, J.; Zhan, K.; Cui, D.; Liu, D.; et al. TubZIP28, a novel bZIP family transcription factor from Triticum urartu, and TabZIP28, its homologue from Triticum aestivum, enhance starch synthesis in wheat. New Phytol. 2020, 226, 1384–1398. [Google Scholar] [CrossRef]
- Slade, A.J.; McGuire, C.; Loeffler, D.; Mullenberg, J.; Skinner, W.; Fazio, G.; Holm, A.; Brandt, K.M.; Steine, M.N.; Goodstal, J.F.; et al. Development of high amylose wheat through TILLING. BMC Plant Biol. 2012, 12, 69. [Google Scholar] [CrossRef] [Green Version]
- Hogg, A.C.; Gause, K.; Hofer, P.; Martin, J.M.; Graybosch, R.A.; Hansen, L.E.; Giroux, M.J. Creation of a high-amylose durum wheat through mutagenesis of starch synthase II (SSIIa). J. Cereal Sci. 2013, 57, 377–383. [Google Scholar] [CrossRef] [Green Version]
- Camerlengo, F.; Frittelli, A.; Sparks, C.A.; Doherty, A.; Martignago, D.; Larre, C.; Lupi, R.; Sestili, F.; Masci, S. CRISPR-Cas9 multiplex editing of the α-amylase/trypsin inhibitor genes to reduce allergen proteins in durum wheat. Front. Sustain. Food Syst. 2020, 4, 104. [Google Scholar] [CrossRef]
- Sestili, F.; Janni, M.; Doherty, A.; Botticella, E.; D’Ovidio, R.; Masci, S.; Jones, H.D.; Lafiandra, D. Increasing the amylose content of durum wheat through silencing of the SBEIIa genes. BMC Plant Biol. 2010, 10, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Guo, H.; Wang, Y.; Xie, Y.; Zhao, L.; Gu, J.; Zhao, S.; Zhao, B.; Wang, G.; Lie, L. Identification of novel alleles induced by EMS-mutagenesis in key genes of kernel hardness and starch biosynthesis in wheat by TILLING. Genes Genom. 2017, 39, 387–395. [Google Scholar] [CrossRef]
- Uauy, C.; Distelfeld, A.; Fahima, T.; Blechl, A.; Dubcovsky, J. A NAC gene regulating senescence improves grain protein, Zinc, and iron content in wheat. Science 2006, 314, 1298–1301. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Song, G.; Gao, J.; Zhang, S.; Zhang, R.; Li, W.; Chen, M.; Liu, M.; Xia, X.; Risacher, T.; et al. Enhancement of grain number per spike by RNA interference of cytokinin oxidase 2 gene in bread wheat. Hereditas 2018, 155, 33. [Google Scholar] [CrossRef] [Green Version]
- Li, J.R.; Zhao, W.; Li, Q.Z.; Ye, X.; An, B.-Y.; Li, X.; Zhang, X.-S. RNA silencing of waxy gene results in low levels of amylose in the seeds of transgenic wheat (Triticum aestivum L.). Acta Genet. Sin. 2005, 32, 846–854. [Google Scholar]
- Yue, S.J.; Li, H.; Li, Y.W.; Zhu, Y.F.; Guo, J.K.; Liu, Y.J.; Chen, Y.; Jia, X. Generation of transgenic wheat lines with altered expression levels of 1Dx5 high-molecular weight glutenin subunit by RNA interference. J. Cereal Sci. 2008, 47, 153–161. [Google Scholar] [CrossRef]
- Schaart, J.G.; van de Wiel, C.C.M.; Lotz, L.A.P.; Smulders, M.J.M. Opportunities for products of new plant breeding techniques. Trends Plant Sci. 2016, 21, 438–449. [Google Scholar] [CrossRef]
- Van de Wiel, C.C.M.; Schaart, J.G.; Lotz, L.A.P.; Smulders, M.J.M. New traits in crops produced by genome editing techniques based on deletions. Plant Biotechnol. Rep. 2017, 11, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Jouanin, A.; Gilissen, L.J.W.J.; Schaart, J.G.; Leigh, F.J.; Cockram, J.; Wallington, E.J.; Boyd, L.A.; van den Broeck, H.C.; van der Meer, I.M.; America, A.H.P.; et al. CRISPR/Cas9 Gene Editing of Gluten in Wheat to Reduce Gluten Content and Exposure—Reviewing Methods to Screen for Coeliac Safety. Front. Nutr. 2020, 7, 51. [Google Scholar] [CrossRef]
- Yotova, R. Regulating genome editing under international human rights law. Int. Comp. Law Q. 2020, 69, 653–684. [Google Scholar] [CrossRef]
- Kim, N.K. Gene-editing: Interpretation of current law and legal policy. Dev. Reprod. 2017, 21, 343–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schiemann, J.; Robienski, J.; Schleissing, S.; Spök, A.; Sprink, T.; Wilhelm, R.A. Editorial: Plant genome editing—Policies and governance. Front. Plant Sci. 2020, 11, 284. [Google Scholar] [CrossRef] [Green Version]
- Yue, J.J.; Hong, C.Y.; Wei, P.; Tsai, Y.C.; Lin, C.S. How to start your monocot CRISPR/Cas project: Plasmid design, efficiency detection, and offspring analysis. Rice 2020, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liang, Z.; Zong, Y.; Wang, Y.; Liu, J.; Chen, K.; Qiu, J.L.; Gao, C. Efficient and transgene-free genome editing in wheat through transient expression of CRISPR/Cas9 DNA or RNA. Nat. Commun. 2016, 7, 12617. [Google Scholar] [CrossRef] [Green Version]
- Asp, N.G. Resistant starch: Proceedings from the 2nd plenary meeting of EURESTA: European Flair Concerted Action no. 11 (COST 911): Physiological implications of the consumption of resistant starch in man. Eur. J. Clin. Nutr. 1992, 46, S1–S148. [Google Scholar]
- Jiang, H.; Lio, J.; Blanco, M.; Campbell, M.; Jane, J.L. Resistant-starch formation in high-amylose maize starch during kernel development. J. Agric. Food Chem. 2010, 58, 8043–8047. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Magliano, D.J.; Zimmet, P.Z. The worldwide epidemiology of type 2 diabetes mellitus—Present and future perspectives. Nat. Rev. Endocrinol. 2012, 8, 228–236. [Google Scholar] [CrossRef]
- Li, C.; Gilbert, R.G. Progress in controlling starch structure by modifying starch-branching enzymes. Planta 2016, 243, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Ball, S.G.; Morell, M.K. From bacterial glycogen to starch: Understanding the biogenesis of the plant starch granule. Annu. Rev. Plant Biol. 2003, 54, 207–233. [Google Scholar] [CrossRef] [PubMed]
- Hanashiro, I.; Abe, J.I.; Hizukuri, S. A periodic distribution of the chain length of amylopectin as revealed by high-performance anion-exchange chromatography. Carbohydr. Res. 1996, 283, 151–159. [Google Scholar] [CrossRef]
- Itoh, K.; Ozaki, H.; Okada, K.; Hori, H.; Takeda, Y.; Mitsui, T. Introduction of Wx transgene into rice wx mutants leads to both high-and low-amylose rice. Plant Cell Physiol. 2003, 44, 473–480. [Google Scholar] [CrossRef]
- Morell, M.K.; Myers, A.M. Towards the rational design of cereal starches. Curr. Opin. Plant Biol. 2005, 8, 204–210. [Google Scholar] [CrossRef]
- Rahman, S.; Bird, A.; Regina, A.; Li, Z.; Ral, J.P.; Mcmaugh, S.; Topping, D.; Morll, M. Resistant starch in cereals: Exploiting genetic engineering and genetic variation. J. Cereal Sci. 2007, 46, 251–260. [Google Scholar] [CrossRef]
- Yano, M.; Okuno, K.; Kawakami, J.; Satoh, H.; Omura, T. High amylose mutants of rice, Oryza sativa L. Theor. Appl. Genet. 1985, 69, 253–257. [Google Scholar] [CrossRef]
- Asaoka, M.; Okuno, K.; Sugimoto, Y.; Yano, M.; Omura, T.; Fuwa, H. Characterization of endosperm starch from high-amylose mutants of rice (Oryza sativa L.). Starch 1986, 38, 114–117. [Google Scholar] [CrossRef]
- Shan, Q.; Zhang, Y.; Chen, K.; Zhang, K.; Gao, C. Creation of fragrant rice by targeted knockout of the OsBADH2 gene using TALEN technology. Plant Biotechnol. J. 2015, 13, 791–800. [Google Scholar] [CrossRef]




| Type of RS | Description | Example | Reference |
|---|---|---|---|
| RS1 | Physically inaccessible starch | Whole or partly milled grains and seeds, legumes | [44] |
| RS2 | Ungelatinized resistant granules with B-type crystallinity and are hydrolyzed slowly by α-amylases | Raw potatoes, green bananas, some legumes, high amylose starches | [44] |
| RS3 | Retrograded starch (e.g., non-granular starch-derived materials) | Cooked and cooled potatoes, bread, cornflakes, food products with prolonged and/or repeated moist heat treatment | [45] |
| RS4 | Chemically modified starches due to cross-bonding with chemical reagents, ethers, esters, etc. | Some fiber drinks, foods in which modified starches have been used (e.g., certain breads and cakes) | [46] |
| RS5 | Amylose–lipid complex | Stearic acid-complexed high-amylose starch | [47] |
| Trait | Gene | Marker | Allele | KASP a | Standard | Reference |
|---|---|---|---|---|---|---|
| Gluten elasticity | Glu-A1 | UMN19 | Glu-A1(Ax1, Ax2 a, AxNull) | gluA1.1_1594; gluA1.1_1883 | Chinese Spring (CS), Opata 85 | [129] |
| Glu-A1 | Ax2 a | Glu-A1b(Ax2 a) | As above | Pavon 76, Opata 85 | [130] | |
| Glu-B1 | TaBAC1215C06-F517/R964 | Glu-B1al(Bx7OE) | Bx7OE | Dorico, ProINTA Colibr1, Klein Jabal | [131] | |
| Glu-B1 | cauBx642 | Glu-B1b(7 + 8); Glu-B1i(17 + 18); Glu-B1h(14 + 15) | NA | CS, Jing771, Pm97034 | [132] | |
| Glu-B1 | ZSBy9F2/R2 | Glu-B1f(13 + 16) | NA | Baxter | [133] | |
| Glu-B1 | ZSBy8F5/By8R5 | Glu-B1(By8) | NA | Sunco | [133] | |
| Glu-D1 | UMN25F/25R | Glu-D1(Dx2, Dx5) | Glu-D1d_SNP | CS, Pavon 76 | [129] | |
| Glu-D1 | UMN26F/26R | Glu-D1(Dy10, Dy12) | Glu-D1d_SNP | CS, Pavon 76 | [129] | |
| Glu-A3 | LA1F/SA1R | Glu-A3a | NA | Neixiang 188, Chinese Spring | [128] | |
| Glu-A3 | LA3F/SA2R | Glu-A3b | NA | Gabo, Pavon 76 | [128] | |
| Glu-A3 | LA1F/SA3R | Glu-A3c | NA | Pitic, Seri 82 | [128] | |
| Glu-A3 | LA3F/SA4R | Glu-A3d | NA | Nidera Baguette 10, Cappelle-Desprez | [128] | |
| Glu-A3 | LA1F/SA5R | Glu-A3e | NA | Amadina, Marquis | [128] | |
| Glu-A3 | LA1F/SA6R | Glu-A3f | NA | Kitanokaori, Renan | [128] | |
| Glu-A3 | LA1F/SA7R | Glu-Ag | NA | Bluesky, Glenlea | [128] | |
| Glu-B3 | SB1F/SB1R | Glu-B3a | NA | Chinese Spring | [134] | |
| Glu-B3 | SB2F/SB2R | Glu-B3b | NA | Renan, Gabo | [134] | |
| Glu-B3 | SB3F/SB4R | Glu-B3c | NA | Insignia, Halberd | [134] | |
| Glu-B3 | SB4F/SB4R | Glu-B3d | NA | Pepital, Ernest | [134] | |
| Glu-B3 | SB5F/SB5R | Glu-B3e | NA | Cheyenne | [134] | |
| Glu-B3 | SB6F/SB6R | Glu-B3fg | NA | Fengmai 27 | [134] | |
| Glu-B3 | SB7F/SB7R | Glu-B3g | NA | Splendor, Cappelle-Desprez | [134] | |
| Glu-B3 | SB8F/SB8R | Glu-B3h | NA | Aca 303, Pavon 76 | [134] | |
| Glu-B3 | SB9F/SB9R | Glu-B3ad | NA | Opata 85 | [134], Ikeda unpublished | |
| Glu-B3 | SB10F/SB10R | Glu-B3bef | NA | Gawain | [134] | |
| Grain texture | Pina-D1 | Pina-N2 | Pina-D1a,b | Pina-D1_INS | Chinese Spring, Zhongyou 9507 | [135] |
| Pinb-D1 | Pinb-D1 | Pinb-D1a,b | Pinb-D1_INS | Chinese Spring, Lorvin10 | [136] | |
| Pinb-D1 | Pinb-DF/Pinb-DR | Pinb-D1p | No | Shannongyoumai 3 | [137] | |
| Pinb-B2 | Pinb-B2v2 | Pinb-B2a, b | Pinb2_IND | Chinese Spring, Zhongmai 175 | [138] | |
| Amylose content | Wx-A1 | AFC/AR2 | Null, Wild-type | NA | Norin 61, Kanton 107 | [139] |
| Wx-B1 | BDFL/BRD | Null, Wild-type | WxB1_SNP | Norin 61, Kanton 107 | [139] | |
| Wx-D1 | BDFL/DRSL | Null, Wild-type | NA | Norin 61, California | [139] | |
| Wheat bread-making quality | Wbm | NWPFor/Rev | Wbm_SNP | Mantol, Aca 601, Insignia | [140] | |
| Species | Target Gene | Target Trait | Results | Mutation System | Reference |
|---|---|---|---|---|---|
| Bread wheat | SBEII | Starch branching enzyme | Increased amylose/resistant starch contents | RNAi | [172] |
| Bread wheat | GBSSI, BMY, SSIII, SBEI, SBEIII, ISA3 | Waxy protein (GBSSI), starch degrading (BMY), starch synthase (SSIII), starch branching enzyme (SBEI, SBEIII), isoamylase (ISA3) | Amylose/resistant starch variation | EMS | [173] |
| Bread wheat | TaWaxy | Granule-bound starch synthase | - Developed the induction of haploids/improved starch quality | SpCas9, lbCpf1, xCas9 | [174] |
| Bread wheat | LMW-N13 | Low-molecular-weight glutenin subunit (LMW-GS) | Superior dough properties (overexpression) | Agro-mediated transformation | [175] |
| Bread wheat | NAC019-A1 | NAC transcription factor | Decreased starch granules | Agro-mediated transformation | [176] |
| Bread wheat | SBEIIa | Starch branching enzyme | Increased amylose/resistant starch contents | Cas9 | [177] |
| Bread wheat | SPA-B | Storage protein activator (member of the bZIP family) | Decreased starch/glutenin content (overexpression) | Agro-mediated transformation | [178] |
| Bread wheat | Pinb-D1x | Puroindoline | Increased the kernel hardness and changed the internal structure of the kernel, flour properties variation (overexpression) | Bombardment | [179] |
| Bread wheat | bZIP28 | Novel basic leucine zipper family | Decreased starch content | Cas9 | [180] |
| Durum/ bread wheat | SBEIIa | Starch branching enzyme | Increased amylose/resistant starch contents | EMS | [181] |
| Durum wheat | SGP-1 | Starch synthase | Increased amylose contents | EMS | [182] |
| Durum wheat | ATI | α-Amylase/Trypsin inhibitor | Reduced amount of potential allergens | Cas9 | [183] |
| Durum wheat | SBEIIa | Starch branching enzyme | Increased amylose/resistant starch contents | RNAi | [184] |
| High-gluten spring wheat | Pinb, waxy, Agp2, SSIIa | Puroindoline (Pinb), waxy, AGPase (Agp2), starch synthase (SSIIa) | Obtained 1 novel allelic variation in the mutant lines-kernel hardness gene Pinb Frame shift and missense mutation of waxy and SSIIa-A: deleterious effects on their functions | EMS | [185] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.-H.; Kim, J.-Y. Understanding Wheat Starch Metabolism in Properties, Environmental Stress Condition, and Molecular Approaches for Value-Added Utilization. Plants 2021, 10, 2282. https://doi.org/10.3390/plants10112282
Kim K-H, Kim J-Y. Understanding Wheat Starch Metabolism in Properties, Environmental Stress Condition, and Molecular Approaches for Value-Added Utilization. Plants. 2021; 10(11):2282. https://doi.org/10.3390/plants10112282
Chicago/Turabian StyleKim, Kyung-Hee, and Jae-Yoon Kim. 2021. "Understanding Wheat Starch Metabolism in Properties, Environmental Stress Condition, and Molecular Approaches for Value-Added Utilization" Plants 10, no. 11: 2282. https://doi.org/10.3390/plants10112282
APA StyleKim, K. -H., & Kim, J. -Y. (2021). Understanding Wheat Starch Metabolism in Properties, Environmental Stress Condition, and Molecular Approaches for Value-Added Utilization. Plants, 10(11), 2282. https://doi.org/10.3390/plants10112282