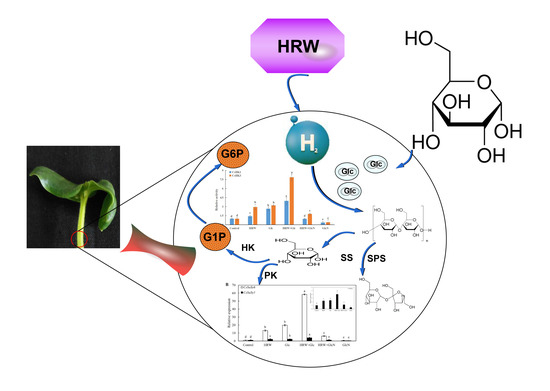
The Involvement of Glucose in Hydrogen Gas-Medicated Adventitious Rooting in Cucumber
Abstract
:1. Introduction
2. Results
2.1. Effect of Different Concentrations Glc on AR Development
2.2. Involvement of Glc in HRW-Regulated AR Development
2.3. Effects of HRW, Glc and GlcN on Glucose, Sucrose, Starch and Total Sugar Contents during Adventitious Rooting
2.4. Effects of HRW, Glc and GlcN on Hexose Phosphate Content during Adventitious Rooting
2.5. Effects of HRW, Glc and GlcN on Key Enzymes of Glucose Metabolism during Adventitious Rooting
2.6. Effects of HRW, Glc and GlcN on the Expression Levels of CsSuSy1, CsSuSy6, CsHK1, CsHK3, CsUDP1, CsUDP1-Like, CsG6P1 and CsG6P1-Like Genes during Adventitious Rooting
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. The Preparation of Hydrogen-Rich Water
4.3. Explant Treatments
4.4. Determination Glucose Content
4.5. Determination Sucrose Content
4.6. Determination Starch Content
4.7. Determination Total Sugar Content
4.8. Hexose Phosphate Content Measurements
4.9. SS, SPS, HK and PK AGPase Enzymes Activity Measurement
4.10. Quantitative Real-Time PCR (qRT-PCR)
4.11. Data Statistics and Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Klerk, G.J.; Van Der Krieken, W.; De Jong, J.C. Review the formation of adventitious roots: New concepts, new possibilities. Vitr. Cell. Dev. Biol. Plant 1999, 35, 189–199. [Google Scholar] [CrossRef]
- Li, S.W.; Leng, Y.; Feng, L.; Zeng, X.Y. Involvement of abscisic acid in regulating antioxidative defense systems and IAA-oxidase activity and improving adventitious rooting in mung bean [Vigna radiata (L.) Wilczek] seedlings under cadmium stress. Environ. Sci. Pollut. Res. 2014, 21, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Pop, T.I.; Pamfil, D.; Bellini, C. Auxin control in the formation of adventitious roots. Not. Bot. Horti. Agrobo. 2011, 39, 307–316. [Google Scholar] [CrossRef] [Green Version]
- Kwak, M.S.; Kim, I.H.; Kim, S.K.; Han, T.J. Effects of brassinolide with naphthalene acetic acid on the formation of adventitious roots, trichome-like roots and calli from cultured tobacco leaf segments, and the expression patterns of CNT103. J. Plant Biol. 2009, 52, 511. [Google Scholar] [CrossRef]
- Steffens, B.; Wang, J.X.; Sauter, M. Interactions between ethylene, gibberellin and abscisic acid regulate emergence and growth rate of adventitious roots in deepwater rice. Planta 2006, 223, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, A.; Hu, Y.M.; Depaepe, T.; Vandenbussche, F.; Boyer, D.F.; Geelen, D. Ethylene controls adventitious root initiation sites in arabidopsis hypocotyls independently of strigolactones. J. Plant Growth Regul. 2017, 36, 897–911. [Google Scholar] [CrossRef] [Green Version]
- Takáč, T.; Obert, B.; Rolčík, J.; Šamaj, J. Improvement of adventitious root formation in flax using hydrogen peroxide. New Biotechnol. 2016, 33, 728–734. [Google Scholar] [CrossRef]
- Jin, X.; Liao, W.B.; Yu, J.H.; Ren, P.J.; Dawuda, M.M.; Wang, M.; Niu, L.J.; Li, X.P.; Xu, X.T. Nitric oxide is involved in ethylene-induced adventitious rooting in marigold (Tagetes erecta L.). CAN. J. Plant Sci. 2017, 97, 620–631. [Google Scholar]
- Chen, Y.; Wang, M.; Hu, L.L.; Liao, W.B.; Dawuda, M.M.; Li, C.L. Carbon monoxide is involved in hydrogen gas-induced adventitious root development in cucumber under simulated drought stress. Front. Plant Sci. 2017, 8, 128. [Google Scholar] [CrossRef]
- Lin, Y.T.; Li, M.Y.; Cui, W.T.; Lu, W.; Shen, W.B. Haem oxygenase-1 is involved in hydrogen sulfide-induced cucumber adventitious root formation. J. Plant Growth Regul. 2012, 31, 519–528. [Google Scholar] [CrossRef]
- Bai, T.H.; Dong, Z.D.; Zheng, X.B.; Song, S.W.; Jiao, J.; Wang, M.M.; Song, C.H. Auxin and its interaction with ethylene control adventitious root formation and development in apple rootstock. Front. Plant Sci. 2020, 11, 574881. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.F.; Hou, Z.; Hao, S.Y.; Wu, W.C.; Mao, X.; Tao, X.G.; Lu, T.; Liu, B.Y. Hydrogen-rich water attenuates bran damage inflammation after traumatic brain injury in rats. Brain Res. 2016, 1637, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, S.M. Molecular hydrogen in sports medicine: New therapeutic perspectives. Int. J. Sports Med. 2015, 36, 273–279. [Google Scholar] [CrossRef] [Green Version]
- Li, C.X.; Gong, T.Y.; Bian, B.T.; Liao, W.B. Roles of hydrogen gas in plants: A review. Funct. Plant Biol. 2018, 45, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.L.; Li, P.X.; Wang, Y.N.; Gu, R.X. Hydrogen-rich water delays postharvest ripening and senescence of kiwifruit. Food Chem. 2014, 156, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.C.; Liao, W.B.; Niu, L.J.; Wang, M.; Ma, Z.J. Nitric oxide is involved in hydrogen gas-induced cell cycle activation during adventitious root formation in cucumber. BMC Plant Biol. 2016, 16, 146. [Google Scholar] [CrossRef] [Green Version]
- Rolland, F.; Baena-Gonzalez, E.; Sheen, J. Sugar sensing and signaling in plants: Conserved and novel mechanisms. Annu. Rev. Plant Biol. 2006, 57, 675–709. [Google Scholar] [CrossRef] [Green Version]
- Xiao, W.Y.; Sheen, J.; Jang, J.C. The role of hexokinase in plant sugar signal transduction and growth and development. Plant Mol. Biol. 2000, 44, 451–461. [Google Scholar] [CrossRef]
- Sturm, A. Invertases. Primary structures, functions, and roles in plant development and sucrose partitioning. Plant Physiol. 1999, 121, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Ren, X.D.; Zhang, J.J. Research progresses on the key enzymes involved in sucrose metabolism in maize. Carbohydr. Res. 2013, 368, 29–34. [Google Scholar] [CrossRef]
- Das, A.; Rushton, P.J.; Rohila, J.S. Metabolomic profiling of soybeans (Glycine max L.) reveals the importance of sugar and nitrogen metabolism under drought and heat stress. Plants 2017, 6, 21. [Google Scholar] [CrossRef] [Green Version]
- Gibson, S.I. Control of plant development and gene expression by sugar signaling. Curr. Opin. Plant Biol. 2005, 8, 93–102. [Google Scholar] [CrossRef]
- Saksena, H.B.; Sharma, M.; Singh, D.; Laxmi, A. The versatile role of glucose signalling in regulating growth, development and stress responses in plants. J. Plant Biochem. Biot. 2020, 29, 687–699. [Google Scholar] [CrossRef]
- Mishra, B.S.; Singh, M.; Aggrawal, P.; Laxmi, A. Glucose and auxin signaling interaction in controlling Arabidopsis thaliana seedlings root growth and development. PLoS ONE 2009, 4, e4502. [Google Scholar] [CrossRef] [PubMed]
- Li, C.X.; Bian, B.T.; Gong, T.Y.; Liao, W.B. Comparative proteomic analysis of key proteins during abscisic acid-hydrogen peroxide-induced adventitious rooting in cucumber (Cucumis sativus L.) under drought stress. J. Plant Physiol. 2018, 229, 185–194. [Google Scholar] [CrossRef]
- Li, C.X.; Huang, D.J.; Wang, C.L.; Wang, N.; Liao, W.B. No is involved in h2-induced adventitious rooting in cucumber by regulating the expression and interaction of plasma membrane h+-atpase and 14-3-3. Planta 2020, 252, 9. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Gupta, A.; Laxmi, A. Glucose control of root growth direction in Arabidopsis thaliana. J. Epx. Bot. 2014, 65, 2981–2993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.J.; Kim, H.K. Anti-diabetic effects of electrolyzed reduced water in streptozotocin-induced and genetic diabetic mice. Life Sci. 2006, 79, 2288–2292. [Google Scholar] [CrossRef]
- Kajiyama, S.; Hasegawa, G.; Asano, M.; Hosoda, H.; Fukui, M.; Nakamura, N.; Kitawaki, J.; Imai, S.; Nakano, K.; Ohta, M.; et al. Supplementation of hydrogen-rich water improves lipid and glucose metabolism in patients with type 2 diabetes or impaired glucose tolerance. Nutr. Res. 2008, 28, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Qi, N.N.; Hou, X.M.; Wang, C.L.; Li, C.X.; Huang, D.J.; Li, Y.H.; Wang, N.; Liao, W.B. Methane-rich water induces bulblet formation of scale cuttings in Lilium davidii var. unicolor by regulating the signal transduction of phytohormones and their levels. Physiol. Plant. 2021, 172, 1919–1930. [Google Scholar] [CrossRef]
- Shi, S.Y.; Wang, W.; Liu, L.Q.; Shu, B.; Wei, Y.Z.; Jue, D.W.; Fu, J.X.; Xie, J.H.; Liu, C.M. Physico-chemical properties of longan fruit during development and ripening. Sci. Hortic. 2016, 207, 160–167. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, M.; Laxmi, A. Multiple interactions between glucose and brassinosteroid signal transduction pathways in Arabidopsis are uncovered by Whole-Genome transcriptional profiling. Plant Physiol. 2015, 168, 1091–1105. [Google Scholar] [CrossRef] [Green Version]
- Bhaji, A.; Li, J.; Ovecka, M.; Ezquer, L.; Muñoz, F.J.; Baroja-Fernández, E.; Romero, J.M.; Almagro, G.; Montero, M.; Hidalgo, M.; et al. Arabidopsis thaliana mutants lacking ADP-glucose pyrophosphorylase accumulate starch and wild-type ADP-glucose content: Further evidence for the occurrence of important sources, other than ADP-glucose pyrophosphorylase, of ADP-glucose linked to leaf starch. Plant Cell Physiol. 2011, 52, 1162–1176. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, M.; Kubota, F.; Saitou, K. Effects of exogenous injection of different sugars on leaf photosynthesis, dry matter production and adenosine 5’-diphosphate glucose pyrophosphorylase (AGPase) activity in sweet potato, ipomoea batatas (Lam.). J. Agron. Crop Sci. 2010, 186, 37–41. [Google Scholar] [CrossRef]
- Tsubone, M.; Kubota, F.; Saitou, K.; Kadowaki, M. Enhancement of tuberous root production and adenosine 5’-diphosphate pyrophosphorylase (AGPase) activity in sweet potato (Ipomoea batatas Lam.) by exogenous injection of sucrose solution. J. Agron. Crop Sci. 2000, 184, 181–186. [Google Scholar] [CrossRef]
- Moore, B.; Zhou, L.; Rolland, F.; Hall, Q.; Cheng, W.H.; Liu, Y.X.; Hwang, I.; Jones, T.; Sheen, J. Role of the Arabidopsis glucose sensor HXK1 in nutrient, light, and hormonal signaling. Science 2003, 300, 332–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, G.; David-Schwartz, R.; Sade, N.; Moshelion, M.; Levi, A.; Alchanatis, V.; Granot, D. The pitfalls of transgenic selection and new roles of AtHXK1: A high level of AtHXK1 expression uncouples hexokinase1-dependent sugar signaling from exogenous sugar. Plant Physiol. 2012, 159, 47–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.W.; Yuan, S.; Xu, F.; Yang, H.; Zhang, N.H.; Cheng, J.; Lin, H.H. The plastid hexokinase pHXK: A node of convergence for sugar and plastid signals in Arabidopsis. FEBS Lett. 2010, 584, 3573–3579. [Google Scholar] [CrossRef] [Green Version]
- Woodward, G.E.; Hudson, M.T. D-glucosamine as an antagonist of glucose in carbohydrate metabolism of yeast. J. Frankl. Inst. 1953, 255, 556–560. [Google Scholar] [CrossRef]
- Tauzin, A.S.; Giardina, T. Sucrose and invertases, a part of the plant defense, response to the biotic stresses. Front. Plant Sci. 2014, 5, 293. [Google Scholar] [CrossRef]
- Smith, A.M.; Zeeman, S.C. Quantification of starch in plant tissues. Nat. Protoc. 2006, 1, 1342–1345. [Google Scholar] [CrossRef]
- Nath, K.; Mahatma, M.K.; Swami, R. Role of total soluble sugar, phenols and defense related enzymes in relation to banana fruit rot by lasiodiplodia theobromae [(path.) griff.and maubl.] during ripening. J. Plant Pathol. 2015, 6, 8. [Google Scholar]
- Nägele, T.; Weckwerth, W. Mathematical modeling reveals that metabolic feedback regulation of SnRK1 and hexokinase is sufficient to control sugar homeostasis from energy depletion to full recovery. Plant Sci. 2014, 5, 365. [Google Scholar]
- Huang, D.J.; Li, W.T.; Dawuda, M.M.; Huo, J.Q.; Wang, C.L.; Liao, W.B. Hydrogen sulfide reduced colour change in lanzhou lily-bulb scales. Postharvest Biol. Tec. 2021, 176, 111520. [Google Scholar] [CrossRef]







| Glc/mM | Root Number | Root Length (mm) |
|---|---|---|
| 0.00 | 3.21 ± 0.18 c | 4.99 ± 0.18 d |
| 0.01 | 3.91 ± 0.12 c | 6.97 ± 0.44 c |
| 0.05 | 4.63 ± 0.14 b | 8.04 ± 0.13 b |
| 0.10 | 6.95 ± 0.05 a | 9.69 ± 005 a |
| 0.50 | 4.37 ± 0.01 b | 7.06 ± 0.25 c |
| 1.00 | 2.58 ± 0.18 d | 4.06 ± 0.44 e |
| Gene Symbol | Accession Number a | Primer Sequence (5′–3′) |
|---|---|---|
| CsSuSy1-F | LOC101213767 | CGTGTGCTAAGGAAGGCGGAAG |
| CsSuSy1-R | CAGTGTCACCCCACCCTCTCTC | |
| CsSuSy6-F | LOC101216865 | TCCAACCGCCACAACTTCATCAC |
| CsSuSy6-R | CCATTCCCACTCTGCCCAAGC | |
| CsHK1-F | LOC101218300 | CGCCATGACCGTCGAGATGC |
| CsHK1-R | TTTGTACCGCCGAGATCCAATGC | |
| CsHK3-F | LOC101215511 | CACGGTCCTAGTCAGTCGGAGAG |
| CsHK3-R | GCCATAGCATCAACCACCTGTCTC | |
| CsUDP1-F | LOC101206505 | TCCAGAGTTCCTTGCTGAGGGTAC |
| CsUDP1-R | AAGCCTGAATTGCCTTGAGACCATC | |
| CsUDP1-like-F | LOC116401645 | AGTTAATGCCATTTCCGCCCTCTG |
| CsUDP1-like-R | TCTTGTATCCGTACCAACCGAATGC | |
| CsG6P1-F | LOC101222586 | AGGTGCGATTGCTAATCCAGATGAG |
| CsG6P1-R | TGCGACTTCAAGAACGAGTTAGGTG | |
| CsG6P1-like-F | LOC101210696 | AGGGTGGAGGTTTAGGGTTTAGGG |
| CsG6P1-like-R | GCCGCTCGTTCATTCCATTGTTC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Li, C.; Liu, H.; Yang, J.; Huang, P.; Liao, W. The Involvement of Glucose in Hydrogen Gas-Medicated Adventitious Rooting in Cucumber. Plants 2021, 10, 1937. https://doi.org/10.3390/plants10091937
Zhao Z, Li C, Liu H, Yang J, Huang P, Liao W. The Involvement of Glucose in Hydrogen Gas-Medicated Adventitious Rooting in Cucumber. Plants. 2021; 10(9):1937. https://doi.org/10.3390/plants10091937
Chicago/Turabian StyleZhao, Zongxi, Changxia Li, Huwei Liu, Jingjing Yang, Panpan Huang, and Weibiao Liao. 2021. "The Involvement of Glucose in Hydrogen Gas-Medicated Adventitious Rooting in Cucumber" Plants 10, no. 9: 1937. https://doi.org/10.3390/plants10091937
APA StyleZhao, Z., Li, C., Liu, H., Yang, J., Huang, P., & Liao, W. (2021). The Involvement of Glucose in Hydrogen Gas-Medicated Adventitious Rooting in Cucumber. Plants, 10(9), 1937. https://doi.org/10.3390/plants10091937