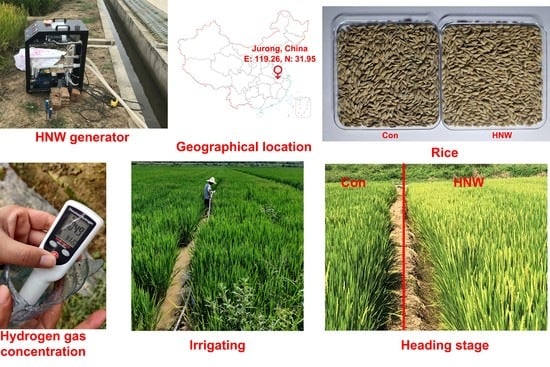
Molecular Hydrogen Increases Quantitative and Qualitative Traits of Rice Grain in Field Trials
Abstract
:1. Introduction
2. Results
2.1. Seed Morphology of Rice Crops Irrigated with HNW
2.2. The Morphology of White Rice
2.3. Qualitative Characters of White Rice Irrigated with HNW
2.4. HNW Influences Contents of the Metal Ions in White Rice
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Field Experiments
4.2. Determination of Qualitative Characters
4.3. Determination of Ion Content
4.4. Determination of Albumin, Globulin, Prolamin, and Glutelin Content
4.5. Real-Time Quantitative Reverse Transcription-PCR (qPCR)
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Law, J.W.-F.; Ser, H.-L.; Khan, T.M.; Chuah, L.-H.; Pusparajah, P.; Chan, K.-G.; Goh, B.-H.; Lee, L.-H. The Potential of Streptomyces as Biocontrol Agents against the Rice Blast Fungus, Magnaporthe oryzae (Pyricularia oryzae). Front. Microbiol. 2017, 8, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Siebenmorgen, T.J.; Griffin, K. Quality Characteristics of Long-Grain Rice Milled in Two Commercial Systems. Cereal Chem. J. 1998, 75, 560–565. [Google Scholar] [CrossRef]
- Ueno, D.; Yamaji, N.; Kono, I.; Huang, C.F.; Ando, T.; Yano, M.; Ma, J.F. Gene limiting cadmium accumulation in rice. Proc. Natl. Acad. Sci. USA 2010, 107, 16500–16505. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Chen, S.; Ren, X.; Lu, Y.; Liu, D.; Cai, X.; Li, Q.; Gao, J.; Liu, Q. Molecular Structure and Physicochemical Properties of Starches from Rice with Different Amylose Contents Resulting from Modification of OsGBSSI Activity. J. Agric. Food Chem. 2017, 65, 2222–2232. [Google Scholar] [CrossRef] [PubMed]
- Soltis, P.S.; Soltis, D.E. The Role of Hybridization in Plant Speciation. Annu. Rev. Plant Biol. 2009, 60, 561–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, L.; Zhang, C.; Zuo, F.; Zheng, L.; Li, D.; Zhang, A.; Zhang, D. Effects of fertilizers and pesticides on the mineral elements used for the geographical origin traceability of rice. J. Food Compos. Anal. 2019, 83, 103276. [Google Scholar] [CrossRef]
- Moose, S.P.; Mumm, R.H. Molecular Plant Breeding as the Foundation for 21st Century Crop Improvement. Plant Physiol. 2008, 147, 969–977. [Google Scholar] [CrossRef] [Green Version]
- Yu, Q.; Liu, S.; Yu, L.; Xiao, Y.; Zhang, S.; Wang, X.; Xu, Y.; Yu, H.; Li, Y.; Yang, J.; et al. RNA demethylation increases the yield and biomass of rice and potato plants in field trials. Nat. Biotechnol. 2021. [Google Scholar] [CrossRef]
- Jiang, L. Commercialization of the gene-edited crop and morality: Challenges from the liberal patent law and the strict GMO law in the EU. New Genet. Soc. 2019, 39, 191–218. [Google Scholar] [CrossRef]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.-I.; Katayama, Y.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Sun, X. Hydrogen biology: It is just beginning. Chin. J. Biochem. Mol. Bio. 2019, 35, 1037–1050. [Google Scholar]
- Jin, Q.; Zhu, K.; Cui, W.; Xie, Y.; Han, B.; Shen, W. Hydrogen gas acts as a novel bioactive molecule in enhancing plant tolerance to paraquat-induced oxidative stress via the modulation of heme oxygenase-1 signalling system. Plant Cell Environ. 2012, 36, 956–969. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Mao, Y.; Lai, D.; Zhang, W.; Shen, W. H2 enhances Arabidopsis salt tolerance by manipulating ZAT10/12-mediated anti-oxidant defence and controlling sodium exclusion. PLoS ONE 2012, 7, e49800. [Google Scholar] [CrossRef] [Green Version]
- Xie, Y.; Mao, Y.; Zhang, W.; Lai, D.; Wang, Q.; Shen, W. Reactive Oxygen Species-Dependent Nitric Oxide Production Contributes to Hydrogen-Promoted Stomatal Closure in Arabidopsis. Plant Physiol. 2014, 165, 759–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Z.; Duan, X.; Yao, P.; Cui, W.; Cheng, D.; Zhang, J.; Jin, Q.; Chen, J.; Dai, T.; Shen, W. Hydrogen Gas Is Involved in Auxin-Induced Lateral Root Formation by Modulating Nitric Oxide Synthesis. Int. J. Mol. Sci. 2017, 18, 2084. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lou, W.; Kong, L.; Shen, W. Hydrogen Commonly Applicable from Medicine to Agriculture: From Molecular Mechanisms to the Field. Curr. Pharm. Des. 2021, 27, 747–759. [Google Scholar] [CrossRef]
- Hu, H.; Zhao, S.; Li, P.; Shen, W. Hydrogen gas prolongs the shelf life of kiwifruit by decreasing ethylene biosynthesis. Postharvest Biol. Technol. 2018, 135, 123–130. [Google Scholar] [CrossRef]
- Hu, H.; Li, P.; Shen, W. Preharvest application of hydrogen-rich water not only affects daylily bud yield but also contributes to the alleviation of bud browning. Sci. Hortic. 2021, 287, 110267. [Google Scholar] [CrossRef]
- Su, J.; Nie, Y.; Zhao, G.; Cheng, D.; Wang, R.; Chen, J.; Zhang, S.; Shen, W. Endogenous hydrogen gas delays petal senescence and extends the vase life of lisianthus cut flowers. Postharvest Biol. Technol. 2019, 147, 148–155. [Google Scholar] [CrossRef]
- Li, L.; Liu, Y.; Wang, S.; Zou, J.; Ding, W.; Shen, W. Magnesium Hydride-Mediated Sustainable Hydrogen Supply Prolongs the Vase Life of Cut Carnation Flowers via Hydrogen Sulfide. Front. Plant Sci. 2020, 11, 595376. [Google Scholar] [CrossRef]
- Jiang, K.; Kuang, Y.; Feng, L.; Liu, Y.; Wang, S.; Du, H.; Shen, W. Molecular Hydrogen Maintains the Storage Quality of Chinese Chive through Improving Antioxidant Capacity. Plants 2021, 10, 1095. [Google Scholar] [CrossRef] [PubMed]
- Nghia, N.H.; van Giang, P.T.; Hanh, N.T.; St-Hilaire, S.; Domingos, A.J. Control of Vibrioparahaemolyticus (AHPND strain) and improvement of water quality using nanobubble technology. Aquac. Res. 2021, 52, 2727–2739. [Google Scholar] [CrossRef]
- Agarwal, A.; Ng, W.J.; Liu, Y. Principle and applications of microbubble and nanobubble technology for water treatment. Chemosphere 2011, 84, 1175–1180. [Google Scholar] [CrossRef]
- Fan, W.; Zhang, Y.; Liu, S.; Li, X.; Li, J. Alleviation of copper toxicity in Daphnia magna by hydrogen nanobubble water. J. Hazard. Mater. 2020, 389, 122155. [Google Scholar] [CrossRef] [PubMed]
- Utsunomiya, Y.; Samejima, C.; Takayanagi, Y.; Izawa, Y.; Yoshida, T.; Sawada, Y.; Fujisawa, Y.; Kato, H.; Iwasaki, Y. Suppression of the rice heterotrimeric G protein β-subunit gene, RGB1, causes dwarfism and browning of internodes and lamina joint regions. Plant J. 2011, 67, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Rao, Y.; Zeng, D.; Yang, Y.; Xu, R.; Zhang, B.; Dong, G.; Qian, Q.; Li, Y. SMALL GRAIN 1, which encodes a mitogen-activated protein kinase kinase 4, influences grain size in rice. Plant J. 2014, 77, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fan, C.; Xing, Y.; Jiang, Y.; Luo, L.; Sun, L.; Shao, D.; Xu, C.; Li, X.; Xiao, J.; et al. Natural variation in GS5 plays an important role in regulating grain size and yield in rice. Nat. Genet. 2011, 43, 1266–1269. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, J.; Huang, J.; Lan, H.; Wang, C.; Yin, C.; Wu, Y.; Tang, H.; Qian, Q.; Li, J.; et al. Rare allele of OsPPKL1 associated with grain length causes extra-large grain and a significant yield increase in rice. Proc. Natl. Acad. Sci. 2012, 109, 21534–21539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, D.; Zhou, H.; Liu, R.; Dan, W.; Li, P.; Wu, B.; Chen, J.; Wang, L.; Gao, G.; Zhang, Q.; et al. GL3.3, a Novel QTL Encoding a GSK3/SHAGGY-like Kinase, Epistatically Interacts with GS3 to Produce Extra-long Grains in Rice. Mol. Plant 2018, 11, 754–756. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Wu, K.; Fu, X.; Liu, Q. Improving coordination of plant growth and nitrogen metabolism for sustainable agriculture. aBIOTECH 2020, 1, 255–275. [Google Scholar] [CrossRef]
- Mora-García, S.; Vert, G.; Yin, Y.; Caño-Delgado, A.; Cheong, H.; Chory, J. Nuclear protein phosphatases with Kelch-repeat domains modulate the response to brassinosteroids in Arabidopsis. Genes Dev. 2004, 18, 448–460. [Google Scholar] [CrossRef] [Green Version]
- Das, T.; Shukla, Y.M.; Poonia, T.C.; Meena, M.; Meena, M.D. Effects of brassinolide on physiological characteristics of rice (Oryza sativa L.) with different salinity levels. Ann. Biol. 2013, 29, 228–231. [Google Scholar]
- Yang, S.; Yuan, D.; Zhang, Y.; Sun, Q.; Xuan, Y.H. BZR1 Regulates Brassinosteroid-Mediated Activation of AMT1;2 in Rice. Front. Plant Sci. 2021, 12, 665883. [Google Scholar] [CrossRef] [PubMed]
- Rayee, R.; Xuan, T.; Khanh, T.; Tran, H.-D.; Kifayatullah, K. Efficacy of Irrigation Interval after Anthesis on Grain Quality, Alkali Digestion, and Gel Consistency of Rice. Agriculture 2021, 11, 325. [Google Scholar] [CrossRef]
- Sasaki, A.; Yamaji, N.; Yokosho, K.; Ma, J.F. Nramp5 Is a Major Transporter Responsible for Manganese and Cadmium Uptake in Rice. Plant Cell 2012, 24, 2155–2167. [Google Scholar] [CrossRef] [Green Version]
- Shimo, H.; Ishimaru, Y.; An, G.; Yamakawa, T.; Nakanishi, H.; Nishizawa, N.K. Low cadmium (LCD), a novel gene related to cadmium tolerance and accumulation in rice. J. Exp. Bot. 2011, 62, 5727–5734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaffron, H. Reduction of Carbon Dioxide with Molecular Hydrogen in Green Algæ. Nat. Cell Biol. 1939, 143, 204–205. [Google Scholar] [CrossRef]
- Gest, H.; Kamen, M.D. Photoproduction of Molecular Hydrogen by Rhodospirillum rubrum. Science 1949, 109, 558–559. [Google Scholar] [CrossRef]
- Renwick, G.M.; Giumarro, C.; Siegel, S.M. Hydrogen Metabolism in Higher Plants. Plant Physiol. 1964, 39, 303–306. [Google Scholar] [CrossRef]
- Zeng, J.; Zhang, M.; Sun, X. Molecular Hydrogen Is Involved in Phytohormone Signaling and Stress Responses in Plants. PLOS ONE 2013, 8, e71038. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Jiang, W.; Han, W.; Li, J.; Liu, Y. Effects of Hydrogen-Rich Water on Fitness Parameters of Rice Plants. Agron. J. 2017, 109, 2033–2039. [Google Scholar] [CrossRef] [Green Version]
- Fu, X.; Ma, L.; Gui, R.; Ashraf, U.; Li, Y.; Yang, X.; Zhang, J.; Imran, M.; Tang, X.; Tian, H.; et al. Differential response of fragrant rice cultivars to salinity and hydrogen rich water in relation to growth and antioxidative defense mechanisms. Int. J. Phytoremediation 2021, 23, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Kong, L.; Gui, R.; Yang, X.; Zhang, J.; Gong, Q.; Qin, D.; Zhuang, M.; Ashraf, U.; Mo, Z. Application of hydrogen-rich water modulates physio-biochemical functions and early growth of fragrant rice under Cd and Pb stress. Environ. Sci. Pollut. Res. 2021, 28, 58558–58569. [Google Scholar] [CrossRef] [PubMed]
- Moles, A.T.; Ackerly, D.D.; Webb, C.O.; Tweddle, J.C.; Dickie, J.B.; Pitman, A.J.; Westoby, M. Factors that shape seed mass evolution. Proc. Natl. Acad. Sci. 2005, 102, 10540–10544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radny, J.; van der Putten, W.H.; Tielbörger, K.; Meyer, K.M. Influence of seed size on performance of non-native annual plant species in a novel community at two planting densities. Acta Oecologica 2018, 92, 131–137. [Google Scholar] [CrossRef]
- Dong, Z.; Wu, L.; Kettlewell, B.; Caldwell, C.D.; Layzell, D.B. Hydrogen fertilization of soils—Is this a benefit of legumes in rotation? Plant Cell Environ. 2003, 26, 1875–1879. [Google Scholar] [CrossRef]
- Zuo, J.; Li, J. Molecular Genetic Dissection of Quantitative Trait Loci Regulating Rice Grain Size. Annu. Rev. Genet. 2014, 48, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Xu, R.; Duan, P.; Li, Y. Control of grain size in rice. Plant Reprod. 2018, 31, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Xu, R.; Li, Y. Molecular Networks of Seed Size Control in Plants. Annu. Rev. Plant Biol. 2019, 70, 435–463. [Google Scholar] [CrossRef]
- Xu, C.; Liu, Y.; Li, Y.; Xu, X.; Xu, C.; Li, X.; Xiao, J.; Zhang, Q. Differential expression of GS5 regulates grain size in rice. J. Exp. Bot. 2015, 66, 2611–2623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oldroyd, G.E.D.; Leyser, O. A plant’s diet, surviving in a variable nutrient environment. Science 2020, 368, 0196. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Wu, W. Potassium and phosphorus transport and signaling in plants. J. Integr. Plant Biol. 2021, 63, 34–52. [Google Scholar] [CrossRef]
- Balakrishna, A.K.; Auckaili, A.; Farid, M. Effect of high pressure impregnation on micronutrient transfer in rice. Food Chem. 2021, 362, 130244. [Google Scholar] [CrossRef]
- Van Dongen, M.V.; van den Berg, M.C.; Vink, N.; Kok, F.J.; de Graaf, C. Taste-nutrient relationships in commonly consumed foods. Br. J. Nutr. 2012, 108, 140–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pogoson, E.; Carey, M.; Meharg, C.; Meharg, A. Reducing the cadmium, inorganic arsenic and dimethylarsinic acid content of rice through food-safe chemical cooking pre-treatment. Food Chem. 2021, 338, 127842. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, A.; Prasad, M.N.V. Cadmium minimization in rice. A review. Agron. Sustain. Dev. 2014, 34, 155–173. [Google Scholar] [CrossRef]
- Dai, C.; Cui, W.; Pan, J.; Xie, Y.; Wang, J.; Shen, W. Proteomic analysis provides insights into the molecular bases of hydrogen gas-induced cadmium resistance in Medicago sativa. J. Proteom. 2017, 152, 109–120. [Google Scholar] [CrossRef]
- He, X.L.; Fan, S.K.; Zhu, J.; Guan, M.Y.; Liu, X.X.; Zhang, Y.S.; Jin, C.W. Iron supply prevents Cd uptake in Arabidopsis by inhibiting IRT1 expression and favoring competition between Fe and Cd uptake. Plant Soil 2017, 416, 453–462. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, M.; Ouwerkerk, P.B.F. Molecular and environmental factors determining grain quality in rice. Food Energy Secur. 2012, 1, 111–132. [Google Scholar] [CrossRef]
- Suo, B.; Li, H.; Wang, Y.; Li, Z.; Pan, Z.; Ai, Z. Effects of ZnO nanoparticle-coated packagingfilm on pork meat quality during cold storage. J. Sci. Food Agric. 2017, 97, 2023–2029. [Google Scholar] [CrossRef]
- Zhu, T.; Jackson, D.S.; Wehling, R.L.; Geera, B. Comparison of Amylose Determination Methods and the Development of a Dual Wavelength Iodine Binding Technique. Cereal Chem. J. 2008, 85, 51–58. [Google Scholar] [CrossRef]
- Cui, W.; Gao, C.; Fang, P.; Lin, G.; Shen, W. Alleviation of cadmium toxicity in Medicago sativa by hydrogen-rich water. J. Hazard. Mater. 2013, 260, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Shen, Z.; Ming, L.; Li, Y.; Dan, W.; Lou, G.; Peng, B.; Wu, B.; Li, Y.; Zhao, D.; et al. Genetic Basis of Variation in Rice Seed Storage Protein (Albumin, Globulin, Prolamin, and Glutelin) Content Revealed by Genome-Wide Association Analysis. Front. Plant Sci. 2018, 9, 612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Yang, B.; Wang, X.M.; Ma, H.Y.; Li, Y.J.; Dai, C.C. Effects of the fungal endophyte Phomopsis liquidambari on nitrogen uptake and me-tabolism in rice. Plant Growth Regul. 2014, 73, 165–179. [Google Scholar]
- Yan, M.; Fan, X.; Feng, H.; Miller, A.J.; Shen, Q.; Xu, G. Rice OsNAR2.1 interacts with OsNRT2.1, OsNRT2.2 and OsNRT2.3a nitrate transporters to provide uptake over high and low concentration ranges. Plant Cell Environ. 2011, 34, 1360–1372. [Google Scholar] [CrossRef]
- Wang, Q.; Su, Q.; Nian, J.; Zhang, J.; Guo, M.; Dong, G.; Hu, J.; Wang, R.; Wei, C.; Li, G.; et al. The Ghd7 transcription factor represses ARE1 expression to enhance nitrogen utilization and grain yield in rice. Mol. Plant 2021, 14, 1012–1023. [Google Scholar] [CrossRef]
- Ye, Y.; Li, P.; Xu, T.; Zeng, L.; Cheng, D.; Yang, M.; Luo, J.; Lian, X. OsPT4 Contributes to Arsenate Uptake and Transport in Rice. Front. Plant Sci. 2017, 8, 2197. [Google Scholar] [CrossRef] [Green Version]
- Okada, T.; Nakayama, H.; Shinmyo, A.; Yoshida, K. Expression of OsHAK genes encoding potassium ion transporters in rice. Plant Biotechnol. 2008, 25, 241–245. [Google Scholar] [CrossRef] [Green Version]
- Sakamoto, T.; Matsuoka, M. Characterization of CONSTITUTIVE PHOTOMORPHOGENESIS AND DWARFISM Homologs in Rice (Oryza sativa L.). J. Plant Growth Regul. 2006, 25, 245–251. [Google Scholar] [CrossRef]
- Tsukagoshi, Y.; Suzuki, H.; Seki, H.; Muranaka, T.; Ohyama, K.; Fujimoto, Y. Ajuga Δ24-Sterol Reductase Catalyzes the Direct Reductive Conversion of 24-Methylenecholesterol to Campesterol. J. Biol. Chem. 2016, 291, 8189–8198. [Google Scholar] [CrossRef] [Green Version]
- Crofts, N.; Satoh, Y.; Miura, S.; Hosaka, Y.; Abe, M.; Fujita, N. Active-type starch synthase (SS) IIa from indica rice partially com-plements the sugary-1 phenotype in japonica rice endosperm. Plant Mol. Biol. 2021. [Google Scholar] [CrossRef]
- Utsumi, Y.; Nakamura, Y. Structural and enzymatic characterization of the isoamylase1 homo-oligomer and the isoamylase1–isoamylase2 hetero-oligomer from rice endosperm. Planta 2006, 225, 75–87. [Google Scholar] [CrossRef]
- Satoh, H.; Nishi, A.; Yamashita, K.; Takemoto, Y.; Tanaka, Y.; Hosaka, Y.; Sakurai, A.; Fujita, N.; Nakamura, Y. Starch-branching enzyme I-deficient mutation spe-cifically affects the structure and properties of starch in rice endosperm. Plant Physiol. 2003, 133, 1111–1121. [Google Scholar]
- Nakamura, Y.; Utsumi, Y.; Sawada, T.; Aihara, S.; Utsumi, C.; Yoshida, M.; Kitamura, S. Characterization of the reactions of starch branching enzymes from rice endosperm. Plant Cell Physiol. 2010, 51, 776–794. [Google Scholar] [CrossRef] [Green Version]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, P.; Wang, J.; Zhao, Z.; Kong, L.; Lou, W.; Zhang, T.; Jing, D.; Yu, J.; Shu, Z.; Huang, L.; et al. Molecular Hydrogen Increases Quantitative and Qualitative Traits of Rice Grain in Field Trials. Plants 2021, 10, 2331. https://doi.org/10.3390/plants10112331
Cheng P, Wang J, Zhao Z, Kong L, Lou W, Zhang T, Jing D, Yu J, Shu Z, Huang L, et al. Molecular Hydrogen Increases Quantitative and Qualitative Traits of Rice Grain in Field Trials. Plants. 2021; 10(11):2331. https://doi.org/10.3390/plants10112331
Chicago/Turabian StyleCheng, Pengfei, Jun Wang, Zhushan Zhao, Lingshuai Kong, Wang Lou, Tong Zhang, Dedao Jing, Julong Yu, Zhaolin Shu, Liqin Huang, and et al. 2021. "Molecular Hydrogen Increases Quantitative and Qualitative Traits of Rice Grain in Field Trials" Plants 10, no. 11: 2331. https://doi.org/10.3390/plants10112331
APA StyleCheng, P., Wang, J., Zhao, Z., Kong, L., Lou, W., Zhang, T., Jing, D., Yu, J., Shu, Z., Huang, L., Zhu, W., Yang, Q., & Shen, W. (2021). Molecular Hydrogen Increases Quantitative and Qualitative Traits of Rice Grain in Field Trials. Plants, 10(11), 2331. https://doi.org/10.3390/plants10112331