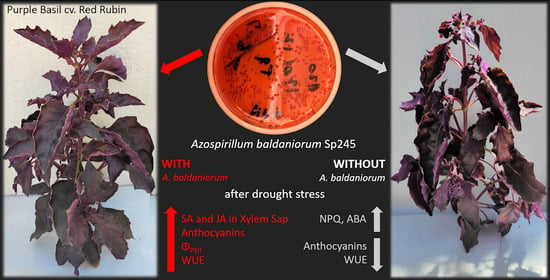
Azospirillum baldaniorum Sp245 Induces Physiological Responses to Alleviate the Adverse Effects of Drought Stress in Purple Basil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Cultivation
2.2. Plant Material and Growth Conditions
2.3. Treatments and Inoculation
2.4. Water Stress and Determination of Leaf Water Potential
2.5. Xylem Sap Sampling and Determination of Hormonal Profiling
2.6. Gas Exchange and Chlorophyll Fluorescence Measurements
2.7. Quantification of Leaf Pigments (Chlorophylls, Total Carotenoids and Total Anthocyanins)
2.8. Carbon Isotope Discrimination of Leaf Dry Matter and Soluble Sugars
2.9. Statistical Analysis
3. Results
3.1. Hormonal Content in the Xylem Sap
3.2. Leaf Water Potential
3.3. Leaf Pigments (Chlorophylls, Total Carotenoids and Total Anthocyanins)
3.4. Photosynthetic Performance
3.5. Carbon Isotope Discrimination
4. Discussion
4.1. Effects of Treatment with A. baldaniorum Sp245 on Hormonal Signaling in the Xylem Sap
4.2. Effect of Treatment with A. baldaniorum Sp245 on Leaf Pigments
4.3. Effects of Treatment with A. baldaniorum Sp245 on Photosynthesis and WUE
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bashan, Y.; de-Bashan, L.E. How the plant growth-promoting bacterium Azospirillum promotes plant growth—A critical assessment. Adv. Agron. 2010, 108, 77–136. [Google Scholar]
- Cohen, A.C.; Bottini, R.; Piccoli, P.N. Azospirillum brasilense Sp245 produces ABA in chemically-defined culture medium and increases ABA content in Arabidopsis plants. Plant Growth Regul. 2008, 54, 97–103. [Google Scholar] [CrossRef]
- Cohen, A.C.; Travaglia, C.N.; Bottini, R.; Piccoli, P.N. Participation of abscisic acid and gibberellins produced by endophytic Azospirillum in the alleviation of drought effects in maize. Botany 2009, 87, 455–462. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef] [Green Version]
- Fukami, J.; Cerezini, P.; Hungria, M. Azospirillum: Benefits that go far beyond biological nitrogen fixation. AMB Express 2018, 8, 73. [Google Scholar] [CrossRef]
- Ferreira, N.S.; Sant’Anna, F.H.; Reis, V.M.; Ambrosini, A.; Volpiano, C.G.; Rothballer, M.; Schwab, S.; Baura, V.A.; Balsanelli, E.; Pedrosa, F.O.; et al. Genome-based reclassification of Azospirillum brasilense Sp245 as the type strain of Azospirillum baldaniorum sp. nov. Int. J. Syst. Evol. Microbiol. 2020, 70, 6203–6212. [Google Scholar] [CrossRef]
- Okon, Y.; Labandera-Gonzales, C.A. Agronomic applications of Azospirillum: An evaluation of 20 years worldwide field inoculation. Soil Biol. Biochem. 1994, 26, 1591–1601. [Google Scholar] [CrossRef]
- Cohen, A.C.; Bottini, R.; Pontina, M.; Berli, F.J.; Moreno, D.; Boccanlandro, H.; Travaglia, C.N.; Piccoli, P.N. Azospirillum brasilense ameliorates the response of Arabidopsis thaliana to drought mainly via enhancement of ABA levels. Physiol. Plant. 2015, 153, 79–90. [Google Scholar] [CrossRef]
- Pereg, L.; de-Bashan, L.E.; Bashan, Y. Assessment of affinity and specificity of Azospirillum for plants. Plant Soil 2016, 399, 389–414. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Bashan, Y.; Holguin, G.; de-Bashan, L.E. Azospirillum-plant relationships: Physiological, molecular, agricultural, and environmental advances (1997–2003). Can. J. Microbiol. 2004, 50, 521–577. [Google Scholar] [CrossRef] [Green Version]
- Vettori, L.; Russo, A.; Felici, C.; Fiaschi, G.; Morini, S.; Toffanin, A. Improving micropropagation: Effect of Azospirillum brasilense Sp245 on acclimatization of rootstocks of fruit tree. J. Plant Interact. 2010, 5, 249–259. [Google Scholar] [CrossRef]
- Bartolini, S.; Carrozza, G.P.; Scalabrelli, G.; Toffanin, A. Effectiveness of Azospirillum brasilens e Sp245 on young plants of Vitis vinifera L. Open Life Sci. 2017, 12, 365–372. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Kopsell, D.E.; Curran-Celentano, J. Carotenoid and chlorophyll pigments in sweet basil grown in the field and greenhouse. HortScience 2005, 40, 1230–1233. [Google Scholar] [CrossRef]
- Flanigan, P.M.; Niemeyer, E.D. Effect of cultivar on phenolic levels, anthocyanin composition, and antioxidant properties in purple basil (Ocimum basilicum L.). Food Chem. 2014, 164, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Sestili, P.; Ismail, T.; Calcabrini, C.; Guescini, M.; Catanzaro, E.; Turrini, E.; Layla, A.; Akhtar, S.; Fimognari, C. The potential effects of Ocimum basilicum on health: A review of pharmacological and toxicological studies. Expert Opin. Drug Metab. Toxicol. 2018, 14, 679–692. [Google Scholar] [CrossRef]
- Phippen, W.B.; Simon, J.E. Anthocyanins in basil (Ocimum basilicum L.). J. Agric. Food Chem. 1998, 46, 1734–1738. [Google Scholar] [CrossRef]
- Filip, S. Basil (Ocimum basilicum L.) a source of valuable phytonutrients. Int. J. Clin. Nutr. Diet 2017, 3, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakabayashi, R.; Yonekura-Sakakibara, K.; Urano, K.; Suzuki, M.; Yamada, Y.; Nishizawa, T.; Matsuda, F.; Kojima, M.; Sakakibara, H.; Shinozaki, K.; et al. Enhancement of oxidative and drought tolerance in Arabidopsis by overaccumulation of antioxidant flavonoids. Plant J. 2014, 77, 367–379. [Google Scholar] [CrossRef]
- Kovinich, N.; Kayanja, G.; Chanoca, A.; Otegui, M.S.; Grotewold, E. Abiotic stresses induce different localizations of anthocyanins in Arabidopsis. Plant Signal Behav. 2015, 10, e1027850. [Google Scholar] [CrossRef] [Green Version]
- Luna, M.C.; Bekhradi, F.; Ferreres, F.; Jordán, M.J.; Delshad, M.; Gil, M.I. Effect of water stress and storage time on anthocyanins and other phenolics of different genotypes of fresh sweet basil. J. Agric. Food Chem. 2015, 63, 9223–9231. [Google Scholar] [CrossRef]
- Damalas, C.A. Improving drought tolerance in sweet basil (Ocimum basilicum) with salicylic acid. Sci. Hortic. 2019, 246, 360–365. [Google Scholar] [CrossRef]
- Saburi, M.; Haj Seyed Hadi, M.; Darzi, M.T. Effects of amino acids and nitrogen fixing bacteria on quantitative yield and essential oil content of basil (Ocimum basilicum). Agric. Sci. Dev. 2014, 3, 265–268. [Google Scholar]
- Mangmang, J.S.; Deaker, R.; Rogers, G. Inoculation effect of Azospirillum brasilense on basil grown under aquaponics production system. Org. Agric. 2016, 6, 65–74. [Google Scholar] [CrossRef]
- Heidari, M.; Golpayegani, A. Effects of water stress and inoculation with plant growth promoting rhizobacteria (PGPR) on antioxidant status and photosynthetic pigments in basil (Ocimum basilicum L.). J. Saudi Soc. Agric. Sci. 2012, 11, 57–61. [Google Scholar] [CrossRef] [Green Version]
- Yaghoub, R.; Sajad, K.; Fardin, G.; Amir Ali, S.; Ghodratallah, S.; Sajad, F. The effect of Azospirilium bacteria and salicylic acid effects on drought stress tolerance in Ocimum basilicum L. medicinal plant. Adv. Biores. 2015, 6, 44–53. [Google Scholar]
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forni, C.; Duca, D.; Glick, B.R. Mechanisms of plant response to salt and drought stress and their alteration by rhizobacteria. Plant Soil 2017, 410, 335–356. [Google Scholar] [CrossRef]
- Fukami, J.; Ollero, F.J.; Megías, M.; Hungria, M. Phytohormones and induction of plant stress tolerance and defense genes by seed and foliar inoculation with Azospirillum brasilense cells and metabolites promote maize growth. AMB Express 2017, 7, 153. [Google Scholar] [CrossRef] [PubMed]
- Morison, J.I.L.; Baker, N.R.; Mullineaux, P.M.; Davies, W.J. Improving water use in crop production. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008, 363, 639–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasim, W.A.; Osman, M.E.; Omar, M.N.; Abd El-Daim, I.A.; Bejai, S.; Meijer, J. Control of drought stress in wheat using plant-growth-promoting bacteria. J. Plant Growth Regul. 2013, 32, 122–130. [Google Scholar] [CrossRef]
- Romero, A.M.; Vega, D.; Correa, O.S. Azospirillum brasilense mitigates water stress imposed by a vascular disease by increasing xylem vessel area and stem hydraulic conductivity in tomato. Appl. Soil Ecol. 2014, 82, 38–43. [Google Scholar] [CrossRef]
- Vurukonda, S.S.; Vardharajula, S.; Shrivastava, M.; SkZ, A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 2016, 184, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Tsukanova, K.A.; Chebotar, V.K.; Meyer, J.J.M.; Bibikova, T.N. Effect of plant growth-promoting Rhizobacteria on plant hormone homeostasis. J. S. Afr. Bot. 2017, 113, 91–102. [Google Scholar] [CrossRef]
- Baldani, V.L.D.; de B. Alvarez, M.A.; Baldani, J.I.; Döbereiner, J. Establishment of inoculated Azospirillum spp. in the rhizosphere and in roots of field grown wheat and sorghum. Plant Soil 1986, 90, 35–46. [Google Scholar] [CrossRef]
- Baldani, J.I.; Reis, V.M.; Videira, S.S.; Lúcia, H.B.; Baldani, V.L.D. The art of isolating nitrogen-fixing bacteria from non-leguminous plants using N-free semi-solid media: A practical guide for microbiologists. Plant Soil 2014, 384, 413–431. [Google Scholar] [CrossRef]
- Kratky, B.A. A suspended pot, non-circulating hydroponic method. Proceedings of the South Pacific Soilless Culture Conference. Acta Hortic. 2004, 648, 83–89. [Google Scholar] [CrossRef]
- Furch, A.; Zimmermann, M.R.; Kogel, K.H.; Reichelt, M.; Mithöfer, A. Direct and individual analysis of stress-related phytohormone dispersion in the vascular system of Cucurbita maxima after flagellin 22 treatment. New Phytol. 2014, 201, 1176–1182. [Google Scholar] [CrossRef]
- Zimmermann, M.R.; Hafke, J.B.; Van Bel, A.J.E.; Furch, A.C.U. Interaction of xylem and phloem during exudation and wound occlusion in Cucurbita maxima. Plant Cell Environ. 2013, 36, 237–247. [Google Scholar] [CrossRef]
- Scartazza, A.; Picciarelli, P.; Mariotti, L.; Curadi, M.; Barsanti, L.; Gualtieri, P. The role of Euglena gracilis paramylon in modulating xylem hormone levels, photosynthesis and water-use efficiency in Solanum lycopersicum L. Physiol. Plant. 2017, 161, 486–501. [Google Scholar] [CrossRef] [PubMed]
- Bilger, W.; Björkman, O. Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth. Res. 1990, 25, 173–185. [Google Scholar] [CrossRef]
- Quach, H.T.; Steeper, R.L.; Griffin, G.W. An improved method for the extraction and thin-layer chromatography of chrorophyll a and b from spinach. J. Chem. Educ. 2004, 81, 385–387. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Meth. Enzymol. 1987, 148, 350–382. [Google Scholar]
- Abdel-Aal, E.S.M.; Hucl, P. A rapid method for quantifying total anthocyanins in blue aleurone and purple pericarp wheats. Cereal. Chem. 1999, 76, 350–354. [Google Scholar] [CrossRef]
- Brugnoli, E.; Hubick, K.T.; von Caemmerer, S.; Wong, S.; Farquhar, G.D. Correlation between the carbon isotope discrimination in leaf starch and sugars of C3 plants and the ratio of intercellular and atmospheric partial pressures of carbon dioxide. Plant Physiol. 1988, 88, 1418–1424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon isotope discrimination and photosynthesis. Annu. Rev. Plant Physiol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Dodd, I.C.; Zinovkina, N.Y.; Safronova, V.I.; Belimov, A.A. Rhizobacterial mediation of plant hormone status. Ann. Appl. Biol. 2010, 157, 361–379. [Google Scholar] [CrossRef]
- Kudoyarova, G.; Arkhipova, T.; Korshunova, T.; Bakaeva, M.; Loginov, O.; Dodd, I.C. Phytohormone mediation of interactions between plants and non-symbiotic growth promoting bacteria under edaphic stresses. Front. Plant Sci. 2019, 10, 1368. [Google Scholar] [CrossRef] [PubMed]
- Van der Ent, S.; Van Wees, S.C.M.; Pieterse, C.M.J. Jasmonate signaling in plant interactions with resistance-inducing beneficial microbes. Phytochemistry 2009, 70, 1581–1588. [Google Scholar] [CrossRef] [Green Version]
- Kordi, S.; Saidi, M.; Ghanbari, F. Induction of drought tolerance in sweet basil (Ocimum basilicum L.) by salicylic acid. Int. J. Agric. Food Res. 2013, 2, 18–26. [Google Scholar] [CrossRef]
- Yang, J.; Kloepper, J.W.; Ryu, C.M. Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci. 2009, 14, 1–4. [Google Scholar] [CrossRef]
- Ahmad, P.; Rasool, S.; Gul, A.; Sheikh, S.A.; Akram, N.A.; Ashraf, M.; Kazi, A.M.; Gucel, S. Jasmonates: Multifunctional roles in stress tolerance. Front. Plant. Sci. 2016, 7, 813. [Google Scholar] [CrossRef] [Green Version]
- Forchetti, G.; Masciarelli, O.; Alemano, S.; Alvarez, D.; Abdala, G. Endophytic bacteria in sunflower (Helianthus annuus L.): Isolation, characterization, and production of jasmonates and abscisic acid in culture medium. Appl. Microbiol. Biotechnol. 2007, 76, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, G.; Vallone, S.; Orsini, F.; Paradiso, R.; De Pascale, S.; Negre-Zakharov, F.; Maggio, A. Stomatal density and metabolic determinants mediate salt stress adaptation and water use efficiency in basil (Ocimum basilicum L.). J. Plant Physiol. 2012, 169, 1737–1746. [Google Scholar] [CrossRef] [PubMed]
- Belimov, A.A.; Dodd, I.C.; Safronova, V.I.; Dumova, V.A.; Shaposhnikov, A.I.; Ladatko, A.G.; Davies, W.J. Abscisic acid metabolizing rhizobacteria decrease ABA concentrations in planta and alter plant growth. Plant Physiol. Biochem. 2014, 74, 84–91. [Google Scholar] [CrossRef]
- Landi, M.; Remorini, D.; Pardossi, A.; Guidi, L. Purple versus green-leafed Ocimum basilicum: Which differences occur with regard to photosynthesis under boron toxicity? J. Soil Sci. Plant Nutr. 2013, 176, 942–951. [Google Scholar] [CrossRef]
- Tattini, M.; Landi, M.; Brunetti, C.; Giordano, C.; Remorini, D.; Gould, K.S.; Guidi, L. Epidermal coumaroyl anthocyanins protect sweet basil against excess light stress: Multiple consequences of light attenuation. Physiol. Plant. 2014, 152, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Tattini, M.; Sebastiani, F.; Brunetti, C.; Fini, A.; Torre, S.; Gori, A.; Centritto, M.; Ferrini, F.; Landi, M.; Guidi, L. Dissecting molecular and physiological response mechanisms to high solar radiation in cyanic and acyanic leaves: A case study on red and green basil. J. Exp. Bot. 2017, 68, 2425–2437. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.; Niu, G.; Gu, M. Pre-harvest UV-B radiation and photosynthetic photon flux density interactively affect plant photosynthesis, growth, and secondary metabolites accumulation in basil (Ocimum basilicum) plants. Agronomy 2019, 9, 434. [Google Scholar] [CrossRef] [Green Version]
- Ferrarezi, R.S.; Bailey, D.S. Basil performance evaluation in aquaponics. Horttechnology 2019, 29, 85–93. [Google Scholar] [CrossRef] [Green Version]
- Bashan, Y.; Bustillos, J.J.; Leyva, L.A.; Hernandez, J.P.; Bacilio, M. Increase in auxiliary photoprotective photosynthetic pigments in wheat seedlings induced by Azospirillum brasilense. Biol. Fertil. Soils 2006, 42, 279–285. [Google Scholar] [CrossRef]
- Mohamed, S.M.; El-Ghait, E.M.A.; Shayeb, N.S.A.; Ghatas, Y.A.; Shahin, A.A. Effect of some fertilizers on improving growth and oil productivity of basil (Ocimum basilicum, L.) cv. Genovese plant. Egypt. J. Appl. Sci. 2015, 30, 384–399. [Google Scholar]
- Moeini Alishah, H.; Heidari, R.; Hassani, A.; Dizaji, A.A. Effect of water stress on some morphological and biochemical characteristics of purple basil (Ocimum basilicum). J. Biol. Sci. 2006, 6, 763–767. [Google Scholar]
- Heidari, M.; Mousavinik, S.M.; Golpayegani, A. Plant growth promoting rhizobacteria (PGPR) effect on physiological parameters and mineral uptake in basil (Ociumum basilicum L.) under water stress. ARPN J. Agric. Biol. Sci. 2011, 6, 6–11. [Google Scholar]
- Foroughi, M.G.; Ashraf, S.; Alipour, Z.T. Effects of two species of mycorrhiza fungi and drought stress on chlorophyll a, b and total of Ocimum basilicum. Int. J. Farm. Allied Sci. 2014, 3, 1104–1108. [Google Scholar]
- Bekhradi, F.; Luna, M.C.; Delshad, M.; Jordan, M.J.; Sotomayor, J.A.; Martínez-Conesa, C.; Gil, M.I. Effect of deficit irrigation on the postharvest quality of different genotypes of basil including purple and green Iranian cultivars and a Genovese variety. Postharvest Biol. Technol. 2015, 100, 127–135. [Google Scholar] [CrossRef]
- Agami, R.A.; Medani, R.A.; Abd El-Mola, I.A.; Taha, R.S. Exogenous application with plant growth promoting rhizobacteria (PGPR) or proline induces stress tolerance in basil plants (Ocimum basilicum L.) exposed to water stress. Int. J. Environ. Agric. Res. 2016, 2, 78–92. [Google Scholar]
- Lobiuc, A.; Vasilache, V.; Pintilie, O.; Stoleru, T.; Burducea, M.; Oroian, M.; Zamfirache, M.M. Blue and red LED illumination improves growth and bioactive compounds contents in acyanic and cyanic Ocimum basilicum L. microgreens. Molecules 2017, 22, 2111. [Google Scholar] [CrossRef] [Green Version]
- Pistelli, L.; Ascrizzi, R.; Giuliani, C.; Cervelli, C.; Ruffoni, B.; Princi, E.; Fontanesi, G.; Flamini, G.; Pistelli, L. Growing basil in the underwater biospheres of Nemo’s Garden®: Phytochemical, physiological and micromorphological analysis. Sci. Hortic. 2020, 259, 108851. [Google Scholar] [CrossRef]
- Lee, J.; Scagel, C.F. Chicoric acid found in basil (Ocimum basilicum L.) leaves. Food Chem. 2009, 115, 650–656. [Google Scholar] [CrossRef]
- McCance, K.R.; Flanigan, P.M.; Quick, M.M.; Niemeyer, E.D. Influence of plant maturity on anthocyanin concentrations, phenolic composition, and antioxidant properties of 3 purple basil (Ocimum basilicum L.) cultivars. J. Food Compos. Anal. 2016, 53, 30–39. [Google Scholar] [CrossRef] [Green Version]
- Chalker-Scott, L. Do anthocyanins function as osmoregulators in leaf tissues? Adv. Bot. Res. 2002, 37, 103–106. [Google Scholar]
- Castellarin, S.D.; Pfeiffer, A.; Sivilotti, P.; Degan, M.; Peterlunger, E.; Di Gaspero, G. Transcriptional regulation of anthocyanin biosynthesis in ripening fruits of grapevine under seasonal water deficit. Plant Cell Environ. 2007, 30, 1381–1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, D.; Sun, D.; Wang, C.; Li, Y.; Guo, T. Expression of flavonoid biosynthesis genes and accumulation of flavonoid in wheat leaves in response to drought stress. Plant Physiol. Biochem. 2014, 80, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Creus, C.M.; Pereyra, M.A.; Casanovas, E.M.; Sueldo, R.J.; Barassi, C.A. Plant growth-promoting effects of rhizobacteria on abiotic stressed plants. Azospirillum -grasses model . Am. J. Plant Sci. Biotechnol. 2010, 4, 49–59. [Google Scholar]
- Złotek, U.; Mikulska, S.; Nagajek, M.; Świeca, M. The effect of different solvents and number of extraction steps on the polyphenol content and antioxidant capacity of basil leaves (Ocimum basilicum L.) extracts. Saudi J. Biol. Sci. 2016, 23, 628–633. [Google Scholar] [CrossRef]
- Kolega, S.; Miras-Moreno, B.; Buffagni, V.; Lucini, L.; Valentinuzzi, F.; Maver, M.; Mimmo, T.; Trevisan, M.; Pii, Y.; Cesco, S. Nutraceutical profiles of two hydroponically grown sweet basil cultivars as affected by the composition of the nutrient solution and the inoculation with Azospirillum brasilense. Front. Plant Sci. 2020, 11, 596000. [Google Scholar] [CrossRef]
- Kannan, T.; Ponmurugan, P. Response of paddy (Oryza sativa L.) varieties to Azospirillum brasilense inoculation. J. Phytol. 2010, 2, 8–13. [Google Scholar]
- Ruíz-Sánchez, M.; Armada, E.; Muñoz, Y.; García de Salamone, I.E.; Aroca, R.; Ruíz-Lozano, J.M.; Azcón, R. Azospirillum and arbuscular mycorrhizal colonization enhance rice growth and physiological traits under well-watered and drought conditions. J. Plant Physiol. 2011, 168, 1031–1037. [Google Scholar] [CrossRef]
- Malekpoor, F.; Ghasemi, P.A.; Salimi, A. Effect of foliar application of chitosan on morphological and physiological characteristics of basil under reduced irrigation. Res. Crop. 2016, 17, 354–359. [Google Scholar] [CrossRef]
- Ekren, S.; Sönmez, Ç.; Özçakal, E.; Kurttaş, Y.S.K.; Bayram, E.; Gürgülü, H. The effect of different irrigation water levels on yield and quality characteristics of purple basil (Ocimum basilicum L.). Agric. Water Manag. 2012, 109, 155–161. [Google Scholar] [CrossRef]
- Kalamartzis, I.; Dordas, C.; Georgiou, P.; Menexes, G. The use of appropriate cultivar of basil (Ocimum basilicum) can increase water use efficiency under water stress. Agronomy 2020, 10, 70. [Google Scholar] [CrossRef] [Green Version]
- Müller, P.; Li, X.; Niyogi, K.K. Non-photochemical quenching. A response to excess light energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef] [Green Version]
- Gharib, F. Effect of salicylic acid on the growth, metabolic activities and oil content of basil and marjoram. Int. J. Agric. Biol. 2006, 4, 485–492. [Google Scholar]
- Sorial, M.E.; El-Gamal, S.M.; Gendy, A.A. Response of sweet basil to jasmonic acid application in relation to different water supplies. Biosci. Res. 2010, 7, 39–47. [Google Scholar]
- Pejić, B.; Adamović, D.; Maksimović, L.; Mačkić, K. Effect of drip irrigation on yield, evapotranspiration and water use efficiency of sweet basil (Ocimum basilicum L.). Ratar. Povrt. 2017, 54, 124–129. [Google Scholar] [CrossRef] [Green Version]
- Hatfield, J.L.; Dold, C. Water-use efficiency: Advances and challenges in a changing climate. Front. Plant Sci. 2019, 10, 103. [Google Scholar] [CrossRef] [Green Version]
- Brugnoli, E.; Farquhar, G.D. Photosynthetic fractionation of carbon isotopes. In Photosynthesis. Advances in Photosynthesis and Respiration; Leegood, R.C., Sharkey, T.D., Caemmerer, S., Eds.; Springer: Dordrecht, The Netherlands, 2000; pp. 399–434. [Google Scholar]
- Brugnoli, E.; Scartazza, A.; Lauteri, M.; Monteverdi, M.C.; Máguas, C. Carbon isotope discrimination in structural and non-structural carbohydrates in relation to productivity and adaptation to unfavourable conditions. In Stable Isotopes: Integration of Biological, Ecological and Geochemical Processes; Griffiths, H., Ed.; Garland Science (Taylor & Francis Group): London, UK, 1998; pp. 133–144. [Google Scholar]
- Samaras, A.; Nikolaidis, M.; Antequera-Gómez, M.L.; Cámara-Almirón, J.; Romero, D.; Moschakis, T.; Amoutzias, G.D.; Karaoglanidis, G.S. Whole genome sequencing and root colonization studies reveal novel insights in the biocontrol potential and growth promotion by Bacillus subtilis MBI 600 on cucumber. Front. Microbiol. 2021, 11, 600393. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariotti, L.; Scartazza, A.; Curadi, M.; Picciarelli, P.; Toffanin, A. Azospirillum baldaniorum Sp245 Induces Physiological Responses to Alleviate the Adverse Effects of Drought Stress in Purple Basil. Plants 2021, 10, 1141. https://doi.org/10.3390/plants10061141
Mariotti L, Scartazza A, Curadi M, Picciarelli P, Toffanin A. Azospirillum baldaniorum Sp245 Induces Physiological Responses to Alleviate the Adverse Effects of Drought Stress in Purple Basil. Plants. 2021; 10(6):1141. https://doi.org/10.3390/plants10061141
Chicago/Turabian StyleMariotti, Lorenzo, Andrea Scartazza, Maurizio Curadi, Piero Picciarelli, and Annita Toffanin. 2021. "Azospirillum baldaniorum Sp245 Induces Physiological Responses to Alleviate the Adverse Effects of Drought Stress in Purple Basil" Plants 10, no. 6: 1141. https://doi.org/10.3390/plants10061141
APA StyleMariotti, L., Scartazza, A., Curadi, M., Picciarelli, P., & Toffanin, A. (2021). Azospirillum baldaniorum Sp245 Induces Physiological Responses to Alleviate the Adverse Effects of Drought Stress in Purple Basil. Plants, 10(6), 1141. https://doi.org/10.3390/plants10061141