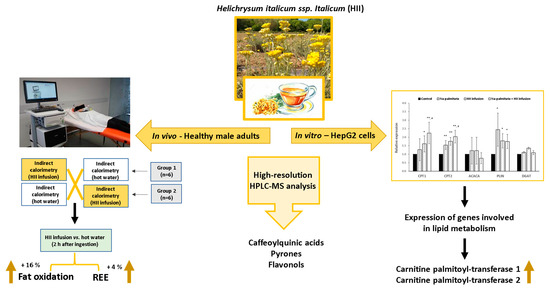
Helichrysum italicum ssp. italicum Infusion Promotes Fat Oxidation in Hepatocytes and Stimulates Energy Expenditure and Fat Oxidation after Acute Ingestion in Humans: A Pilot Study
Abstract
:1. Introduction
2. Results
2.1. Chemical Analysis and Sensory Evaluation of HII Infusion
2.2. Baseline Characteristics of Study Participants
2.3. Acute Effects of a Single Ingestion of HII Infusion on Energy Expenditure and Blood Pressure
2.3.1. Resting Energy Expenditure
2.3.2. RQ and Fat Oxidation
2.3.3. Blood Pressure
2.4. In Vitro Experiments in Hepatocytes
3. Discussion
4. Materials and Methods
4.1. Test Beverages’ Preparation and Chemical Analysis
4.2. Sensory Evaluation of Tea Samples
4.3. Human Pilot Study
4.4. In Vitro Experiments
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antunes Viegas, D.; Palmeira-de-Oliveira, A.; Salgueiro, L.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, R. Helichrysum Italicum: From Traditional Use to Scientific Data. J. Ethnopharmacol. 2014, 151, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Kramberger, K.; Barlič-Maganja, D.; Bandelj, D.; Baruca Arbeiter, A.; Peeters, K.; Miklavčič Višnjevec, A.; Jenko Pražnikar, Z. HPLC-DAD-ESI-QTOF-MS Determination of Bioactive Compounds and Antioxidant Activity Comparison of the Hydroalcoholic and Water Extracts from Two Helichrysum Italicum Species. Metabolites 2020, 10, 403. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-Y.; Fu, L.; Li, C.-Y.; Xu, L.-P.; Zhang, L.-X.; Zhang, W.-M. Quercetin, Hyperin, and Chlorogenic Acid Improve Endothelial Function by Antioxidant, Antiinflammatory, and ACE Inhibitory Effects. J. Food Sci. 2017, 82, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhang, Y.; Guo, J.; You, Y.; Zhan, J.; Huang, W. Chlorogenic Acid Stimulates the Thermogenesis of Brown Adipocytes by Promoting the Uptake of Glucose and the Function of Mitochondria. J. Food Sci. 2019, 84, 3815–3824. [Google Scholar] [CrossRef]
- Wang, Z.; Lam, K.-L.; Hu, J.; Ge, S.; Zhou, A.; Zheng, B.; Zeng, S.; Lin, S. Chlorogenic Acid Alleviates Obesity and Modulates Gut Microbiota in High-Fat-Fed Mice. Food Sci. Nutr. 2019, 7, 579–588. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Llorens, S.; Pourbagher-Shahri, A.M.; Ashrafizadeh, M.; Talebi, M.; Shakibaei, M.; Samarghandian, S. An Overview of the Role of Adipokines in Cardiometabolic Diseases. Molecules 2020, 25, 5218. [Google Scholar] [CrossRef]
- Petelin, A.; Bizjak, M.; Černelič-Bizjak, M.; Jurdana, M.; Jakus, T.; Jenko-Pražnikar, Z. Low-Grade Inflammation in Overweight and Obese Adults Is Affected by Weight Loss Program. J. Endocrinol. Investig. 2014, 37, 745–755. [Google Scholar] [CrossRef]
- Saltiel, A.R. New Therapeutic Approaches for the Treatment of Obesity. Sci. Transl. Med. 2016, 8, 323rv2. [Google Scholar] [CrossRef]
- Brown, L.; Caligiuri, S.P.B.; Brown, D.; Pierce, G.N. Clinical Trials Using Functional Foods Provide Unique Challenges. J. Funct. Foods 2018, 45, 233–238. [Google Scholar] [CrossRef]
- Konstantinidi, M.; Koutelidakis, A.E. Functional Foods and Bioactive Compounds: A Review of Its Possible Role on Weight Management and Obesity’s Metabolic Consequences. Medicines 2019, 6, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monteiro, M.; Farah, A.; Perrone, D.; Trugo, L.C.; Donangelo, C. Chlorogenic Acid Compounds from Coffee Are Differentially Absorbed and Metabolized in Humans. J. Nutr. 2007, 137, 2196–2201. [Google Scholar] [CrossRef] [Green Version]
- Farah, A.; Monteiro, M.; Donangelo, C.M.; Lafay, S. Chlorogenic Acids from Green Coffee Extract Are Highly Bioavailable in Humans. J. Nutr. 2008, 138, 2309–2315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stalmach, A.; Steiling, H.; Williamson, G.; Crozier, A. Bioavailability of Chlorogenic Acids Following Acute Ingestion of Coffee by Humans with an Ileostomy. Arch. Biochem. Biophys. 2010, 501, 98–105. [Google Scholar] [CrossRef]
- Loader, T.B.; Taylor, C.G.; Zahradka, P.; Jones, P.J.H. Chlorogenic Acid from Coffee Beans: Evaluating the Evidence for a Blood Pressure-Regulating Health Claim. Nutr. Rev. 2017, 75, 114–133. [Google Scholar] [CrossRef]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The Potential Effects of Chlorogenic Acid, the Main Phenolic Components in Coffee, on Health: A Comprehensive Review of the Literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef] [PubMed]
- Park, I.; Ochiai, R.; Ogata, H.; Kayaba, M.; Hari, S.; Hibi, M.; Katsuragi, Y.; Satoh, M.; Tokuyama, K. Effects of Subacute Ingestion of Chlorogenic Acids on Sleep Architecture and Energy Metabolism through Activity of the Autonomic Nervous System: A Randomised, Placebo-Controlled, Double-Blinded Cross-over Trial. Br. J. Nutr. 2017, 117, 979–984. [Google Scholar] [CrossRef] [Green Version]
- Soga, S.; Ota, N.; Shimotoyodome, A. Stimulation of Postprandial Fat Utilization in Healthy Humans by Daily Consumption of Chlorogenic Acids. Biosci. Biotechnol. Biochem. 2013, 77, 1633–1636. [Google Scholar] [CrossRef]
- Thom, E. The Effect of Chlorogenic Acid Enriched Coffee on Glucose Absorption in Healthy Volunteers and Its Effect on Body Mass When Used Long-Term in Overweight and Obese People. J. Int. Med. Res. 2007, 35, 900–908. [Google Scholar] [CrossRef]
- Watanabe, T.; Arai, Y.; Mitsui, Y.; Kusaura, T.; Okawa, W.; Kajihara, Y.; Saito, I. The Blood Pressure-Lowering Effect and Safety of Chlorogenic Acid from Green Coffee Bean Extract in Essential Hypertension. Clin. Exp. Hypertens. 2006, 28, 439–449. [Google Scholar] [CrossRef]
- Bruckbauer, A.; Zemel, M.B. Synergistic Effects of Polyphenols and Methylxanthines with Leucine on AMPK/Sirtuin-Mediated Metabolism in Muscle Cells and Adipocytes. PLoS ONE 2014, 9, e89166. [Google Scholar] [CrossRef] [Green Version]
- Boschmann, M.; Steiniger, J.; Hille, U.; Tank, J.; Adams, F.; Sharma, A.M.; Klaus, S.; Luft, F.C.; Jordan, J. Water-Induced Thermogenesis. J. Clin. Endocrinol. Metab. 2003, 88, 6015–6019. [Google Scholar] [CrossRef] [Green Version]
- Shimoda, H.; Seki, E.; Aitani, M. Inhibitory Effect of Green Coffee Bean Extract on Fat Accumulation and Body Weight Gain in Mice. BMC Complement. Altern Med. 2006, 6, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, K.; Liang, X.; Zhong, Y.; He, W.; Wang, Z. 5-Caffeoylquinic Acid Decreases Diet-Induced Obesity in Rats by Modulating PPARα and LXRα Transcription. J. Sci. Food Agric. 2015, 95, 1903–1910. [Google Scholar] [CrossRef]
- Mills, C.E.; Flury, A.; Marmet, C.; Poquet, L.; Rimoldi, S.F.; Sartori, C.; Rexhaj, E.; Brenner, R.; Allemann, Y.; Zimmermann, D.; et al. Mediation of Coffee-Induced Improvements in Human Vascular Function by Chlorogenic Acids and Its Metabolites: Two Randomized, Controlled, Crossover Intervention Trials. Clin. Nutr. 2017, 36, 1520–1529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, N.C.; Hodgson, J.M.; Woodman, R.J.; Zimmermann, D.; Poquet, L.; Leveques, A.; Actis-Goretta, L.; Puddey, I.B.; Croft, K.D. Acute Effects of Chlorogenic Acids on Endothelial Function and Blood Pressure in Healthy Men and Women. Food Funct. 2016, 7, 2197–2203. [Google Scholar] [CrossRef] [Green Version]
- Rosa, A.; Pollastro, F.; Atzeri, A.; Appendino, G.; Melis, M.P.; Deiana, M.; Incani, A.; Loru, D.; Dessì, M.A. Protective Role of Arzanol against Lipid Peroxidation in Biological Systems. Chem. Phys. Lipids 2011, 164, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Kothavade, P.S.; Nagmoti, D.M.; Bulani, V.D.; Juvekar, A.R. Arzanol, a Potent MPGES-1 Inhibitor: Novel Anti-Inflammatory Agent. Sci. World J. 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Jiang, H.; Wu, X.; Fang, J. Therapeutic Effects of Quercetin on Inflammation, Obesity, and Type 2 Diabetes. Mediat. Inflamm. 2016, 2016, 9340637. [Google Scholar] [CrossRef]
- Pfeuffer, M.; Auinger, A.; Bley, U.; Kraus-Stojanowic, I.; Laue, C.; Winkler, P.; Rüfer, C.E.; Frank, J.; Bösch-Saadatmandi, C.; Rimbach, G.; et al. Effect of Quercetin on Traits of the Metabolic Syndrome, Endothelial Function and Inflammation in Men with Different APOE Isoforms. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 403–409. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, Q.Q.; Granato, D.; Xu, Y.Q.; Ho, C.T. Association between Chemistry and Taste of Tea: A Review. Trends Food Sci. Technol. 2020, 101, 139–149. [Google Scholar] [CrossRef]
- Alasalvar, C.; Grigor, J.M.; Zhang, D.; Quantick, P.C.; Shahidi, F. Comparison of Volatiles, Phenolics, Sugars, Antioxidant Vitamins, and Sensory Quality of Different Colored Carrot Varieties. J. Agric. Food Chem. 2001, 49, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- Kreutzmann, S.; Christensen, L.P.; Edelenbos, M. Investigation of Bitterness in Carrots (Daucus carota L.) Based on Quantitative Chemical and Sensory Analyses. LWT Food Sci. Technol. 2008, 2, 193–205. [Google Scholar] [CrossRef]
- Herrando Moraira, S.; Blanco Moreno, J.M.; Sáez, L.; Galbany Casals, M. Re-Evaluation of Helichrysum Italicum Complex (Compositae: Gnaphalieae): A New Species from Majorca (Balearic Islands). Collect. Bot. 2016, 35, e009. [Google Scholar]
- Baruca Arbeiter, A.; Hladnik, M.; Jakše, J.; Bandelj, D. First Set of Microsatellite Markers for Immortelle (Helichrysum Italicum (Roth) G. Don): A Step towards the Selection of the Most Promising Genotypes for Cultivation. Ind. Crop. Prod. 2021, 162, 113298. [Google Scholar] [CrossRef]
- Kurobe, K.; Nakao, S.; Nishiwaki, M.; Matsumoto, N. Combined Effect of Coffee Ingestion and Repeated Bouts of Low-Intensity Exercise on Fat Oxidation. Clin. Physiol. Funct. Imaging 2017, 37, 148–154. [Google Scholar] [CrossRef]
- Mansour, M.S.; Ni, Y.-M.; Roberts, A.L.; Kelleman, M.; RoyChoudhury, A.; St-Onge, M.-P. Ginger Consumption Enhances the Thermic Effect of Food and Promotes Feelings of Satiety without Affecting Metabolic and Hormonal Parameters in Overweight Men: A Pilot Study. Metab. Clin. Exp. 2012, 61, 1347–1352. [Google Scholar] [CrossRef] [Green Version]
- Zemestani, M.; Rafraf, M.; Asghari-Jafarabadi, M. Chamomile Tea Improves Glycemic Indices and Antioxidants Status in Patients with Type 2 Diabetes Mellitus. Nutrition 2016, 32, 66–72. [Google Scholar] [CrossRef]
- Magalhães, L.M.; Santos, F.; Segundo, M.A.; Reis, S.; Lima, J.L.F.C. Rapid Microplate High-Throughput Methodology for Assessment of Folin-Ciocalteu Reducing Capacity. Talanta 2010, 83, 441–447. [Google Scholar] [CrossRef]
- Adnan, M.; Ahmad, A.; Ahmed, A.; Khalid, N.; Hayat, I.; Ahmed, I. Chemical Composition and Sensory Evaluation of Tea (Camellia Sinensis) Commercialized in Pakistan. Pak. J. Bot. 2013, 45, 901–907. [Google Scholar]
- Theron, K.A.; Muller, M.; van der Rijst, M.; Cronje, J.C.; le Roux, M.; Joubert, E. Sensory Profiling of Honeybush Tea (Cyclopia Species) and the Development of a Honeybush Sensory Wheel. Food Res. Int. 2014, 66, 12–22. [Google Scholar] [CrossRef]
- Mayer, C.; Côme, M.; Blanckaert, V.; Chini Zittelli, G.; Faraloni, C.; Nazih, H.; Ouguerram, K.; Mimouni, V.; Chénais, B. Effect of Carotenoids from Phaeodactylum Tricornutum on Palmitate-Treated HepG2 Cells. Molecules 2020, 25, 2845. [Google Scholar] [CrossRef] [PubMed]
- Hoang, N.A.; Richter, F.; Schubert, M.; Lorkowski, S.; Klotz, L.-O.; Steinbrenner, H. Differential Capability of Metabolic Substrates to Promote Hepatocellular Lipid Accumulation. Eur. J. Nutr. 2019, 58, 3023–3034. [Google Scholar] [CrossRef] [PubMed]






| HII Infusion M ± SD (Min–Max) | Hot Water | |
|---|---|---|
| Water (g) | 200 | 200 |
| Total identified phenolic compounds (mg) | 15.5 ± 4.2 (10.0–20.0) | 0 |
| Total hydroxycinnamic acids (mg) | 7.6 ± 1.3 (6.0–9.0) | 0 |
| Caffeoylquinic acids (mg) | 3.0 ± 0.8 (2.0–4.0) | 0 |
| Total hydroxybenzoic acids (mg) | 0.53 ± 0.17 (0.30–0.70) | 0 |
| Total flavonoids (mg) | 1.4 ± 0.4 (1.0–2.0) | 0 |
| Total flavonols (mg) | 0.7 ± 0.2 (0.5–0.9) | 0 |
| Quercetin and its derivatives (mg) | 0.10 ± 0.05 (0.05–0.15) | 0 |
| Kaempferol and its derivatives (mg) | 0.2 ± 0.1 (0.1–0.3) | 0 |
| Total arzanol derivatives and other pyrones (mg) | 2.4 ± 0.9 (1.5–3.5) | 0 |
| Arzanol (mg) | 0.2 ± 0.1 (0.1–0.3) | 0 |
| Mean ± SD All (n = 11) | Mean ± SD Group 1 (n = 6) | Mean ± SD Group 2 (n = 5) | |
|---|---|---|---|
| Age (years) | 34.7 ± 8.8 | 34.0 ± 8.7 | 35.2 ± 8.8 |
| Height (cm) | 182.8 ± 7.4 | 183.3 ± 6.1 | 181.8 ± 9.4 |
| Weight (kg) | 76.2 ± 10.6 | 74.5 ± 10.4 | 78.2 ± 10.2 |
| BMI (kg/m2) | 22.8 ± 2.1 | 22.3 ± 2.4 | 23.2 ± 1.6 |
| Body fat (%) | 13.9 ± 3.1 | 14.2 ± 2.7 | 13.8 ± 4.5 |
| Fat mass (kg) | 11.1 ± 3.1 | 11.2 ± 3.5 | 11.1 ± 4.3 |
| FFM (kg) | 65.6 ± 9.1 | 63.8 ± 8.7 | 68.3 ± 8.9 |
| Blood pressure (mmHg) | 121 ± 8/74 ± 6 | 122 ± 8/75 ± 7 | 119 ± 6/74 ± 3 |
| HII Infusion | Hot Water | Effect | |||||
|---|---|---|---|---|---|---|---|
| Baseline | 30 min | 120 min | Baseline | 30 min | 120 min | Trial × Time | |
| REE (kcal/day) | 1676 ± 56 | 1699 ± 45 | 1738 ± 42 * | 1708 ± 61 | 1669 ± 65 * | 1713 ± 62 | <0.01 |
| RQ | 0.88 ± 0.02 | 0.82 ± 0.03 * | 0.82 ± 0.02 * | 0.87 ± 0.02 | 0.83 ± 0.02 | 0.84 ± 0.03 | <0.01 |
| Fat (%) | 38 ± 8 | 56 ± 10 * | 59 ± 6 * | 41 ± 8 | 52 ± 9 | 50 ± 7 | <0.01 |
| SBP (mmHg) | 120 ± 3 | 118 ± 3 | 120 ± 3 | 124 ± 3 | 121 ± 3 | 120 ± 3 | 0.345 |
| DBP (mmHg) | 74 ± 2 | 70 ± 2 * | 71 ± 2 | 72 ± 3 | 71 ± 3 | 69 ± 3 | 0.657 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kenig, S.; Kramberger, K.; Petelin, A.; Bandelj, D.; Baruca Arbeiter, A.; Miklavčič Višnjevec, A.; Peeters, K.; Mohorko, N.; Šik Novak, K.; Jenko Pražnikar, Z. Helichrysum italicum ssp. italicum Infusion Promotes Fat Oxidation in Hepatocytes and Stimulates Energy Expenditure and Fat Oxidation after Acute Ingestion in Humans: A Pilot Study. Plants 2021, 10, 1516. https://doi.org/10.3390/plants10081516
Kenig S, Kramberger K, Petelin A, Bandelj D, Baruca Arbeiter A, Miklavčič Višnjevec A, Peeters K, Mohorko N, Šik Novak K, Jenko Pražnikar Z. Helichrysum italicum ssp. italicum Infusion Promotes Fat Oxidation in Hepatocytes and Stimulates Energy Expenditure and Fat Oxidation after Acute Ingestion in Humans: A Pilot Study. Plants. 2021; 10(8):1516. https://doi.org/10.3390/plants10081516
Chicago/Turabian StyleKenig, Saša, Katja Kramberger, Ana Petelin, Dunja Bandelj, Alenka Baruca Arbeiter, Ana Miklavčič Višnjevec, Kelly Peeters, Nina Mohorko, Karin Šik Novak, and Zala Jenko Pražnikar. 2021. "Helichrysum italicum ssp. italicum Infusion Promotes Fat Oxidation in Hepatocytes and Stimulates Energy Expenditure and Fat Oxidation after Acute Ingestion in Humans: A Pilot Study" Plants 10, no. 8: 1516. https://doi.org/10.3390/plants10081516
APA StyleKenig, S., Kramberger, K., Petelin, A., Bandelj, D., Baruca Arbeiter, A., Miklavčič Višnjevec, A., Peeters, K., Mohorko, N., Šik Novak, K., & Jenko Pražnikar, Z. (2021). Helichrysum italicum ssp. italicum Infusion Promotes Fat Oxidation in Hepatocytes and Stimulates Energy Expenditure and Fat Oxidation after Acute Ingestion in Humans: A Pilot Study. Plants, 10(8), 1516. https://doi.org/10.3390/plants10081516